An Efficient Prephenate Dehydrogenase Gene for the Biosynthesis of L-tyrosine: Gene Mining, Sequence Analysis, and Expression Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Recombinant Expression of the Prephenate Dehydrogenase Gene tyrA
2.3. Chemicals
2.4. Determination of L-tyrosine
2.5. L-tyrosine Fermentation
2.6. Statistical Analysis
3. Results and Discussion
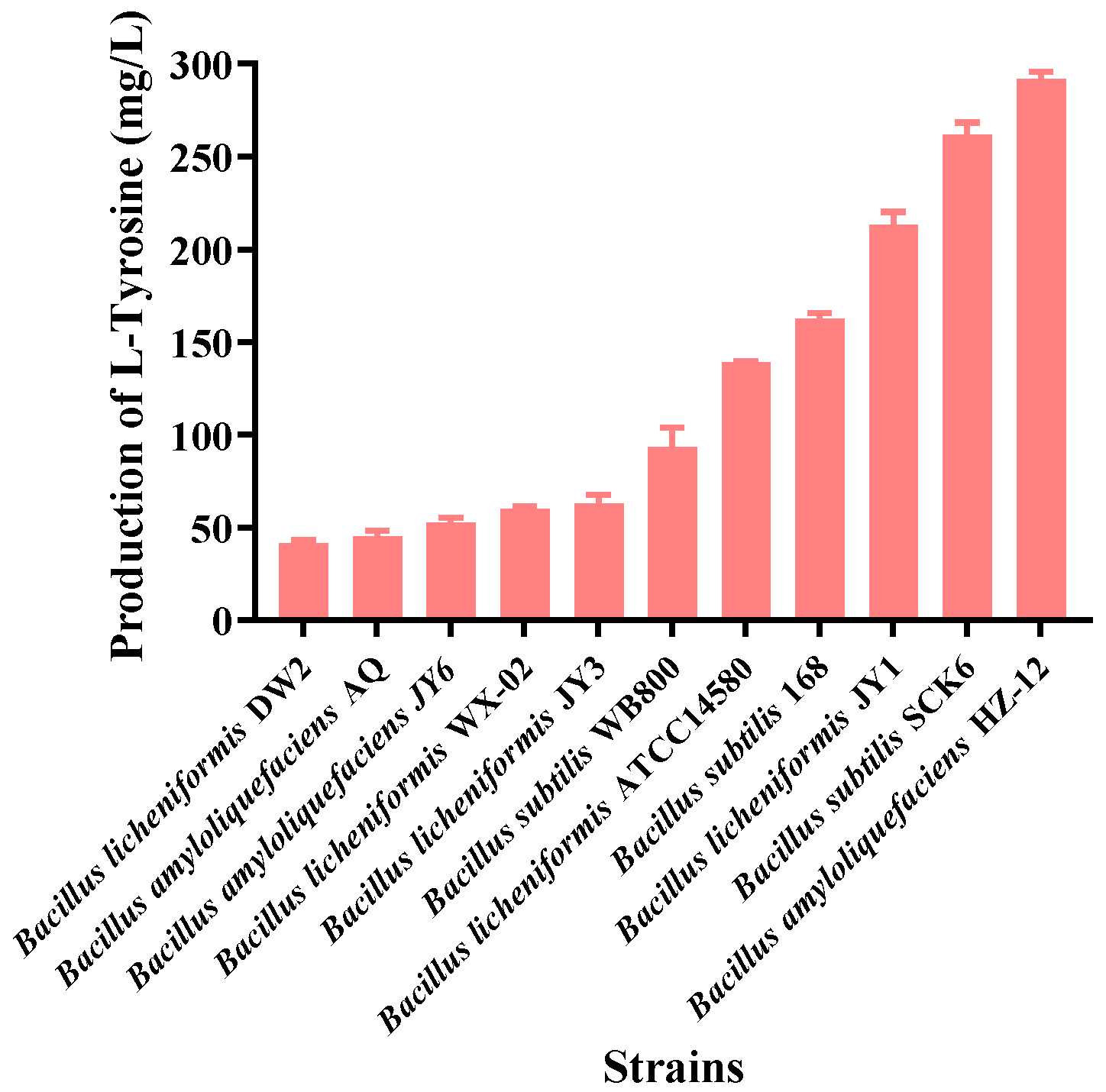
3.1. Screening the Optimal Host Strain for the Synthesis of the Platform Compound L-tyrosine
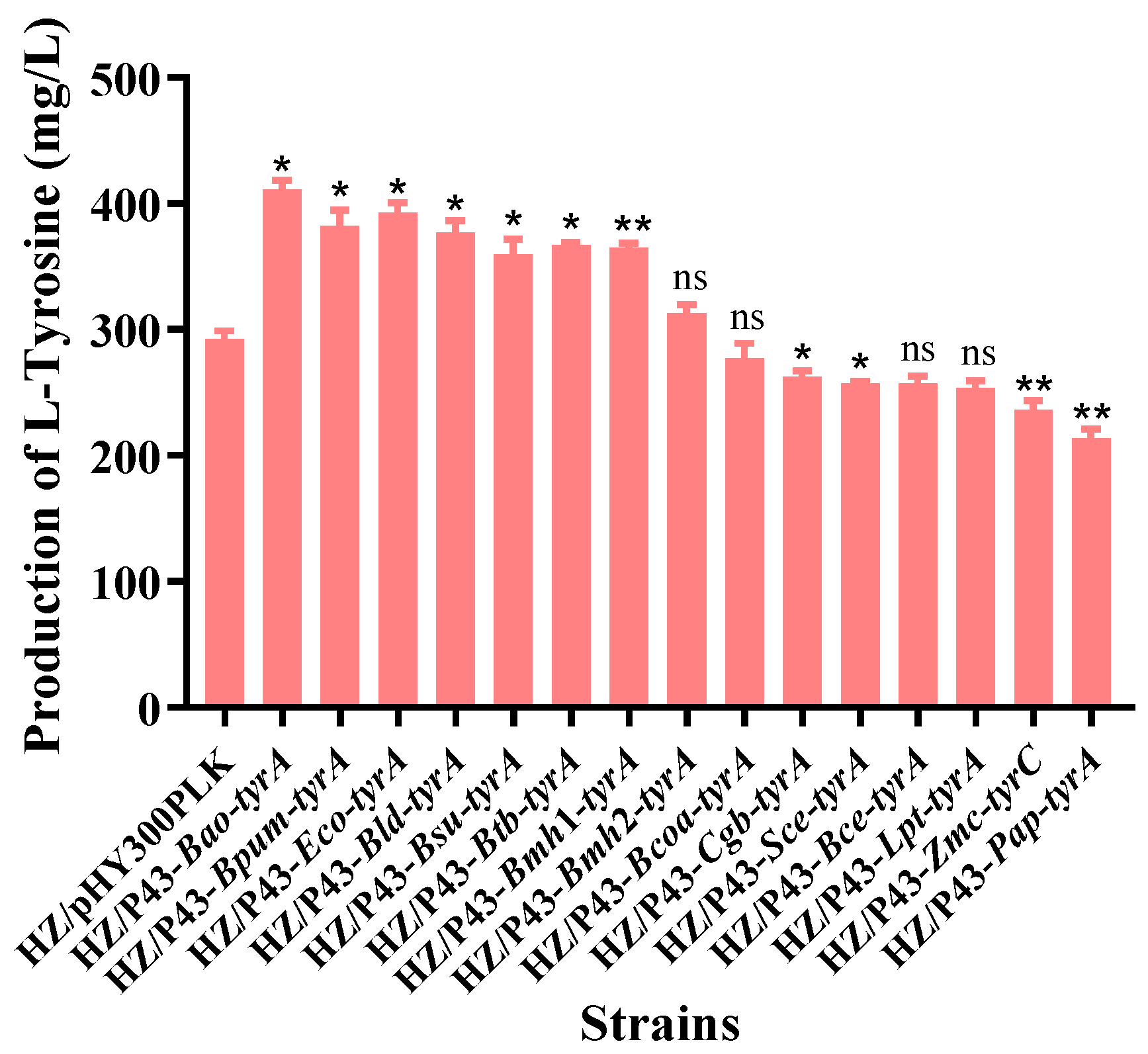
3.2. Effects of Different Prephenate Dehydrogenase Genes on Biosynthesis of L-tyrosine
3.3. Amino Acid Sequence Analysis and Possible Catalytic Mechanism
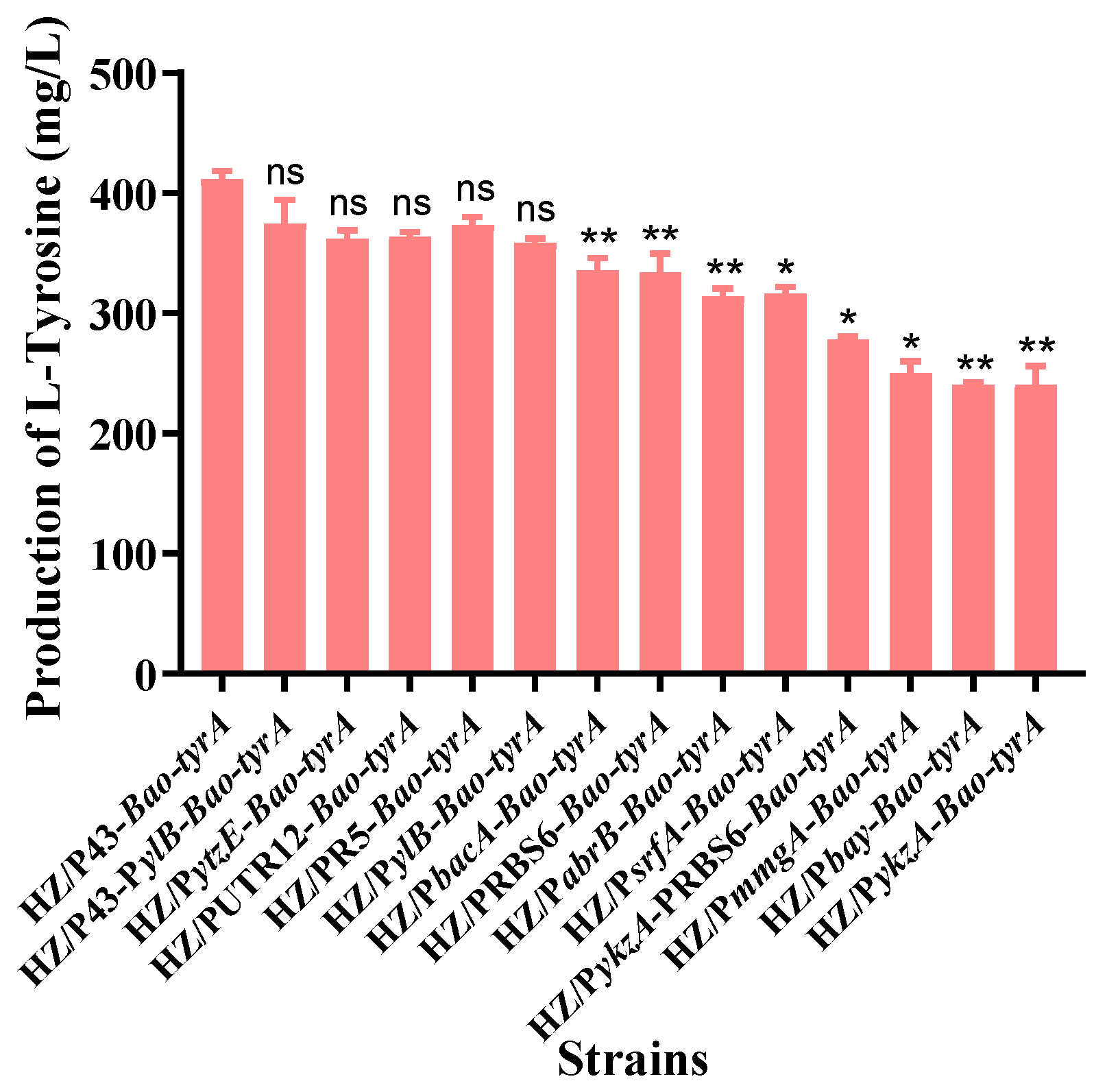
3.4. Effects of Different Promoters of Bao-tyrA on Biosynthesis of L-tyrosine
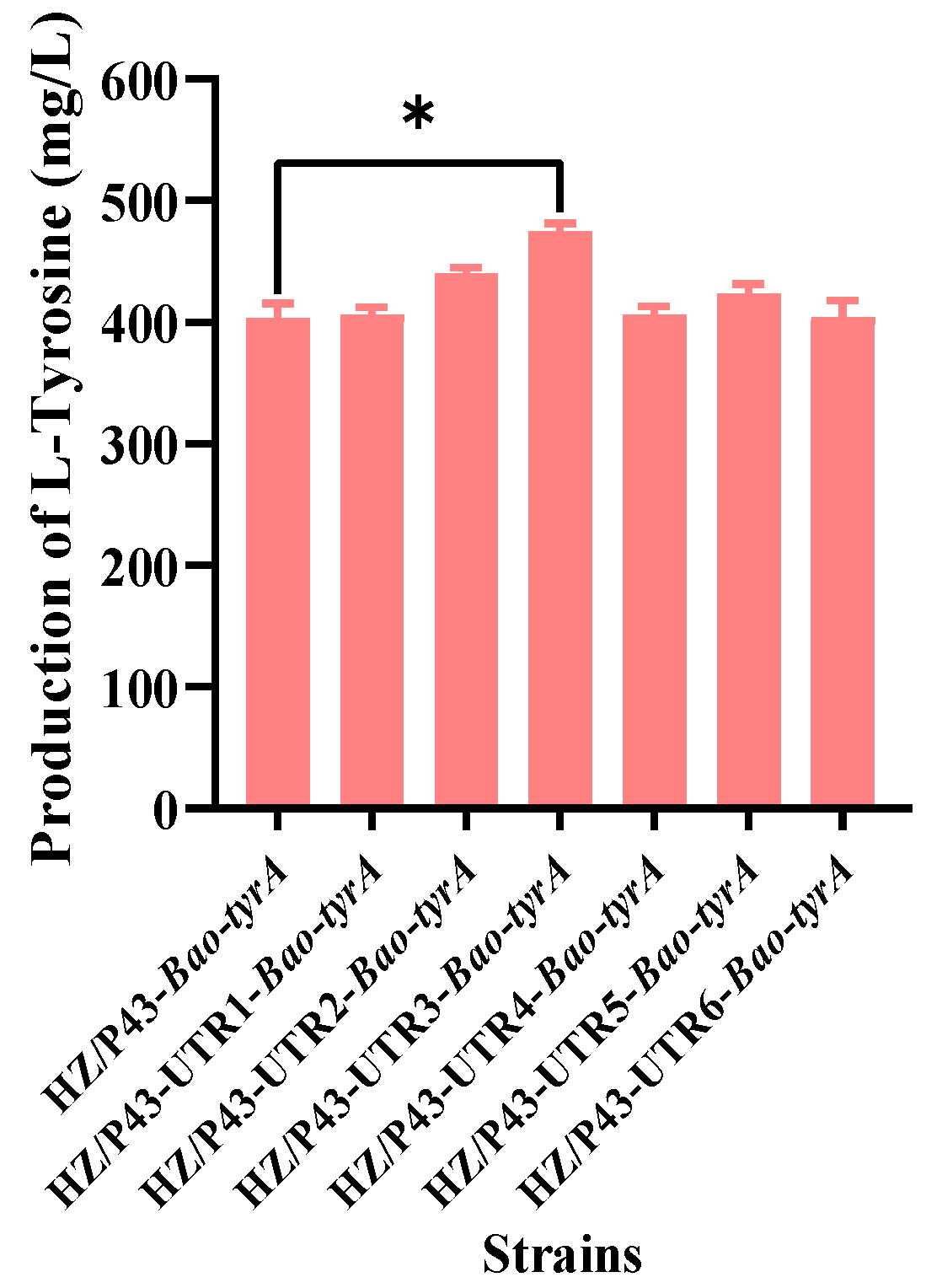
3.5. Redesign of the 5′-UTR of the Gene Bao-tyrA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lutke-Eversloh, T.; Stephanopoulos, G. L-tyrosine production by deregulated strains of Escherichia coli. Appl. Microbiol. Biotechnol. 2007, 75, 103–110. [Google Scholar] [CrossRef]
- Kim, S.C.; Min, B.E.; Hwang, H.G.; Seo, S.W.; Jung, G.Y. Pathway optimization by re-design of untranslated regions for L-tyrosine production in Escherichia coli. Sci. Rep. 2015, 5, 13853. [Google Scholar]
- Wang, J.; Shen, X.; Rey, J.; Yuan, Q.; Yan, Y. Recent advances in microbial production of aromatic natural products and their derivatives. Appl. Microbiol. Biotechnol. 2018, 102, 47–61. [Google Scholar] [CrossRef]
- Kumokita, R.; Bamba, T.; Inokuma, K.; Yoshida, T.; Ito, Y.; Kondo, A.; Hasunuma, T. Construction of an L-Tyrosine Chassis in Pichia pastoris Enhances Aromatic Secondary Metabolite Production from Glycerol. ACS Synth. Biol. 2022, 11, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.F.; Wang, C.S.; Qiao, J.; Zhao, G.R. Metabolic engineering of Escherichia coli for production of salvianic acid A via an artificial biosynthetic pathway. Metab. Eng. 2013, 19, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yao, J.; Meng, J.; Han, W.; Tao, Y.; Chen, Y.; Guo, Y.; Shi, G.; He, Y.; Jin, J.