Expression of Ice Nucleation Protein in Bacillus amyloliquefaciens and Its Application in Food Freezing Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Construction of Bacillus amyloliquefaciens Expression Plasmids and Transformation
2.3. Bacillus Amyloliquefaciens BAX-9 Expression Analysis
2.4. Determination of the Supercooling Point Using Differential Scanning Calorimetry
2.5. Ice nucleation Activity Assays in Liquid Model Food System
2.6. Measurement of Freezing Curves in Liquid Model Food System
2.7. Freezing Curves of Real Food System
2.8. Microscopic Observation of Ice Crystal
2.9. Statistical Analysis
3. Results and Discussion
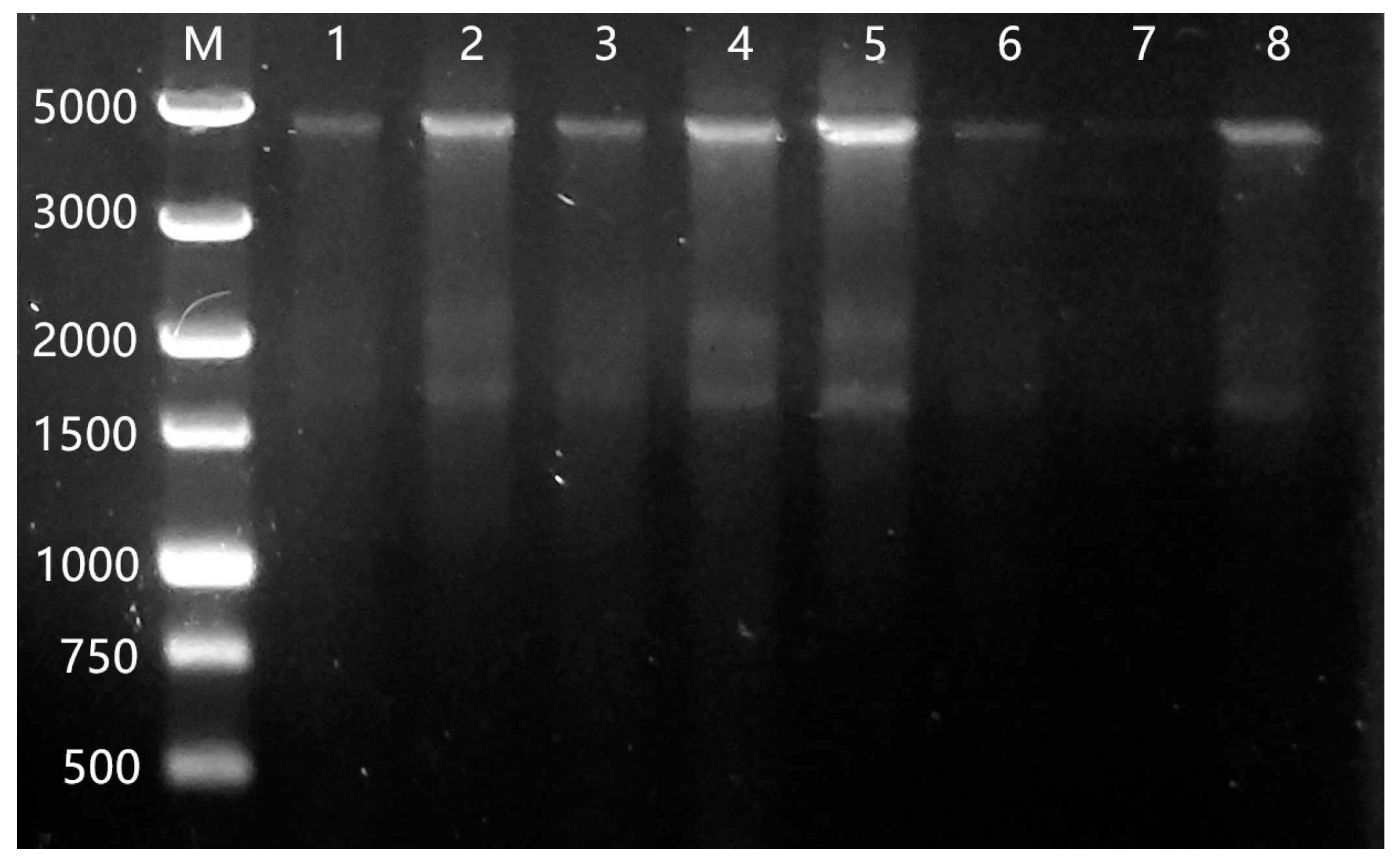
3.1. The Amplification of IceE Gene and Identification of Recombinant Clones
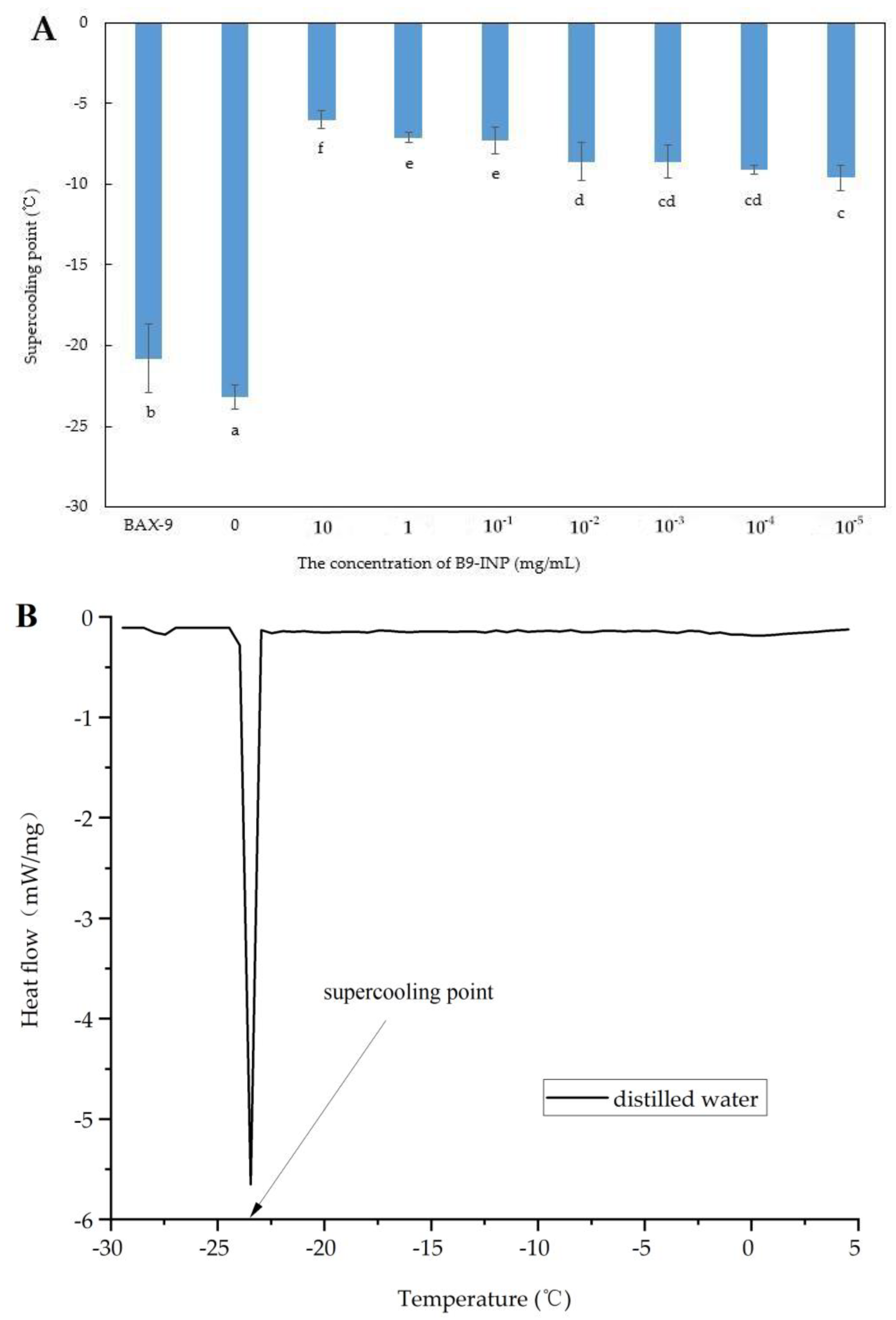
3.2. The Impact of B9-INP on Supercooling Point of Distilled Water
3.3. Effect of B9-INP on Supercooling Point of Liquid Model Food System
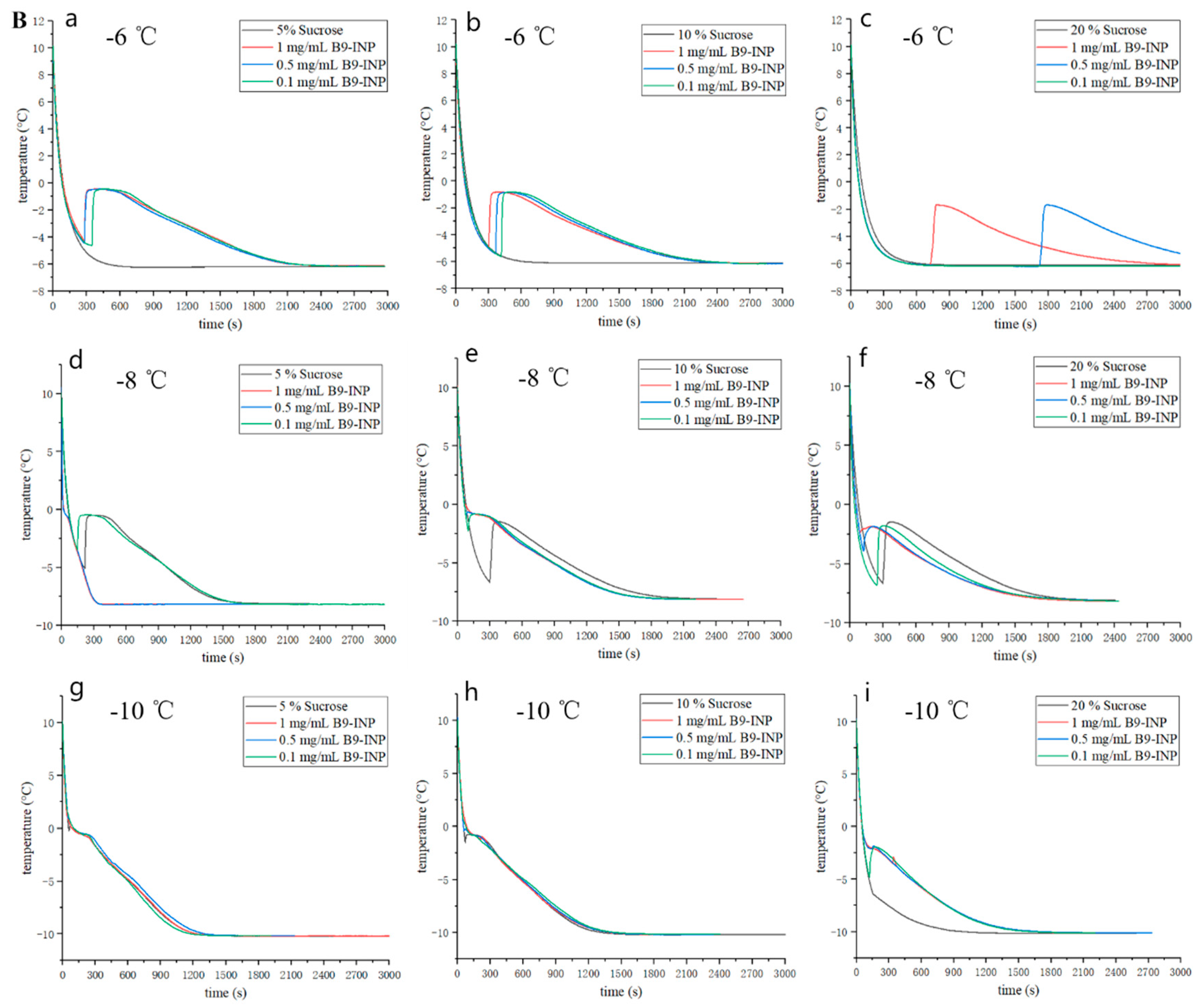
3.4. Freezing Curves of Liquid Model Food System
3.5. Freezing Curves of Real Food System
3.6. Microscopic Observation of Ice Crystal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maeda, N. Brief Overview of Ice Nucleation. Molecules 2021, 26, 392. [Google Scholar] [CrossRef] [PubMed]
- Kanji, Z.A.; Ladino, L.A.; Wex, H.; Boose, Y.; Burkert-Kohn, M.; Cziczo, D.J.; Krämer, M. Overview of Ice Nucleating Particles. Meteorol. Monogr. 2017, 58, 1.1–1.33. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Huang, S.; Ren, L.; Yue, S.; Li, P.; Xie, Q.; Zhao, W.; Wei, L.; Ren, H.; et al. Biological Aerosol Particles in Polluted Regions. Curr. Pollut. Rep. 2020, 6, 65–89. [Google Scholar] [CrossRef]
- Šantl-Temkiv, T.; Sikoparija, B.; Maki, T.; Carotenuto, F.; Amato, P.; Yao, M.; Morris, C.E.; Schnell, R.; Jaenicke, R.; Pöhlker, C.; et al. Bioaerosol Field Measurements: Challenges and Perspectives in Outdoor Studies. Aerosol Sci. Technol. 2019, 54, 520–546. [Google Scholar] [CrossRef]
