Postharvest Quality Improvement of Bell Pepper (Capsicum annuum L. cv Nagano) with Forced-Air Precooling and Modified Atmosphere Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Greenhouse Climate Condition
2.2. Chemicals and Reagents Used for the Study
2.3. Quality Assessment of Bell Pepper cv. Nagano
2.4. Extraction Procedure and HPLC Analysis of Soluble Sugars
2.5. Extraction Procedure and Spectrophotometric Analysis of Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activities
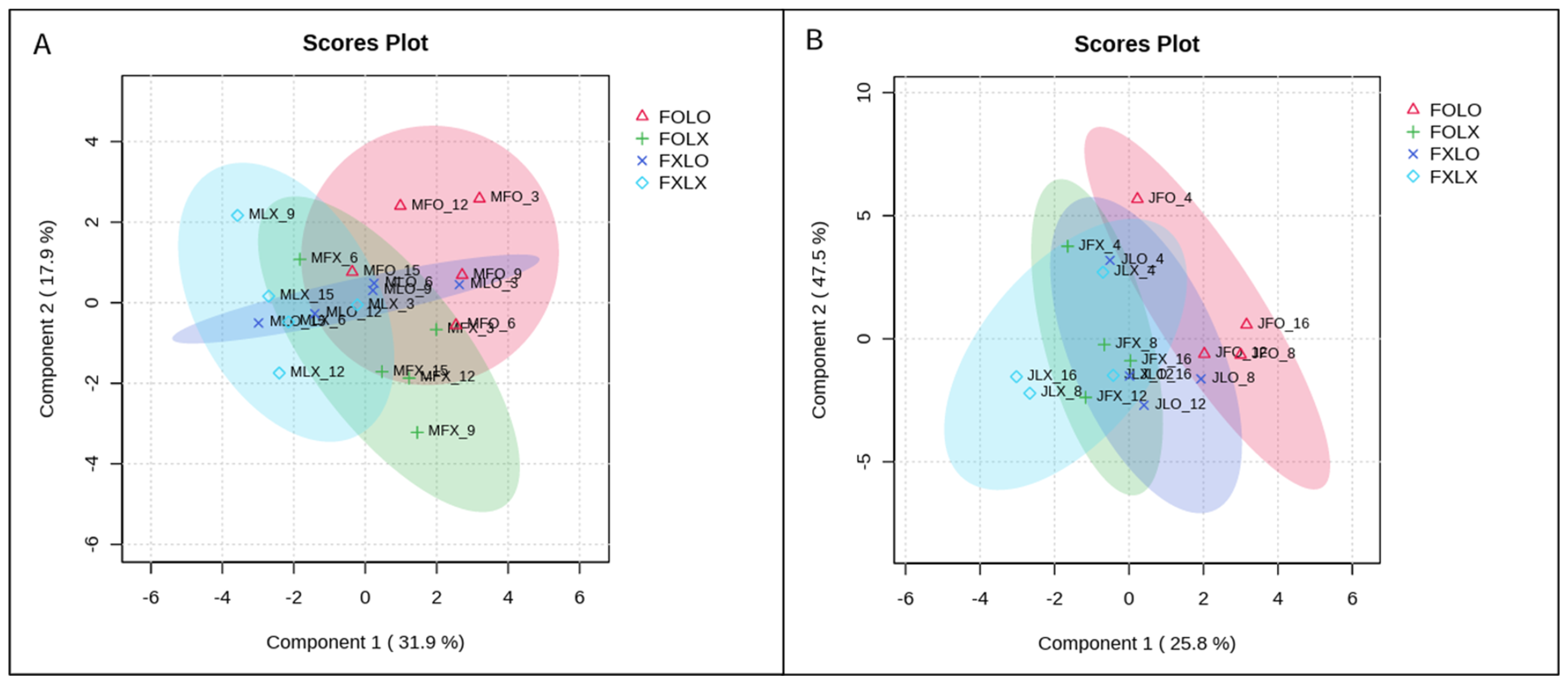
2.6. Experimental Design and Statistical Analysis
3. Results
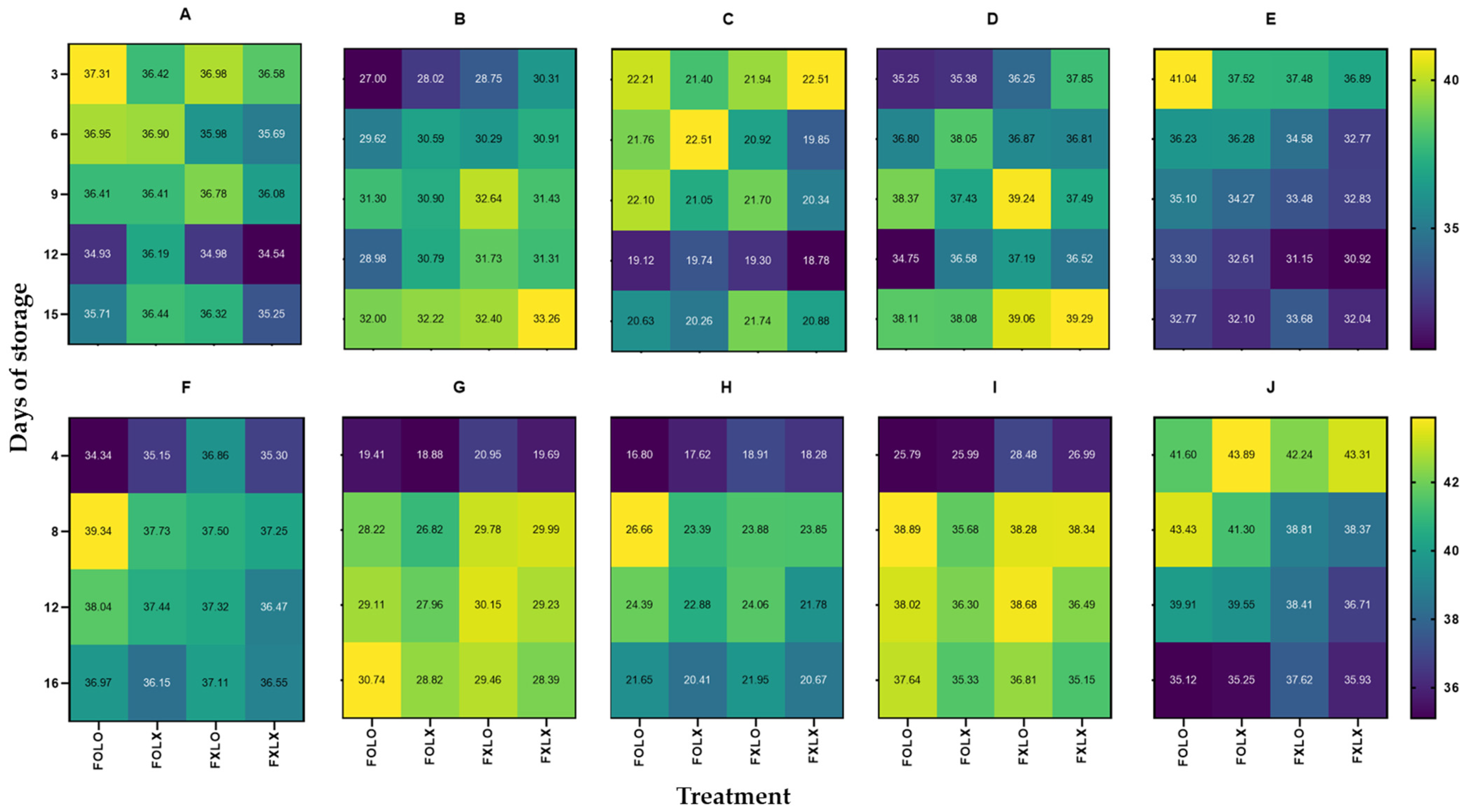
3.1. Weight Loss (WL)
3.2. Firmness
3.3. Fruit Color
3.4. Soluble Solids Content (SSC)
3.5. Soluble Sugar Contents
3.6. Bioactive Compounds of Bell Pepper cv. Nagano
3.7. Antioxidant Activity
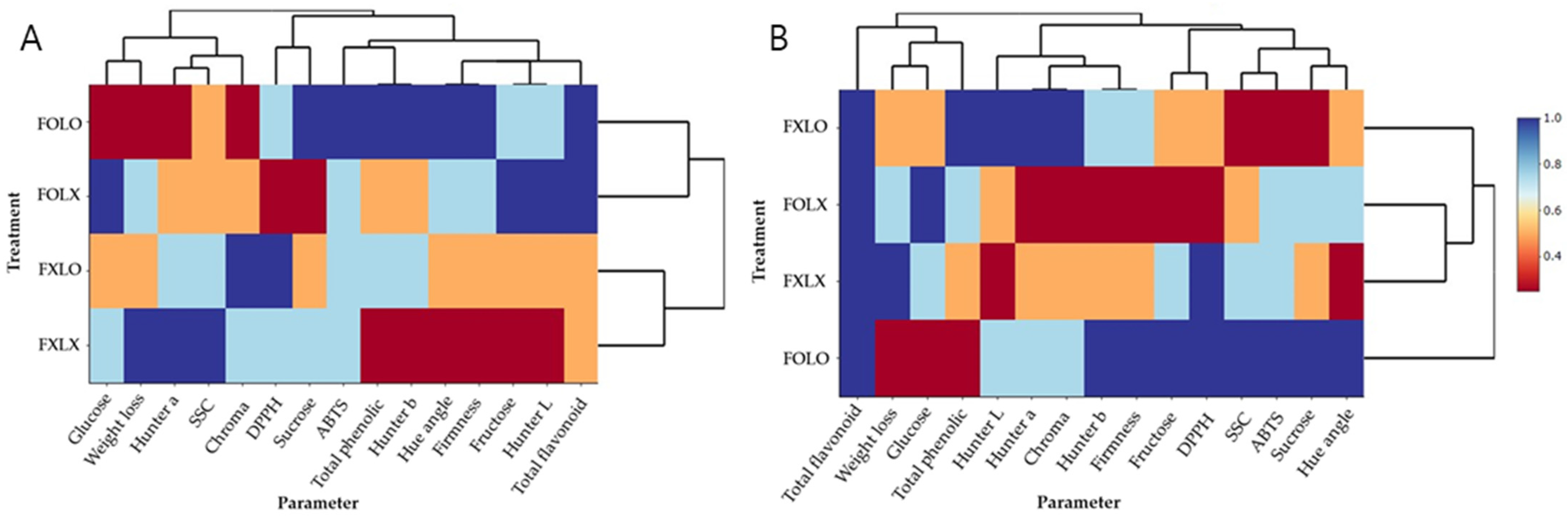
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tiamiyu, Q.O.; Adebayo, S.E.; Ibrahim, N. Recent advances on postharvest technologies of bell pepper: A review. Heliyon 2023, 9, e15302. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Abbasi, N.A.; Shafique, M.; Qureshi, A.A. Influence of Edible Coatings on Biochemical Fruit Quality and Storage Life of Bell Pepper cv. ‘Yolo Wonder’. J. Food Qual. 2017, 2017, 2142409. [Google Scholar] [CrossRef]
- Olveira-Bouzas, V.; Pita-Calvo, C.; Romero-Rodríguez, M.Á.; Vázquez-Odériz, M.L. Evaluation of a Packaging System in Pallets Under Modified Atmosphere to Extend the Shelf-life of ‘Padrón’ Peppers Stored at Refrigeration Temperature. Food Bioprocess Technol. 2023, 16, 785–803. [Google Scholar] [CrossRef]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef]
