Biofortified Beverage with Chlorogenic Acid from Stressed Carrots: Anti-Obesogenic, Antioxidant, and Anti-Inflammatory Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Plant Material
2.2. Juice Preparation and Storage Studies
2.3. Microbiological Validation
2.4. Physicochemical Analyses
2.5. Proximal and Dietary Fiber Analysis
2.6. Extraction, Identification, and Quantification of Phytochemicals
2.6.1. Phenolics
2.6.2. Carotenoids
2.6.3. Glucosinolates
2.6.4. Reduced, Oxidized, and Total Ascorbic Acid
2.7. Cell Culture
2.7.1. MMT Assay
2.7.2. Evaluation of Anti-Inflammatory Potential
2.7.3. Cellular Antioxidant Activity (CAA)
2.7.4. Evaluation of Antiadipogenic Potential
3T3-L1 Differentiation and ORO Staining
Free Glycerol Assay
Triglyceride Enzymatic Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Proximate and Dietary Fiber Composition
3.2. Physicochemical Characterization and Changes during Storage
3.3. Phytochemical Composition and Changes during Storage
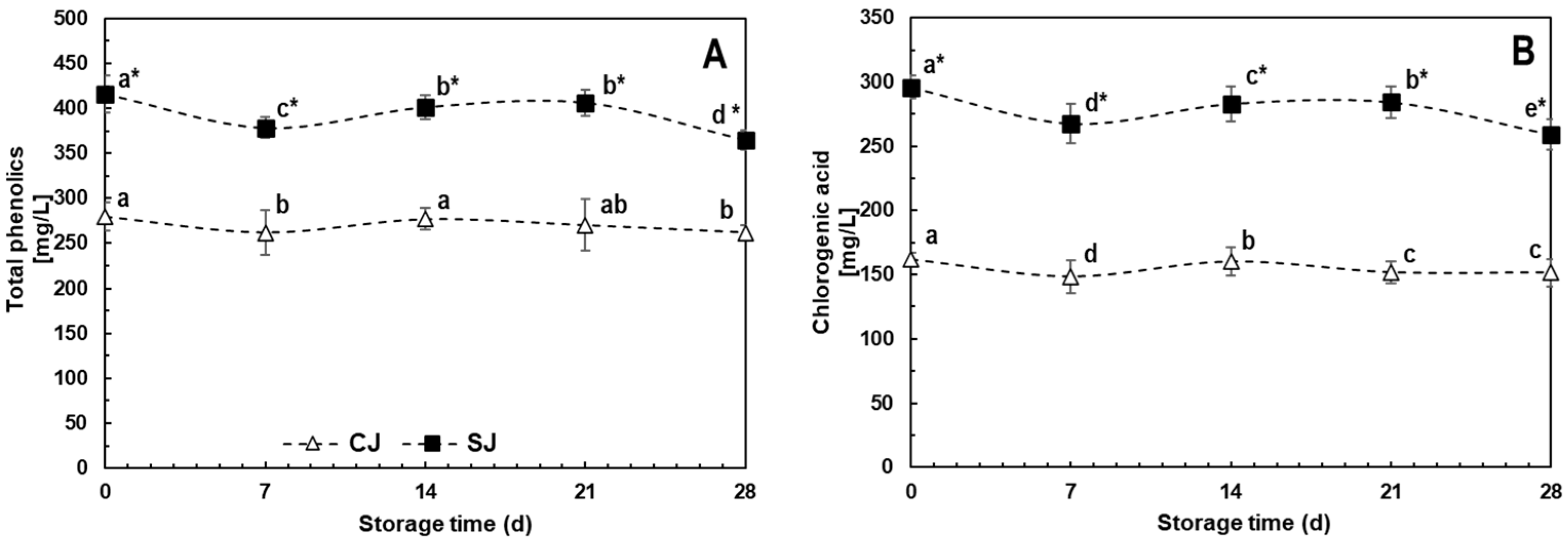
3.3.1. Phenolics
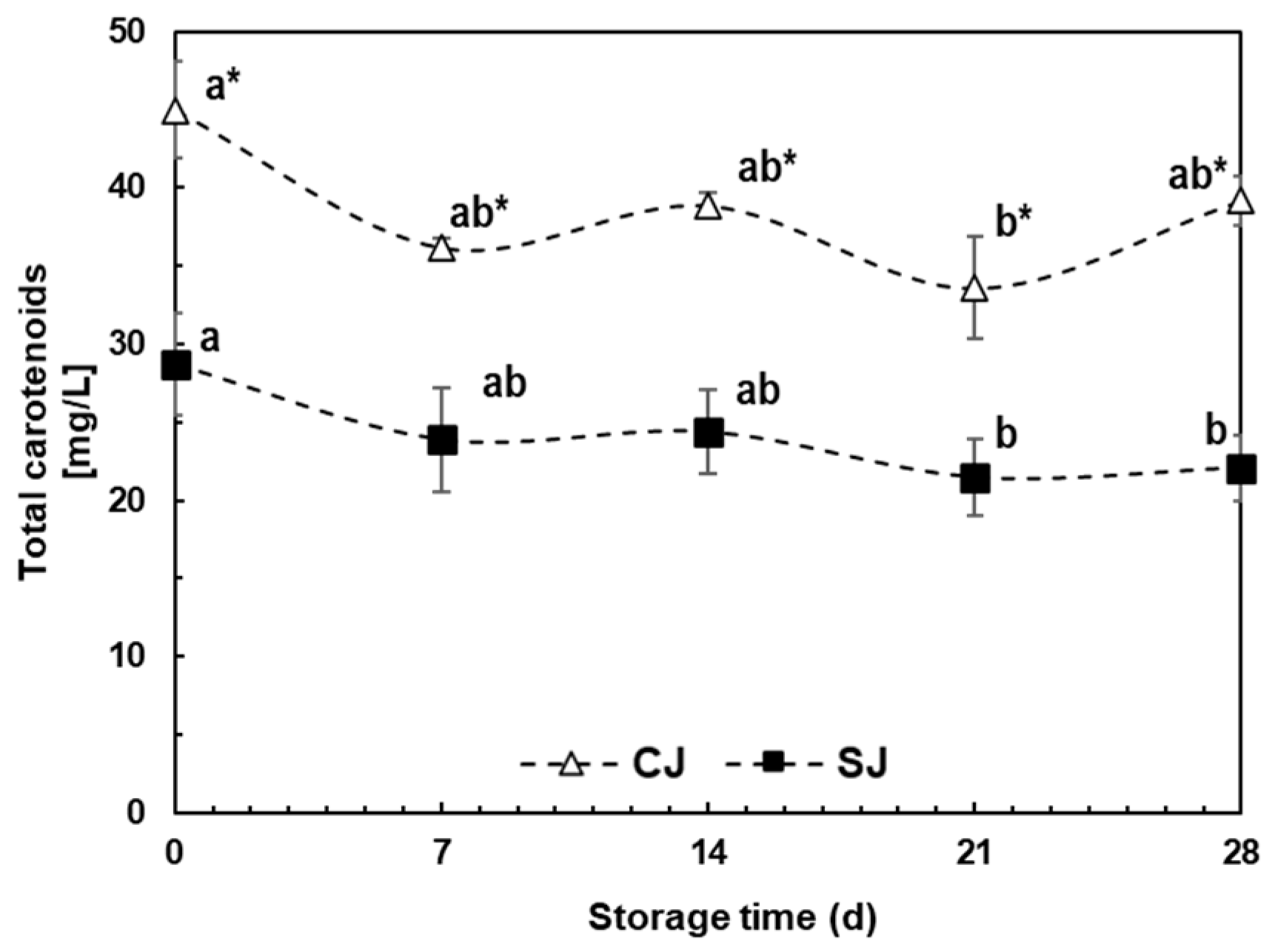
3.3.2. Carotenoids
3.3.3. Ascorbic Acid
3.3.4. Glucosinolates
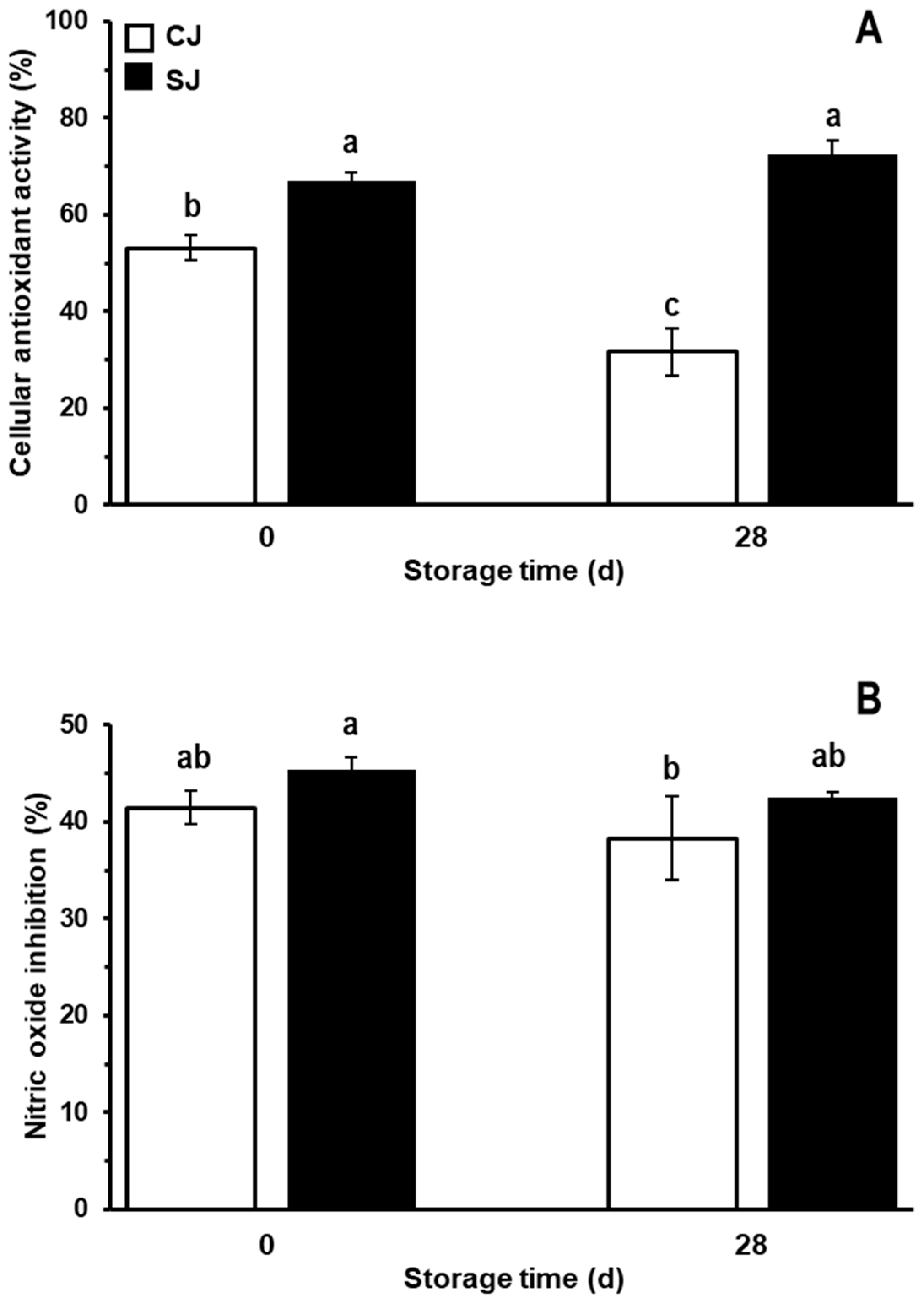
3.4. Antioxidant and Anti-Inflammatory Activity