- Huang, S.; Hu, W.; Chen, J.; Wu, Z.; Fu, P. Overview of Biological Ice Nucleating Particles in the Atmosphere. Environ. Int. 2021, 146, 106197. [Google Scholar] [CrossRef]
- Hartmann, S.; Ling, M.; Dreyer, L.S.A.; Zipori, A.; Finster, K.; Grawe, S.; Jensen, L.Z.; Borck, S.; Reicher, N.; Drace, T.; et al. Structure and Protein-Protein Interactions of Ice Nucleation Proteins Drive Their Activity. Front. Microbiol. 2022, 13, 872306. [Google Scholar] [CrossRef]
- Akila, M.; Priyamvada, H.; Ravikrishna, R.; Gunthe, S.S. Characterization of Bacterial Diversity and Ice-Nucleating Ability during Different Monsoon Seasons over a Southern Tropical Indian Region. Atmos. Environ. 2018, 191, 387–394. [Google Scholar] [CrossRef]
- Obata, H.; Saeki, Y.; Tanishita, J.; Tokuyama, T.; Hori, H.; Higashi, Y. Identification of an Ice-Nucleating Bacterium KUIN-1 as Pseudomonas Fluorescens and Its Ice Nucleation Properties. J. Agric. Chem. Soc. Jpn. 2014, 51, 1761–1766. [Google Scholar]
- Karimi, B.; Nosrati, R.; Fazly Bazzaz, B.S.; Mirpour, M.; Malboobi, M.; Owlia, P. A Comparative Evaluation of Freezing Criteria and Molecular Characterization of Epiphytic Ice-Nucleating (Ice+) and Non-Ice-Nucleating (Ice−) Pseudomonas Syringae and Pseudomonas Fluorescens. J. Plant Pathol. 2019, 102, 169–178. [Google Scholar] [CrossRef]
- Kunert, A.T.; Pöhlker, M.L.; Tang, K.; Krevert, C.S.; Wieder, C.; Speth, K.R.; Hanson, L.E.; Morris, C.E.; Schmale III, D.G.; Pöschl, U.; et al. Macromolecular fungal ice nuclei in Fusarium: Effects of physical and chemical processing. Biogeosciences 2019, 16, 4647–4659. [Google Scholar] [CrossRef]
- Nesvadba, P. Thermal Properties and Ice Crystal Development in Frozen Foods. In Frozen Food Science and Technology; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 1–25. [Google Scholar]