- Tsegay, D.; Tesfaye, B.; Mohammed, A.; Yirga, H.; Bayleyegn, A. Effects of harvesting stage and storage duration on postharvest quality and shelf life of sweet bell pepper (Capsicum annuum L.) varieties under passive refrigeration system. Int. J. Biotechnol. Mol. Biol. Res. 2013, 4, 60–70. [Google Scholar] [CrossRef]
- Rives-Castillo, S.C.H.; Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Hernández-López, M.; Barrera-Necha, L.L.; Ventura-Aguilar, R.I.; Bautista-Baños, S. The Effect of Netting Bags on the Postharvest Quality, Bioactive and Nutritional Compounds, and the Spoilage Microorganisms Content of Bell Peppers. Foods 2023, 12, 2071. [Google Scholar] [CrossRef]
- Young, E.; Mirosa, M.; Bremer, P. A systematic review of consumer perceptions of smart packaging technologies for food. Front. Sustain. Food Syst. 2020, 4, 63. [Google Scholar] [CrossRef]
- Tadesse, T.; Hewett, E.W.; Nichols, M.A.; Fisher, K.J. Changes in physicochemical attributes of sweet pepper cv. Domino during fruit growth and development. Sci. Hortic. 2002, 93, 91–103. [Google Scholar] [CrossRef]
- Frank, C.A.; Nelson, R.G.; Simonne, E.H.; Behe, B.K.; Simonne, A.H. Consumer preferences for color, price, and vitamin C content of bell peppers. HortScience 2001, 36, 795–800. [Google Scholar] [CrossRef]
- Danojević, D.; Glogovac, S.K.; Moravčević, Đ.; Medić Pap, S.S. Preferences of Serbian Consumers towards Different Pepper Fruits. Food Feed Res. 2021, 48, 155–163. [Google Scholar] [CrossRef]
- Sánchez Toledano, B.I.; Camarena Gómez, D.M.J.; López Santiago, M.A.; Cuevas Reyes, V. Consumer Preferences of Jalapeño Pepper in the Mexican Market. Horticulturae 2023, 9, 684. [Google Scholar] [CrossRef]