3.5. Anti-Obesogenic Potential of Juice Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barquera, S.; Rivera, J.A. Obesity in Mexico: Rapid Epidemiological Transition and Food Industry Interference in Health Policies. Lancet Diabetes Endocrinol. 2020, 8, 746–747. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Hamernig, I.; Jian, L.; James, A.P.; Johnson, S.K.; Tapsell, L.C. Vegetables Containing Phytochemicals with Potential Anti-Obesity Properties: A Review. Food Res. Int. 2013, 52, 323–333. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical Composition, Functional Properties and Processing of Carrot—A Review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, A.K.; BØhn, S.K.; Smeland, S.; Thoresen, M.; Smedshaug, G.B.; TausjØ, J.; Svilaas, A.; Karlsen, A.; Russnes, K.M.; Svilaas, T.; et al. Postradiotherapy Plasma Lutein, α-Carotene, and β-Carotene Are Positively Associated with Survival in Patients with Head and Neck Squamous Cell Carcinoma. Nutr. Cancer 2010, 62, 322–328. [Google Scholar] [CrossRef]
- Sharma, K.D.; Stähler, K.; Smith, B.; Melton, L. Antioxidant Capacity, Polyphenolics and Pigments of Broccoli-Cheese Powder Blends. J. Food Sci. Technol. 2011, 48, 510–514. [Google Scholar] [CrossRef][Green Version]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, Medicinal Properties and Their Application in Food and Nutraceutical Industry. J. Med. Plants Res. 2011, 5, 322–328. [Google Scholar] [CrossRef]
- Kasperczyk, S.; Dobrakowski, M.; Kasperczyk, J.; Ostałowska, A.; Zalejska-Fiolka, J.; Birkner, E. Beta-Carotene Reduces Oxidative Stress, Improves Glutathione Metabolism and Modifies Antioxidant Defense Systems in Lead-Exposed Workers. Toxicol. Appl. Pharmacol. 2014, 280, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hamauzu, Y. Phenolic Compounds and Their Antioxidant Properties in Different Tissues of Carrots (Daucus carota L.). J. Food Agric. Environ. 2004, 2, 95–100. [Google Scholar]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Does Consumption of Tortured Fruit and Vegetables Improve Health, and Do They Taste Good? ACS Food Sci. Technol. 2023, 3, 1311–1313. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled Abiotic Stresses Revisited: From Homeostasis through Hormesis to Extreme Stresses and the Impact on Nutraceuticals and Quality during Pre- and Postharvest Applications in Horticultural Crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A. Transformation of Carrots into Novel Food Ingredients and Innovative Healthy Foods. Appl. Food Res. 2023, 3, 100303. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-154902-8.