- Li, D.; Zhu, Z.; Sun, D.-W. Effects of Freezing on Cell Structure of Fresh Cellular Food Materials: A Review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Li, J.; Izquierdo, M.P.; Lee, T. Effects of Ice-nucleation Active Bacteria on the Freezing of Some Model Food Systems. Int. J. Food Sci. Technol. 1997, 32, 41–49. [Google Scholar] [CrossRef]
- Zasypkin, D.V.; Lee, T.-C. Extracellular Ice Nucleators from Pantoea ananas: Effects on Freezing of Model Foods. J. Food Sci. 1999, 64, 473–478. [Google Scholar] [CrossRef]
- Li, J.; Lee, T.-C. Bacterial Extracellular Ice Nucleator Effects on Freezing of Foods. J. Food Sci. 1998, 63, 375–381. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Chen, G. Addition of Ice-Nucleation Active Bacteria: Pseudomonas syringae pv. panici on Freezing of Solid Model Food. LWT Food Sci. Technol. 2010, 43, 1414–1418. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, Z.; Sun, D.-W. Naturally Sourced Biosubstances for Regulating Freezing Points in Food Researches: Fundamentals, Current Applications and Future Trends. Trends Food Sci. Technol. 2020, 95, 131–140. [Google Scholar] [CrossRef]
- Watanabe, M.; Arai, S. Bacterial Ice-Nucleation Activity and Its Application to Freeze Concentration of Fresh Foods for Modification of Their Properties. J. Food Eng. 1994, 22, 453–473. [Google Scholar] [CrossRef]
- Zhu, X.; Lee, T.-C. Application of a Biogenic Extra Cellular Ice Nucleator for Food Processing: Effects on the Freeze-Thaw Stability of Fish Actomyosin from Tilapia. Int. J. Food Sci. Technol. 2007, 42, 768–772. [Google Scholar] [CrossRef]
- Li, J.; Lee, T.-C. Enhanced Production of Extracellular Ice Nucleators from Erwinia herbicola. J. Gen. Appl. Microbiol. 1998, 44, 405–413. [Google Scholar] [CrossRef]