- Cheema, A.; Padmanabhan, P.; Amer, A.; Parry, M.J.; Lim, L.-T.; Subramanian, J.; Paliyath, G. Postharvest hexanal vapor treatment delays ripening and enhances shelf life of greenhouse grown sweet bell pepper (Capsicum annum L.). Postharvest Biol. Technol. 2018, 136, 80–89. [Google Scholar] [CrossRef]
- Correia, A.F.K.; Loro, A.C.; Zanatta, S.; Spoto, M.H.F.; Vieira, T.M.F.S. Effect of temperature, time, and material thickness on the dehydration process of tomato. Int. J. Food Sci. 2015, 2015, 970724. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Zaidi, S.; Arshad, M. Postharvest quality assessment of apple during storage at ambient temperature. Heliyon 2021, 7, e07714. [Google Scholar] [CrossRef]
- Hong, S.J.; Eum, H.L. Determination of the Harvest Date and Ripening Phase of ‘Seolhyang’ Strawberry. Prot. Hortic. Plant Fact. 2020, 29, 62–72. [Google Scholar] [CrossRef]
- Antoniali, S.; Leal, P.A.M.; de Magalhães, A.M.; Sanches, J. Forced-air pre-cooling of yellow bell pepper. Cienc. Rural 2012, 42, 1110–1116. [Google Scholar] [CrossRef]
- Nkolisa, N.; Magwaza, L.S.; Workneh, T.S.; Chimphango, A.; Sithole, N.J. Postharvest quality and bioactive properties of tomatoes (Solanum lycopersicum) stored in a low-cost and energy-free evaporative cooling system. Heliyon 2019, 5, e02266. [Google Scholar] [CrossRef]
- Park, H.-W.; Kim, S.-H.; Cha, H.-S.; Kim, Y.-H.; Choi, J.-Y. Effect of Quality Change of Fuji Apple by Pressure Cooling. Korean J. Food Preserv. 2006, 13, 427–431. [Google Scholar] [CrossRef]
- Nkolisa, N.; Magwaza, L.S.; Workneh, T.S.; Chimpango, A. Evaluating evaporative cooling system as an energy-free and cost-effective method for postharvest storage of tomatoes (Solanum lycopersicum L.) for smallholder farmers. Sci. Hortic. 2018, 241, 131–143. [Google Scholar] [CrossRef]