-M. Promiscuous enzymatic activity-aided multiple-pathway network design for metabolic flux rearrangement in hydroxytyrosol biosynthesis. Nat. Commun. 2019, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yan, Y. Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex. Microb. Cell Factories 2012, 11, 42. [Google Scholar] [CrossRef]
- Yuan, S.F.; Yi, X.; Johnston, T.G.; Alper, H.S. De novo resveratrol production through modular engineering of an Escherichia coli–Saccharomyces cerevisiae co-culture. Microb. Cell Factories 2020, 19, 143. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Zhou, Y.; Kan, Y.; Wu, T.; Xiao, W.; Luo, Y. Multi-modular engineering of Saccharomyces cerevisiae for high-titre production of tyrosol and salidroside. Microb. Biotechnol. 2021, 14, 2605–2616. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Guo, Y.-G.; Ding, Y.-H.; Wu, G.-J.; Zhu, S.-L.; Sun, Y.-F.; Yan, S.-K.; Qian, F.; Jin, H.-Z.; Zhang, W.-D. Three new alkaloids from Xylopia vielana and their antiinflammatory activities. Fitoterapia 2018, 127, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guleria, S.; Koffas, M.A.; Yan, Y. Microbial production of value-added nutraceuticals. Curr. Opin. Biotechnol. 2016, 37, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-F.; Alper, H.S. Metabolic engineering of microbial cell factories for production of nutraceuticals. Microb. Cell Factories 2019, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects-A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Guo, X.; Wu, X.; Ma, H.; Liu, H.; Luo, Y. Yeast: A platform for the production of L-tyrosine derivatives. Yeast 2023, 40, 214–230. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Chen, J.; Qin, Z.; Yu, S.; Zhou, J. Coordinating caffeic acid and salvianic acid a pathways for efficient production of rosmarinic acid in Escherichia coli. Metab. Eng. 2023, 76, 29–38. [Google Scholar] [CrossRef]