- Shi, K.; Yu, H.; Lee, T.-C. A Novel Approach for Improving Yeast Viability and Baking Quality of Frozen Dough by Adding Biogenic Ice Nucleators from Erwinia herbicola. J. Cereal Sci. 2013, 57, 237–243. [Google Scholar] [CrossRef]
- Jin, J.; Yurkow, E.J.; Adler, D.; Lee, T.-C. Improved Freeze Drying Efficiency by Ice Nucleation Proteins with Ice Morphology Modification. Food Res. Int. 2018, 106, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yurkow, E.J.; Adler, D.; Lee, T.-C. A Novel Approach to Improve the Efficiency of Block Freeze Concentration Using Ice Nucleation Proteins with Altered Ice Morphology. J. Agric. Food Chem. 2017, 65, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Lindow, S.E.; Arny, D.C.; Upper, C.D. Bacterial Ice Nucleation: A Factor in Frost Injury to Plants. Plant Physiol. 1982, 70, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Zalila-Kolsi, I.; Ben-Mahmoud, A.; Al-Barazie, R. Bacillus Amyloliquefaciens: Harnessing Its Potential for Industrial, Medical, and Agricultural Applications—A Comprehensive Review. Microorganisms 2023, 11, 2215. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic Assembly of DNA Molecules up to Several Hundred Kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Southworth, M.W.; Wolber, P.K.; Warren, G.J. Nonlinear Relationship between Concentration and Activity of a Bacterial Ice Nucleation Protein. J. Biol. Chem. 1988, 263, 15211–15216. [Google Scholar] [CrossRef]
- Ling, M.L.; Wex, H.; Grawe, S.; Jakobsson, J.; Löndahl, J.; Hartmann, S.; Finster, K.; Boesen, T.; Šantl-Temkiv, T. Effects of Ice Nucleation Protein Repeat Number and Oligomerization Level on Ice Nucleation Activity. J. Geophys. Res. Atmos. 2018, 123, 1802–1810. [Google Scholar] [CrossRef]
- Schmid, D.; Pridmore, D.; Capitani, G.; Battistutta, R.; Neeser, J.-R.; Jann, A. Molecular Organisation of the Ice Nucleation Protein InaV from Pseudomonas syringae. FEBS Lett. 1997, 414, 590–594. [Google Scholar] [CrossRef]