- Adhikari, P.; Paneru, N.; Thapa, K.; Dhakal, A. Effects of Perforation Mediated-modified Atmospheric Packaging (MAP) on Shelf Life and Quality of Calcium Chloride Treated Bell Pepper (Capsicum annum). Asian J. Dairy Food Res. 2020, 39, 354–358. [Google Scholar] [CrossRef]
- Singh, R.; Giri, S.K.; Kotwaliwale, N. Shelf-life enhancement of green bell pepper (Capsicum annuum L.) under active modified atmosphere storage. Food Packag. Shelf Life 2014, 1, 101–112. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Dynamics and Physical and Physiological Quality of Pomegranate Fruit during Cold Storage. Foods 2021, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Kumarihami, H.M.P.C.; Shin, M.H.; Jang, K.E.; Kim, Y.H.; Ma, K.B.; Kim, J.G. Modified Atmosphere Packaging and 1-Methylcyclopropene Treatments Maintain the Fruit Quality of ‘Wonmi’ Persimmons during Export Simulation. Foods 2022, 11, 4004. [Google Scholar] [CrossRef]
- Sharma, K.; Cardona, J.; Sibomana, M.; Herrera, N.; Nampeera, E.; Fallik, E. Quality attributes of modified atmosphere packaged bell pepper (Capsicum annuum L.) during storage. J. Nutr. Food Res. Technol. 2018, 1, 56–62. [Google Scholar] [CrossRef]
- Dirpan, A.; Ainani, A.F.; Djalal, M. A bibliometrics visualization analysis of active packaging system for food packaging. Heliyon 2023, 9, e18457. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plant Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive characteristics and antioxidant activities of nine peppers. J. Funct. Foods 2012, 4, 331–338. [Google Scholar] [CrossRef]
- Smith, D.L.; Stommel, J.R.; Fung, R.W.M.; Wang, C.Y.; Whitaker, B.D. Influence of cultivar and harvest method on postharvest storage quality of pepper (Capsicum annuum L.) fruit. Postharvest Biol. Technol. 2006, 42, 243–247. [Google Scholar] [CrossRef]