- Miralles, P.; Chisvert, A.; Salvador, A. Determination of hydroxytyrosol and tyrosol by liquid chromatography for the quality control of cosmetic products based on olive extracts. J. Pharm. Biomed. Anal. 2015, 102, 157–161. [Google Scholar] [CrossRef]
- Luo, Z.W.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of benzoic acid from glucose. Metab. Eng. 2020, 62, 298–311. [Google Scholar] [CrossRef]
- Sadler, J.C.; Wallace, S. Microbial synthesis of vanillin from waste poly (ethylene terephthalate). Green Chem. 2021, 23, 4665–4672. [Google Scholar] [CrossRef]
- Kim, J.; Hoang Nguyen Tran, P.; Lee, S.-M. Current Challenges and Opportunities in Non-native Chemical Production by Engineered Yeasts. Front. Bioeng. Biotechnol. 2020, 8, 594061. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ding, Q.; Jiang, G.-Z.; Liu, Z.-N.; Wang, H.-Y.; Zhao, G.-R. Chromosome engineering of Escherichia coli for constitutive production of salvianic acid A. Microb. Cell Factories 2017, 16, 84. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, T.; Du, G.; Zhou, J.; Chen, J. Modular optimization of heterologous pathways for de novo synthesis of (2S)-naringenin in Escherichia coli. PLoS ONE 2014, 9, e101492. [Google Scholar] [CrossRef]
- Kim, B.; Binkley, R.; Kim, H.U.; Lee, S.Y. Metabolic engineering of Escherichia coli for the enhanced production of L-tyrosine. Biotechnol. Bioeng. 2018, 115, 2554–2564. [Google Scholar] [CrossRef]
- Rodriguez, A.; Martnez, J.A.; Flores, N.; Escalante, A.; Gosset, G.; Bolivar, F. Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds. Microb. Cell Factories 2014, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Juminaga, D.; Baidoo, E.; Redding-Johanson, A.M.; Batth, T.S.; Burd, H.; Mukhopadhyay, A.; Petzold, C.J.; Keasling, J.D. Modular Engineering of L-Tyrosine Production in Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Schenck, C.A.; Holland, C.K.; Schneider, M.R.; Men, Y.; Lee, S.G.; Jez, J.M.; Maeda, H.A. Molecular basis of the evolution of alternative tyrosine biosynthetic routes in plants. Nat. Chem. Biol. 2017, 13, 1029–1035. [Google Scholar] [CrossRef]