- Mueller, G.M.; Wolber, P.K.; Warren, G.J. Clustering of Ice Nucleation Protein Correlates with Ice Nucleation Activity. Cryobiology 1990, 27, 416–422. [Google Scholar] [CrossRef]
- Green, R.L.; Corotto, L.V.; Warren, G.J. Deletion Mutagenesis of the Ice Nucleation Gene from Pseudomonas syringae S203. Mol. Gen. Genet. 1988, 215, 165–172. [Google Scholar] [CrossRef]
- Warren, G.J. Identification and Analysis of Ice Nucleation Active (Ina) Genes and Proteins. In Biological Ice Nucleation and Its Applications; APS Press: St. Paul, MN, USA, 1995. [Google Scholar]
- Attard, E.; Yang, H.; Delort, A.-M.; Amato, P.; Pöschl, U.; Glaux, C.; Koop, T.; Morris, C.E. Effects of atmospheric conditions on ice nucleation activity of Pseudomonas. Atmos. Chem. Phys. 2012, 12, 10667–10677. [Google Scholar] [CrossRef]
- Lukas, M.; Schwidetzky, R.; Kunert, A.T.; Pöschl, U.; Fröhlich-Nowoisky, J.; Bonn, M.; Meister, K. Electrostatic Interactions Control the Functionality of Bacterial Ice Nucleators. J. Am. Chem. Soc. 2020, 142, 6842–6846. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzam, M.; Saidur, R.; Masjuki, H.H. Investigation of Energy Consumption and Energy Savings of Refrigerator-Freezer During Open and Closed Door Condition. J. Appl. Sci. 2008, 8, 1822–1831. [Google Scholar] [CrossRef]
- Saidur, R.S.; Masjuki, H.H.; Mahlia, T.M.I.; Nasrudin, A.R. Factors Affecting Refrigerator-freezers Energy Consumption. ASEAN J. Sci. Technol. Dev. 2017, 19, 57–67. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y. Effect of Fat in the Ice Cream Mix on Freezing Point and Water Form. Food Res. Dev. 2018, 39, 10–13. [Google Scholar] [CrossRef]
- Hwang, W.-Z.; Coetzer, C.; Tumer, N.E.; Lee, T.-C. Expression of a Bacterial Ice Nucleation Gene in a Yeast Saccharomyces cerevisiae and Its Possible Application in Food Freezing Processes. J. Agric. Food Chem. 2001, 49, 4662–4666. [Google Scholar] [CrossRef]