- Kabir, M.S.N.; Chowdhury, M.; Lee, W.H.; Hwang, Y.S.; Cho, S.I.; Chung, S.O. Influence of delayed cooling on quality of bell pepper (Capsicum annuum L.) stored in a controlled chamber. Emirates J. Food Agric. 2019, 31, 271–280. [Google Scholar] [CrossRef]
- Antoniali, S.; Leal, P.A.M.; De Magalhães, A.M.; Fuziki, R.T.; Sanches, J. Physico-chemical characterization of ‘Zarco HS’ yellow bell pepper for different ripeness stages. Sci. Agric. 2007, 64, 19–22. [Google Scholar] [CrossRef]
- Qin, Y.; Zhuang, Y.; Wu, Y.; Li, L. Quality evaluation of hot peppers stored in biodegradable poly(lactic acid)-based active packaging. Sci. Hortic. 2016, 202, 1–8. [Google Scholar] [CrossRef]
- Chitravathi, K.; Chauhan, O.P.; Raju, P.S. Influence of modified atmosphere packaging on shelf-life of green chillies (Capsicum annuum L.). Food Packag. Shelf Life 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, R.; Li, B.; Tian, S. Characterisation of genes encoding key enzymes involved in sugar metabolism of apple fruit in controlled atmosphere storage. Food Chem. 2013, 141, 3323–3328. [Google Scholar] [CrossRef]
- Yamaki, S. Metabolism and accumulation of sugars translocated to fruit and their regulation. J. Jpn. Soc. Hortic. Sci. 2010, 79, 1–15. [Google Scholar] [CrossRef]
- Hu, W.; Sun, D.W.; Pu, H.; Pan, T. Recent Developments in Methods and Techniques for Rapid Monitoring of Sugar Metabolism in Fruits. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1067–1079. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling during Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Bucheli, P.; Dévaud, S. Sugar accumulation in tomato and partial purification of buffer-insoluble invertase. Phytochemistry 1994, 36, 837–841. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 2014, 65, 821–832. [Google Scholar] [CrossRef]