- Hudson, G.S.; Wong, V.; Davidson, B.E. Chorismate mutase/prephenate dehydrogenase from Escherichia coli K12: Purification, characterization, and identification of a reactive cysteine. Biochemistry 1984, 23, 6240–6249. [Google Scholar] [CrossRef]
- Bonner, C.A.; Disz, T.; Hwang, K.; Song, J.; Vonstein, V.; Overbeek, R.; Jensen, R.A. Cohesion group approach for evolutionary analysis of TyrA, a protein family with wide-ranging substrate specificities. Microbiol. Mol. Biol. Rev. 2008, 72, 13–53. [Google Scholar] [CrossRef]
- Zhao, G.; Xia, T.; Ingram, L.O.; Jensen, R.A. An allosterically insensitive class of cyclohexadienyl dehydrogenase from Zymomonas mobilis. Eur. J. Biochem. 1993, 212, 157–165. [Google Scholar] [CrossRef]
- Chavez-Bejar, M.I.; Lara, A.R.; Lopez, H.; Hernandez-Chavez, G.; Martinez, A.; Ramirez, O.T.; Bolivar, F.; Gosset, G. Metabolic engineering of Escherichia coli for L-tyrosine production by expression of genes coding for the chorismate mutase domain of the native chorismate mutase-prephenate dehydratase and a cyclohexadienyl dehydrogenase from Zymomonas mobilis. Appl. Environ. Microbiol. 2008, 74, 3284–3290. [Google Scholar] [CrossRef] [PubMed]
- Bonner, C.A.; Jensen, R.A.; Gander, J.E.; Keyhani, N.O. A core catalytic domain of the TyrA protein family: Arogenate dehydrogenase from Synechocystis. Biochem. J. 2004, 382, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Wu, Z.; Lu, Y.; Tao, G.; Zhang, L.; Ding, Z.; Shi, G. Combining Precursor-Directed Engineering with Modular Designing: An Effective Strategy for De Novo Biosynthesis of L-DOPA in Bacillus licheniformis. ACS Synth. Biol. 2022, 11, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Zhang, L.; Ding, Z.; Gu, Z.; Shi, G. Unraveling the specific regulation of the shikimate pathway for tyrosine accumulation in Bacillus licheniformis. J. Ind. Microbiol. Biotechnol. 2019, 46, 1047–1059. [Google Scholar] [CrossRef]