- Malik, V.S.; Hu, F.B. The Role of Sugar-Sweetened Beverages in the Global Epidemics of Obesity and Chronic Diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Fahey, J.W.; Holtzclaw, W.D.; Prestera, T.; Zhang, Y. Chemoprotection against Cancer by Phase 2 Enzyme Induction. Toxicol. Lett. 1995, 82–83, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Villela Castrejón, J.; Serna-Saldívar, S.O.; Jacobo-Velázquez, D.A. Anticancer Potential of Dihydrocaffeic Acid: A Chlorogenic Acid Metabolite. CyTA-J. Food 2020, 18, 245–248. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Postharvest Wounding Stress in Horticultural Crops as a Tool for Designing Novel Functional Foods and Beverages with Enhanced Nutraceutical Content: Carrot Juice as a Case Study. J. Food Sci. 2019, 84, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- NOM-092-SSA1-1994; Secretaría de Salud. Bienes y Servicios. Método Para la Cuenta de Bacterias Aerobias en Placa. Diario Oficial de la Federación: Mexico City, Mexico, 1994.
- NOM-210-SSA1-2014; Secretaría de Salud. Productos y Servicios. Métodos de Prueba Microbiológicos. Determinación de Microorganismos Indicadores. Determinación de Microorganismos Patógenos. Diario Oficial de la Federación: Mexico City, Mexico, 2014.
- NOM-111-SSA1-1994; Secretaría de Salud. Bienes y Servicios. Método Para La Cuenta de Mohos y Levaduras En Alimentos. Diario Oficial de la Federación: Mexico City, Mexico, 1994.
- NMX-F-083-1986; Secretaría de Economía. Alimentos. Determinación de Humedad En Productos Alimenticios. Dirección General de Normas: Mexico City, Mexico, 1986.
- NOM-F-68-S-1980; Secretaría de Economía. Alimentos Determinación de Proteínas. Diario Oficial de la Federación: Mexico City, Mexico, 1980.
- NMX-F-615-NORMEX-2004; NORMEX. Alimentos—Determinación de Extracto Etéreo. Sociedad Mexicana de Normalización y Certificación, S.C: Mexico City, Mexico, 2004.
- NOM-F-90-S-1978; Secretaría de Economía. Determinación de Fibra Cruda En Alimentos. Dirección General de Normas: Mexico City, Mexico, 1978.
- NMX-F-607-NORMEX-2020; NORMEX. Alimentos. Determinación de Cenizas en Alimentos—Método de Prueba. Sociedad Mexicana de Normalización y Certificación S.C.: Mexico City, Mexico, 2020.
- NMX-F-622-NORMEX-2008; NORMEX. Alimentos. Determinación de Fibra Dietética Fracción Insoluble y Fracción Soluble (Método Gravimétrico—Enzimático) en Alimentos—Método de Prueba. Sociedad Mexicana de Normalización y Certificación S.C.: Mexico City, Mexico, 2008.