- Lee, Y.; Howard, L.R.; Villalon, B. Flavonoids and Antioxidant Activity of Fresh Pepper (Capsicum annuum) Cultivars. J. Food Sci. 1995, 60, 473–476. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.d.J.; Cira-Chávez, L.A.; Gardea-Béjar, A.A.; Guevara-Arauza, J.C.; Sepúlveda, D.R.; Reyes-Hernández, J.; Ruiz-Cruz, S. Effect of heat treatment on the content of some bioactive compounds and free radical-scavenging activity in pungent and non-pungent peppers. Food Res. Int. 2013, 50, 519–525. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; López, J.; Parada, G.; Sanders, M.; Aranda, M.; Uribe, E.; Di Scala, K. Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa Willd.). Ind. Crops Prod. 2010, 32, 258–263. [Google Scholar] [CrossRef]
- Duma, M.; Alsina, I.; Dubova, L.; Erdberga, I. Quality of tomatoes during storage. Foodbalt 2017, 130–133. [Google Scholar] [CrossRef]
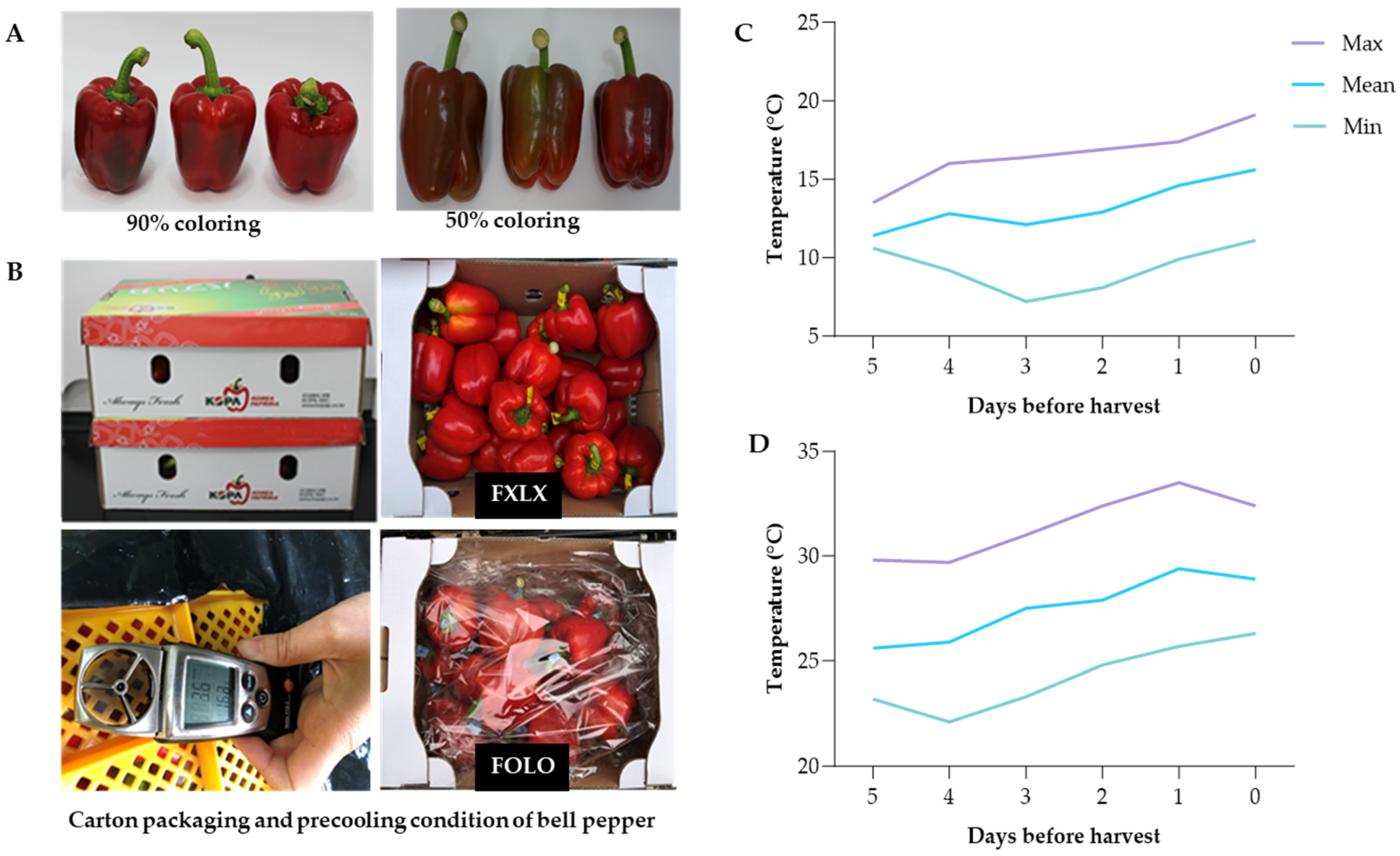

| 90% Coloring | 50% Coloring | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Treatment | 3 Days | 6 Days | 9 Days | 12 Days | 15 Days | 4 Days | 8 Days | 12 Days | 16 Days |
| SSC (°Brix) | FOLO | 6.6 ± 0.3a | 6.3 ± 0.2b | 6.5 ± 0.4bc | 6.3 ± 0.4b | 6.3 ± 0.3bc | 6.9 ± 0.8a | 6.0 ± 0.3b | 6.4 ± 0.7a | 6.8 ± 0.5a |
| FOLX | 6.4 ± 0.4ab | 6.4 ± 0.3b | 6.9 ± 0.6a | 6.3 ± 0.1b | 6.1 ± 0.7c | 6.7 ± 0.7a | 6.7 ± 0.6a | 5.6 ± 0.3b | 6.4 ± 0.2ab | |
| FXLO | 6.1 ± 0.6b | 6.3 ± 0.3b | 6.3 ± 0.6c | 6.9 ± 0.2a | 6.6 ± 0.4b | 6.1 ± 0.1b | 6.5 ± 0.3ab | 5.4 ± 0.9b | 6.3 ± 0.4b | |
| FXLX | 6.7 ± 0.2a | 6.8 ± 0.6a | 6.8 ± 0.4ab | 6.9 ± 0.6a | 7.1 ± 0.7a | 6.7 ± 0.5a | 6.5 ± 0.3ab | 6.4 ± 0.3a | 6.2 ± 0.3b | |