- Ouyang, X.; Liu, Y.; Qu, R.; Tian, M.; Yang, T.; Zhu, R.; Gao, H.; Jin, M.; Huang, J. Optimizing protein-glutaminase expression in Bacillus subtilis. Curr. Microbiol. 2021, 78, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Shin, H.-d.; Chen, R.R.; Wang, N.S.; Li, J.; Du, G.; Chen, J. Developing Bacillus spp. as a cell factory for production of microbial enzymes and industrially important biochemicals in the context of systems and synthetic biology. Appl. Microbiol. Biotechnol. 2013, 97, 6113–6127. [Google Scholar] [CrossRef]
- Cai, D.; Rao, Y.; Zhan, Y.; Wang, Q.; Chen, S. Engineering Bacillus for efficient production of heterologous protein: Current progress, challenge and prospect. J. Appl. Microbiol. 2019, 126, 1632–1642. [Google Scholar] [CrossRef]
- Corrêa, G.G.; Lins, M.R.d.C.R.; Silva, B.F.; de Paiva, G.B.; Zocca, V.F.B.; Ribeiro, N.V.; Picheli, F.P.; Mack, M.; Pedrolli, D.B. A modular autoinduction device for control of gene expression in Bacillus subtilis. Metab. Eng. 2020, 61, 326–334. [Google Scholar] [CrossRef]
- Zou, D.; Min, Y.; Liu, Y.; Wei, X.; Wang, J. Identification of a Spermidine Synthase Gene from Soybean by Recombinant Expression, Transcriptional Verification, and Sequence Analysis. J. Agric. Food Chem. 2020, 68, 2366–2372. [Google Scholar] [CrossRef]
- Jiang, C.; Ruan, L.; Wei, X.; Guo, A. Enhancement of S-adenosylmethionine production by deleting thrB gene and overexpressing SAM2 gene in Bacillus amyloliquefaciens. Biotechnol. Lett. 2020, 42, 2293–2298. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Liu, Y.; Zhu, Y.; Li, J.; Du, G.; Chen, J.; Ledesma-Amaro, R.; Liu, L. Pyruvate-responsive genetic circuits for dynamic control of central metabolism. Nat. Chem. Biol. 2020, 16, 1261–1268. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, X.; Wu, Y.; Niu, T.; Liu, Y.; Li, J.; Du, G.; Liu, L. Advances and prospects of Bacillus subtilis cellular factories: From rational design to industrial applications. Metab. Eng. 2018, 50, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Yang, J.S.; Cho, H.S.; Yang, J.; Kim, S.C.; Park, J.M.; Kim, S.; Jung, G.Y. Predictive combinatorial design of mRNA translation initiation regions for systematic optimization of gene expression levels. Sci. Rep. 2014, 4, 4515. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.N.S.; Xiao, W.; Stephanopoulos, G. Rational, combinatorial, and genomic approaches for engineering L-tyrosine production in Escherichia coli. Proc. Natl. Acad. Sci. USA 2012, 109, 13538–13543. [Google Scholar] [CrossRef] [PubMed]
- Christendat, D.; Turnbull, J. Identification of active site residues of chorismate mutase-prephenate dehydrogenase from Escherichia coli. Biochemistry 1996, 35, 4468. [Google Scholar] [CrossRef] [PubMed]
- Lütke-Eversloh, T.; Stephanopoulos, G. Feedback inhibition of chorismate mutase/prephenate dehydrogenase (TyrA) of Escherichia coli: Generation and characterization of tyrosine-insensitive mutants. Appl. Environ. Microbiol. 2005, 71, 7224–7228. [Google Scholar] [CrossRef]
- Christendat, D.; Turnbull, J.L. Identifying groups involved in the binding of prephenate to prephenate dehydrogenase from Escherichia coli. Biochemistry 1999, 38, 4782–4793. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.; Galburt, E.A. The context-dependent influence of promoter sequence motifs on transcription initiation kinetics and regulation. J. Bacteriol. 2021, 203, e00512–e00520. [Google Scholar] [CrossRef]
- Cazier, A.P.; Blazeck, J. Advances in promoter engineering: Novel applications and predefined transcriptional control. Biotech. J. 2021, 16, 2100239. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Bechthold, A.; Ma, Z.; Yu, X. Selection of an efficient promoter and its application in toyocamycin production improvement in Streptomyces diastatochromogenes 1628. World J. Microbiol. Biotechnol. 2017, 33, 30. [Google Scholar] [CrossRef]