- Passot, S.; Tréléa, I.C.; Marin, M.; Galan, M.; Morris, G.J.; Fonseca, F. Effect of Controlled Ice Nucleation on Primary Drying Stage and Protein Recovery in Vials Cooled in a Modified Freeze-Dryer. J. Biomech. Eng. 2009, 131, 074511. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, J.; Chen, X.; Zhang, Q.; Cai, X.; Ding, Y.; Zhou, X.; Wang, S. Dual Cryoprotective Strategies for Ice-Binding and Stabilizing of Frozen Seafood: A Review. Trends Food Sci. Technol. 2021, 111, 223–232. [Google Scholar] [CrossRef]
- Obadi, M.; Zhang, J.; Shi, Y.; Xu, B. Factors Affecting Frozen Cooked Noodle Quality: A Review. Trends Food Sci. Technol. 2021, 109, 662–673. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Jiang, C.; Zhu, J.; Liu, J.; Zhang, L.; Zuo, J.; Zheng, W.; Liu, S.; Huang, Q.; Wei, X.; et al. Expression of Ice Nucleation Protein in Bacillus amyloliquefaciens and Its Application in Food Freezing Process. Foods 2023, 12, 3896. https://doi.org/10.3390/foods12213896
Song R, Jiang C, Zhu J, Liu J, Zhang L, Zuo J, Zheng W, Liu S, Huang Q, Wei X, et al. Expression of Ice Nucleation Protein in Bacillus amyloliquefaciens and Its Application in Food Freezing Process. Foods. 2023; 12(21):3896. https://doi.org/10.3390/foods12213896
Chicago/Turabian StyleSong, Rong, Cong Jiang, Jing Zhu, Jia Liu, Li Zhang, Jingnan Zuo, Wei Zheng, Shilin Liu, Qingrong Huang, Xuetuan Wei, and et al. 2023. "Expression of Ice Nucleation Protein in Bacillus amyloliquefaciens and Its Application in Food Freezing Process" Foods 12, no. 21: 3896. https://doi.org/10.3390/foods12213896
APA StyleSong, R., Jiang, C., Zhu, J., Liu, J., Zhang, L., Zuo, J., Zheng, W., Liu, S., Huang, Q., Wei, X., & Chen, Y. (2023). Expression of Ice Nucleation Protein in Bacillus amyloliquefaciens and Its Application in Food Freezing Process. Foods, 12(21), 3896. https://doi.org/10.3390/foods12213896