| ** | ** | ** | *** | *** | * | ** | *** | ** | ||
| TPC (mg GAE/g DW) | FOLO | 4.74 ± 0.60a | 3.97 ± 0.14a | 4.31 ± 0.29ab | 4.72 ± 0.43a | 4.34 ± 0.27a | 4.63 ± 0.43a | 4.30 ± 0.30b | 4.16 ± 0.23b | 4.62 ± 0.36a |
| FOLX | 4.12 ± 0.31bc | 4.34 ± 0.21a | 3.51 ± 0.13c | 4.19 ± 0.64b | 4.00 ± 0.40a | 4.38 ± 0.06ab | 4.50 ± 0.28ab | 4.30 ± 0.24b | 4.89 ± 0.57a | |
| FXLO | 4.55 ± 0.24ab | 4.38 ± 0.68a | 4.35 ± 0.23a | 4.01 ± 0.29b | 4.09 ± 0.31a | 4.41 ± 0.14ab | 4.95 ± 0.26a | 4.68 ± 0.08a | 4.40 ± 0.72a | |
| FXLX | 4.01 ± 0.24c | 3.98 ± 0.13a | 4.09 ± 0.02b | 3.74 ± 0.13b | 4.21 ± 0.75a | 4.17 ± 0.14b | 4.72 ± 0.66ab | 4.21 ± 0.37b | 4.82 ± 0.17a | |
| ** | ns | *** | ** | ns | * | ns | ** | ns | ||
| TFC (mg QE/g DW) | FOLO | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.01 a | 0.01 ± 0.00b | 0.02 ± 0.00ab | 0.02 ± 0.02a | 0.01 ± 0.01a | 0.02 ± 0.00a | 0.03 ± 0.01a |
| FOLX | 0.01 ± 0.00a | 0.01 ± 0.00b | 0.02 ± 0.00a | 0.03 ± 0.01a | 0.03 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.01 ± 0.01a | 0.02 ± 0.01a | |
| FXLO | 0.02 ± 0.01a | 0.01 ± 0.00b | 0.01 ± 0.01a | 0.02 ± 0.01b | 0.01 ± 0.01b | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.01 ± 0.01a | 0.02 ± 0.01a | |
| FXLX | 0.02 ± 0.00a | 0.01 ± 0.00b | 0.02 ± 0.01a | 0.02 ± 0.01b | 0.01 ± 0.01b | 0.01 ± 0.00a | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.00a | |
| ns | ** | ns | * | ** | ns | ns | ns | ns | ||
| DPPH Inhibition rate (%) | FOLO | 43 ± 4a | 42 ± 7b | 46 ± 3a | 52 ± 7a | 48 ± 4a | 48 ± 4a | 50 ± 6a | 47 ± 3a | 50 ± 3a |
| FOLX | 49 ± 5a | 49 ± 2a | 37 ± 4b | 42 ± 6b | 44 ± 5a | 41 ± 3b | 49 ± 3a | 50 ± 3a | 49 ± 2ab | |
| FXLO | 45 ± 7a | 53 ± 5a | 49 ± 3a | 45 ± 5b | 43 ± 5a | 47 ± 3a | 51 ± 3a | 48 ± 3a | 46 ± 3b | |