- Song, Y.; Nikoloff, J.M.; Fu, G.; Chen, J.; Li, Q.; Xie, N.; Zheng, P.; Sun, J.; Zhang, D. Promoter Screening from Bacillus subtilis in Various Conditions Hunting for Synthetic Biology and Industrial Applications. PLoS ONE 2016, 11, e0158447. [Google Scholar] [CrossRef] [PubMed]
- Hartz, P.; Mattes, C.; Schad, M.; Bernhardt, R.; Hannemann, F. Expanding the promoter toolbox of Bacillus megaterium. J. Bacteriol. 2019, 294, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Cheng, J.; Guo, D.; Mao, L.; Li, J.; Lu, L.; Zhang, Y.; Yang, J.; Jiang, H. Engineering the 5′UTR-Mediated Regulation of Protein Abundance in Yeast Using Nucleotide Sequence Activity Relationships. ACS Synth. Biol. 2018, 7, 2709–2714. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Yang, J.-S.; Kim, I.; Yang, J.; Min, B.E.; Kim, S.; Jung, G.Y. Predictive design of mRNA translation initiation region to control prokaryotic translation efficiency. Metab. Eng. 2013, 15, 67–74. [Google Scholar] [CrossRef]
- Noh, M.H.; Lim, H.G.; Park, S.; Seo, S.W.; Jung, G.Y. Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli. Metab. Eng. 2017, 43, 1–8. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cha, S.; Lee, J.H.; Lim, H.G.; Noh, M.H.; Kang, C.W.; Jung, G.Y. Plug-in repressor library for precise regulation of metabolic flux in Escherichia coli. Metab. Eng. 2021, 67, 365–372. [Google Scholar] [CrossRef]
- Jiang, A.; Song, Y.; You, J.; Zhang, X.; Xu, M.; Rao, Z. High-yield ectoine production in engineered Corynebacterium glutamicum by fine metabolic regulation via plug-in repressor library. Bioresour. Technol. 2022, 362, 127802. [Google Scholar] [CrossRef]


| Strains or Plasmids | Characteristics | Source |
|---|---|---|
| Bacillus amyloliquefaciens HZ-12 | Wild type | stored in lab |
| Bacillus subtilis 168 | trpC2 | stored in lab |
| Bacillus subtilis SCK6 | ErmR, 1A751 derivate, lacA::PxylA-comK | stored in lab |
| Bacillus subtilis WB800 | nprE aprE epr bpr mpr::ble nprB::bsr Δvpr wprA::hyg | stored in lab |