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as Biofactories: Postharvest Stress-Induced Accumulation of Phenolic Compounds and Glucosinolates in Broccoli Subjected to Wounding Stress and Exogenous Phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Ainsworth, E.A. Measurement of Reduced, Oxidized and Total Ascorbate Content in Plants. Nat. Protoc. 2007, 2, 871–874. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Park, E.-J.; Rostama, B.; Pezzuto, J.M.; Chang, L.C. Inhibition of Nitric Oxide (NO) Production in Lipopolysaccharide (LPS)-Activated Murine Macrophage RAW 264.7 Cells by the Norsesterterpene Peroxide, Epimuqubilin A. Mar. Drugs 2010, 8, 429–437. [Google Scholar] [CrossRef]
- Davies, C.A.; Rocks, S.A.; O’ Shaughnessy, M.C.; Perrett, D.; Winyard, P.G. Analysis of Nitrite and Nitrate in the Study of Inflammation. In Inflammation Protocols; Winyard, P.G., Willoughby, D.A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2003; pp. 305–320. ISBN 978-1-59259-374-3. [Google Scholar]
- Tsikas, D. Analysis of Nitrite and Nitrate in Biological Fluids by Assays Based on the Griess Reaction: Appraisal of the Griess Reaction in the l-Arginine/Nitric Oxide Area of Research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Kellett, M.E.; Greenspan, P.; Pegg, R.B. Modification of the Cellular Antioxidant Activity (CAA) Assay to Study Phenolic Antioxidants in a Caco-2 Cell Line. Food Chem. 2018, 244, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for Effective Differentiation of 3T3-L1 Cells to Adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, Hydration, and Health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef]
- Lee, S.R.; Yi, S.A.; Nam, K.H.; Ryoo, R.; Lee, J.; Kim, K.H. Pantheric Acids A–C from a Poisonous Mushroom, Amanita Pantherina, Promote Lipid Accumulation in Adipocytes. J. Nat. Prod. 2019, 82, 3489–3493. [Google Scholar] [CrossRef] [PubMed]
- Olalude, C.B.; Oyedeji, F.O.; Adegboyega, A.M. Physico-Chemical Analysis of Daucus Carota (Carrot) Juice for Possible Industrial Applications. IOSR J. Appl. Chem. 2015, 8, 110–113. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Abaire, K.E. Quality Acceptability, Nutritional Composition and Antioxidant Properties of Carrot-Cucumber Juice. Beverages 2019, 5, 15. [Google Scholar] [CrossRef]
- Chuku, E.C.; Akani, N.P. Determination of Proximate Composition and Microbial Contamination of Fresh Juice from Three Citrus Species. IIARD Int. J. Biol. Med. Res. 2015, 1, 1–8. [Google Scholar]
- Banigo, E.B.; Kiin-Kabari, D.B.; Owuno, F. Physicochemical and Sensory Evaluation of Soy/Carrot Drinks Flavoured with Beetroot. Afr. J. Food Sci. Technol. 2015, 6, 136–140. [Google Scholar] [CrossRef]
- Dima, F.; Istrati, D.; Garnai, M.; Serea, V.; Vizireanu, C. Study on Obtaining Vegetables Juices with High Antioxidant Potential, Preserved by Ohmic Pasteurization. J. Agroaliment. Process. Technol. 2015, 21, 67–74. [Google Scholar]
- Braide, W.; Oranusi, S.U.; Otali, C.C. Nutritional, Antinutritional, Minerals and Vitamin Compositions of Fourteen Branfs of Fruit Juice Sold in Onitsha Main Market. Res. Basic Appl. Sci. 2012, 1, 4–6. [Google Scholar]
- Yanaka, A. Daily Intake of Sulforaphane-Rich Broccoli Sprouts Normalizes Bowel Habits in Healthy Human Subjects. FASEB J. 2017, 31, 972.20. [Google Scholar] [CrossRef]
- Bokić, J.; Škrobot, D.; Tomić, J.; Šeregelj, V.; Abellán-Victorio, Á.; Moreno, D.A.; Ilić, N. Broccoli Sprouts as a Novel Food Ingredient: Nutritional, Functional and Sensory Aspects of Sprouts Enriched Pasta. LWT 2022, 172, 114203. [Google Scholar] [CrossRef]
- Popoola-Akinola, O.O.; Raji, T.J.; Olawoye, B. Lignocellulose, Dietary Fibre, Inulin and Their Potential Application in Food. Heliyon 2022, 8, e10459. [Google Scholar] [CrossRef]