| FXLX | 48 ± 2a | 49 ± 3a | 45 ± 2a | 41 ± 2b | 46 ± 7a | 47 ± 3a | 50 ± 3a | 50 ± 3a | 50 ± 3a | |
| ns | ** | *** | ** | ns | ** | ns | ns | ns | ||
| ABTS Inhibition rate (%) | FOLO | 23 ± 2b | 25 ± 3ab | 26 ± 1ab | 27 ± 2a | 28 ± 1a | 30 ± 1a | 27 ± 2a | 26 ± 2a | 30 ± 1a |
| FOLX | 25 ± 2ab | 27 ± 1a | 22 ± 3c | 23 ± 1b | 22 ± 2b | 25 ± 1c | 28 ± 3a | 27 ± 1a | 28 ± 2a | |
| FXLO | 26 ± 1a | 20 ± 1c | 28 ± 2a | 24 ± 1b | 22 ± 3b | 25 ± 1c | 28 ± 1a | 26 ± 1a | 24 ± 4b | |
| FXLX | 24 ± 2ab | 24 ± 1b | 24 ± 3bc | 23 ± 4b | 24 ± 3b | 27 ± 1b | 28 ± 4a | 26 ± 4a | 27 ± 1ab | |
| ns | *** | ** | * | ** | *** | ns | ns | ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeboah, S.; Hong, S.J.; Park, Y.; Choi, J.H.; Eum, H.L. Postharvest Quality Improvement of Bell Pepper (Capsicum annuum L. cv Nagano) with Forced-Air Precooling and Modified Atmosphere Packaging. Foods 2023, 12, 3961. https://doi.org/10.3390/foods12213961
Yeboah S, Hong SJ, Park Y, Choi JH, Eum HL. Postharvest Quality Improvement of Bell Pepper (Capsicum annuum L. cv Nagano) with Forced-Air Precooling and Modified Atmosphere Packaging. Foods. 2023; 12(21):3961. https://doi.org/10.3390/foods12213961
Chicago/Turabian StyleYeboah, Samuel, Sae Jin Hong, Yeri Park, Jeong Hee Choi, and Hyang Lan Eum. 2023. "Postharvest Quality Improvement of Bell Pepper (Capsicum annuum L. cv Nagano) with Forced-Air Precooling and Modified Atmosphere Packaging" Foods 12, no. 21: 3961. https://doi.org/10.3390/foods12213961
APA StyleYeboah, S., Hong, S. J., Park, Y., Choi, J. H., & Eum, H. L. (2023). Postharvest Quality Improvement of Bell Pepper (Capsicum annuum L. cv Nagano) with Forced-Air Precooling and Modified Atmosphere Packaging. Foods, 12(21), 3961. https://doi.org/10.3390/foods12213961