- Niu, L.; Wu, J.; Liao, X.; Chen, F.; Wang, Z.; Zhao, G.; Hu, X. Physicochemical Characteristics of Orange Juice Samples From Seven Cultivars. Agric. Sci. China 2008, 7, 41–47. [Google Scholar] [CrossRef]
- Romeo, R.; Bruno, A.D.; Piscopo, A.; Medina, E.; Ramírez, E.; Brenes, M.; Poiana, M. Effects of Phenolic Enrichment on Vitamin C and Antioxidant Activity of Commercial Orange Juice. Braz. J. Food Technol. 2020, 23, e2019130. [Google Scholar] [CrossRef]
- Salehi, F. Physico-Chemical and Rheological Properties of Fruit and Vegetable Juices as Affected by High Pressure Homogenization: A Review. Int. J. Food Prop. 2020, 23, 1136–1149. [Google Scholar] [CrossRef]
- Yu, L.J.; Rupasinghe, H.P.V. Effect of Acidification on Quality and Shelf-Life of Carrot Juice. Can. J. Plant Sci. 2012, 92, 1113–1120. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Brunton, N.; Rai, D.K.; Balagueró, E.; Hossain, M.B.; Valverde, J. Polyacetylene Levels in Carrot Juice, Effect of pH and Thermal Processing. Food Chem. 2014, 152, 370–377. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B. Non-Enzymatic Browning in Citrus Juice: Chemical Markers, Their Detection and Ways to Improve Product Quality. J. Food Sci. Technol. 2014, 51, 2271–2288. [Google Scholar] [CrossRef]
- Zhu, D.; Shen, Y.; Xu, L.; Cao, X.; Lv, C.; Li, J. Browning and Flavor Changing of Cloudy Apple Juice during Accelerated Storage. IOP Conf. Ser. Mater. Sci. Eng. 2019, 611, 012061. [Google Scholar] [CrossRef]
- Munsch, M.H.; Simard, R.E.; Girard, J.-M. Relationships in Colour and Carotene Content of Carrot Juices. Can. Inst. Food Sci. Technol. J. 1983, 16, 173–178. [Google Scholar] [CrossRef]
- Leahu, A.; Damian, C.; Carpiuc, N.; Oroian, M.; Avramiuc, M. Change in Colour and Physicochemical Quality of Carrot Juice Mixed with Other Fruits. J. Agroaliment. Process. Technol. 2013, 19, 241–246. [Google Scholar]
- Ferrario, M.; Guerrero, S.; Char, C. Optimisation of Minimal Processing Variables to Preserve the Functional Quality and Colour of Carrot Juice by Means of the Response Surface Methodology. Int. J. Food Sci. Technol. 2017, 52, 864–871. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Colour Degradation and Quality Parameters of Sonicated Orange Juice Using Response Surface Methodology. LWT-Food Sci. Technol. 2008, 41, 1876–1883. [Google Scholar] [CrossRef]
- Sirichan, T.; Kijpatanasilp, I.; Asadatorn, N.; Assatarakul, K. Optimization of Ultrasound Extraction of Functional Compound from Makiang Seed by Response Surface Methodology and Antimicrobial Activity of Optimized Extract with Its Application in Orange Juice. Ultrason. Sonochem. 2022, 83, 105916. [Google Scholar] [CrossRef] [PubMed]
- Blanco Gomis, D.; Sánchez Núñez, N.; Dolores Gutiérrez Álvarez, M. High Speed Liquid Chromatography for In-Process Control. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 931–948. [Google Scholar] [CrossRef]
- Ianni, F.; Barola, C.; Blasi, F.; Moretti, S.; Galarini, R.; Cossignani, L. Investigation on Chlorogenic Acid Stability in Aqueous Solution after Microwave Treatment. Food Chem. 2022, 374, 131820. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, M.; Santana-Gálvez, J.; Santacruz, A.; Carranza-Montealvo, L.D.; Ortega-Hernández, E.; Tirado-Escobosa, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Using a Functional Carrot Powder Ingredient to Produce Sausages with High Levels of Nutraceuticals. J. Food Sci. 2018, 83, 2351–2361. [Google Scholar] [CrossRef]
- Arvayo-Enríquez, H.; Mondaca-Fernández, I.; Gortárez-Moroyoqui, P.; López-Cervantes, J.; Rodríguez-Ramírez, R. Carotenoids Extraction and Quantification: A Review. Anal. Methods 2013, 5, 2916–2924. [Google Scholar] [CrossRef]
- Chen, H.E.; Peng, H.Y.; Chen, B.H. Stability of Carotenoids and Vitamin A during Storage of Carrot Juice. Food Chem. 1996, 57, 497–503. [Google Scholar] [CrossRef]
- Bell, T.; Alamzad, R.; Graf, B.A. Effect of pH on the Chemical Stability of Carotenoids in Juice. Proc. Nutr. Soc. 2016, 75, E94. [Google Scholar] [CrossRef]
- Chanson-Rolle, A.; Braesco, V.; Chupin, J.; Bouillot, L. Nutritional Composition of Orange Juice: A Comparative Study between French Commercial and Home-Made Juices. Food Nutr. Sci. 2016, 07, 252. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, J.-Y.; Qian, P.; Meng, J.; Zhang, X.-J.; Lu, R.-R. Comparative Study of the Microbial Stability and Quality of Carrot Juice Treated by High-Pressure Processing Combined with Mild Temperature and Conventional Heat Treatment. J. Food Process Eng. 2015, 38, 395–404. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Glucosinolates in Broccoli Sprouts (Brassica oleracea Var. Italica) as Conditioned by Sulphate Supply during Germination. J. Food Sci. 2010, 75, C673–C677. [Google Scholar] [CrossRef]
- Oliviero, T.; Verkerk, R.; Van Boekel, M.A.J.S.; Dekker, M. Effect of Water Content and Temperature on Inactivation Kinetics of Myrosinase in Broccoli (Brassica oleracea Var. Italica). Food Chem. 2014, 163, 197–201. [Google Scholar] [CrossRef]
- Burmeister, W.P.; Cottaz, S.; Rollin, P.; Vasella, A.; Henrissat, B. High Resolution X-Ray Crystallography Shows That Ascorbate Is a Cofactor for Myrosinase and Substitutes for the Function of the Catalytic Base. J. Biol. Chem. 2000, 275, 39385–39393. [Google Scholar] [CrossRef]
- Wang, J.; Barba, F.J.; Frandsen, H.B.; Sørensen, S.; Olsen, K.; Sørensen, J.C.; Orlien, V. The Impact of High Pressure on Glucosinolate Profile and Myrosinase Activity in Seedlings from Brussels Sprouts. Innov. Food Sci. Emerg. Technol. 2016, 38, 342–348. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.a.; Cisneros-Zevallos, L. Correlations of Antioxidant Activity against Phenolic Content Revisited: A New Approach in Data Analysis for Food and Medicinal Plants. J. Food Sci. 2009, 74, R107–R113. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Tsao, R. Anthocyanin-Rich Phenolic Extracts of Purple Root Vegetables Inhibit pro-Inflammatory Cytokines Induced by H2O2 and Enhance Antioxidant Enzyme Activities in Caco-2 Cells. J. Funct. Foods 2016, 22, 363–375. [Google Scholar] [CrossRef]
- Ning, W.; Peng, X.; Ma, L.; Cui, L.; Lu, X.; Wang, J.; Tian, J.; Li, X.; Wang, W.; Zhang, L. Enhanced Secondary Metabolites Production and Antioxidant Activity in Postharvest Lonicera Japonica Thunb. in Response to UV Radiation. Innov. Food Sci. Emerg. Technol. 2012, 13, 231–243. [Google Scholar] [CrossRef]
- Villamil-Galindo, E.; Antunes-Ricardo, M.; Piagentini, A.M.; Jacobo-Velázquez, D.A. Adding Value to Strawberry Agro-Industrial by-Products through Ultraviolet A-Induced Biofortification of Antioxidant and Anti-Inflammatory Phenolic Compounds. Front. Nutr. 2022, 9, 1080147. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic Anti-Inflammatory Effects and Mechanisms of Combined Phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Anzano, A.; Bonanomi, G.; Mazzoleni, S.; Lanzotti, V. Plant Metabolomics in Biotic and Abiotic Stress: A Critical Overview. Phytochem. Rev. 2022, 21, 503–524. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Yen, G.-C. Effects of Flavonoids and Phenolic Acids on the Inhibition of Adipogenesis in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2007, 55, 8404–8410. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.; Sasikumar, S.J.; Silambanan, S.; Natarajan, D.; Ramakrishnan, R.; Nair, A.J.; Kiran, M.S. Chlorogenic Acid Promotes Development of Brown Adipocyte-like Phenotype in 3T3-L1 Adipocytes. J. Funct. Foods 2020, 74, 104161. [Google Scholar] [CrossRef]




| Determinations | Samples i,ii,iii | |
|---|---|---|
| Control Juice (CJ, %) | Stressed Juice (SJ, %) | |
| Moisture | 90.6 ± 0.01 | 90.65 ± 0.02 |
| Ash | 0.44 ± 0.01 | 0.46 ± 0.001 |
| Total carbohydrates | 7.88 ± 0.01 | 7.94 ± 0.01 |
| Total fat | 0.14 ± 0.01 a | <0.10 b |
| Protein | 0.94 ± 0.00 a | 0.95 ± 0.01 b |
| Crude fiber | 0.25 ± 0.00 | 0.38 ± 0.01 |
| Total dietary fiber | 0.82 ± 0.01 | 0.80 ± 0.01 |
| Soluble dietary fiber | 0.55 ± 0.01 | 0.54 ± 0.01 |
| Insoluble dietary fiber | 0.27 ± 0.00 a | 0.25 ± 0.01 b |
| Samples i | Storage Time (Days) | Parameters ii,iii | |||
|---|---|---|---|---|---|
| Acidity (g/L Citric Acid) | pH | Soluble Solids (°Bx) | Insoluble Solids (g/L) | ||
| Control Juice (CJ) | 0 | 0.23 ± 0.001 b, A | 4.9 ± 0.03 a, A | 8.8 ± 0.03 a, A | 90.6 ± 0.06 a, A |
| 14 | 0.24 ± 0.001 a, A | 4.8 ± 0.001 b, A | 8.7 ± 0.001 b, A | 89.5 ± 0.18 b, A | |
| 28 | 0.23 ± 0.001 b, A | 4.8 ± 0.001 b, A | 8.1 ± 0.001 c, A | 90.09 ± 0.01 a, A | |
| Stressed Juice (SJ) | 0 | 0.23 ± 0.001 a, A | 4.8 ± 0.03 a, B | 8.7 ± 0.06 a, B | 91.2 ± 0.12 a, A |
| 14 | 0.27 ± 0.001 c, B | 4.7 ± 0.001 b, B | 8.7 ± 0.03 a, A | 90.6 ± 0.44 a, b, A | |
| 28 | 0.25 ± 0.001 b, B | 4.7 ± 0.01 b, B | 8.4 ± 0.03 b, B | 90.9 ± 0.08 b, A | |
| Samples i | Storage Time (Days) | Parameters ii,iii | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | Chroma | Hue | ||
| Control Juice (CJ) | 0 | 7.43 ± 0.008 a, A | 26.77 ± 0.006 a, A | 12.57 ± 0.02 a, A | 29.57 ± 0.003 a, A | 25.16 ± 0.04 a, A |
| 14 | 8.64 ± 0.017 b, A | 27.68 ± 0.008 b, A | 14.73 ± 0.006 b, A | 31.35 ± 0.01 b, A | 28.01 ± 0.007 b, A | |
| 28 | 14.01 ± 0.02 c, A | 31.19 ± 0.021 c, A | 24.02 ± 0.08 c, A | 39.36 ± 0.06 c, A | 37.59 ± 0.07 c, A | |
| Stressed Juice (SJ) | 0 | 11.47 ± 0.01 a, B | 26.11 ± 0.001 a, A | 19.3 ± 0.02 a, B | 32.46 ± 0.01 a, B | 36.47 ± 0.03 a, B |
| 14 | 17.24 ± 0.003 b, B | 15.14 ± 0.006 b, B | 7.99 ± 0.01 b, B | 17.12 ± 0.006 b, B | 27.80 ± 0.04 b, B | |
| 28 | 10.15 ± 0.04 c, B | 26.94 ± 0.05 c, B | 17.41 ± 0.07 c, B | 31.24 ± 0.08 c, B | 33.85 ± 0.06 c, B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastélum-Estrada, A.; Rabadán-Chávez, G.; Reza-Zaldívar, E.E.; de la Cruz-López, J.L.; Fuentes-Palma, S.A.; Mojica, L.; Díaz de la Garza, R.I.; Jacobo-Velázquez, D.A. Biofortified Beverage with Chlorogenic Acid from Stressed Carrots: Anti-Obesogenic, Antioxidant, and Anti-Inflammatory Properties. Foods 2023, 12, 3959. https://doi.org/10.3390/foods12213959
Gastélum-Estrada A, Rabadán-Chávez G, Reza-Zaldívar EE, de la Cruz-López JL, Fuentes-Palma SA, Mojica L, Díaz de la Garza RI, Jacobo-Velázquez DA. Biofortified Beverage with Chlorogenic Acid from Stressed Carrots: Anti-Obesogenic, Antioxidant, and Anti-Inflammatory Properties. Foods. 2023; 12(21):3959. https://doi.org/10.3390/foods12213959
Chicago/Turabian StyleGastélum-Estrada, Alejandro, Griselda Rabadán-Chávez, Edwin E. Reza-Zaldívar, Jessica L. de la Cruz-López, Sara A. Fuentes-Palma, Luis Mojica, Rocío I. Díaz de la Garza, and Daniel A. Jacobo-Velázquez. 2023. "Biofortified Beverage with Chlorogenic Acid from Stressed Carrots: Anti-Obesogenic, Antioxidant, and Anti-Inflammatory Properties" Foods 12, no. 21: 3959. https://doi.org/10.3390/foods12213959
APA StyleGastélum-Estrada, A., Rabadán-Chávez, G., Reza-Zaldívar, E. E., de la Cruz-López, J. L., Fuentes-Palma, S. A., Mojica, L., Díaz de la Garza, R. I., & Jacobo-Velázquez, D. A. (2023). Biofortified Beverage with Chlorogenic Acid from Stressed Carrots: Anti-Obesogenic, Antioxidant, and Anti-Inflammatory Properties. Foods, 12(21), 3959. https://doi.org/10.3390/foods12213959