| Bacillus licheniformis JY1 | Wild type | stored in lab |
| Bacillus licheniformis JY3 | Wild type | stored in lab |
| Bacillus licheniformis DW2 | Wild type | stored in lab |
| Bacillus licheniformis WX-02 | Wild type | stored in lab |
| B. licheniformis ATCC14580 | Wild type | stored in lab |
| B. amyloliquefaciens JY6 | Wild type | stored in lab |
| B. amyloliquefaciens AQ | Wild type | stored in lab |
| HZ/pHY300PLK | HZ-12 with pHY300PLK | this study |
| HZ/P43-Eco-tyrA | HZ-12 with pHY-P43-Eco-tyrA | this study |
| HZ/P43-Bao-tyrA | HZ-12 with pHY-P43-Bao-tyrA | this study |
| HZ/P43-Bld-tyrA | HZ-12 with pHY-P43-Bld-tyrA | this study |
| HZ/P43-Bsu-tyrA | HZ-12 with pHY-P43-Bsu-tyrA | this study |
| HZ/P43-Btb-tyrA | HZ-12 with pHY-P43-Btb-tyrA | this study |
| HZ/P43-Bpum-tyrA | HZ-12 with pHY-P43-Bpum-tyrA | this study |
| HZ/P43-Cgb-tyrA | HZ-12 with pHY-P43-Cgb-tyrA | this study |
| HZ/P43-Sce-tyrA | HZ-12 with pHY-P43-Sce-tyrA | this study |
| HZ/P43-Lpt-tyrA | HZ-12 with pHY-P43-Lpt-tyrA | this study |
| HZ/P43-Bcoa-tyrA | HZ-12 with pHY-P43-Bcoa-tyrA | this study |
| HZ/P43-Bce-tyrA | HZ-12 with pHY-P43-Bce-tyrA | this study |
| HZ/P43-Bmh1-tyrA | HZ-12 with pHY-P43-Bmh1-tyrA | this study |
| HZ/P43-Bmh2-tyrA | HZ-12 with pHY-P43-Bmh2-tyrA | this study |
| HZ/P43-Zmc-tyrA | HZ-12 with pHY-P43-Zmc-tyrA | this study |
| HZ/P43-Pap-tyrA | HZ-12 with pHY-P43-Pap-tyrA | this study |
| HZ/PsrfA-Bao-tyrA | HZ-12 with pHY-PsrfA-Bao-tyrA | this study |
| HZ/PytzE-Bao-tyrA | HZ-12 with pHY-PytzE-Bao-tyrA | this study |
| HZ/PylB-Bao-tyrA | HZ-12 with pHY-PylB-Bao-tyrA | this study |
| HZ/Pbay-Bao-tyrA | HZ-12 with pHY-Pbay-Bao-tyrA | this study |
| HZ/PykzA-Bao-tyrA | HZ-12 with pHY-PykzA-Bao-tyrA | this study |
| HZ/PykzA-PRBS6-Bao-tyrA | HZ-12 with pHY-PykzA-PRBS6-Bao-tyrA | this study |
| HZ/PmmgA-Bao-tyrA | HZ-12 with pHY-PmmgA-Bao-tyrA | this study |
| HZ/PabrB-Bao-tyrA | HZ-12 with pHY-PabrB-Bao-tyrA | this study |
| HZ/PbacA-Bao-tyrA | HZ-12 with pHY-PbacA-Bao-tyrA | this study |
| HZ/P12-Bao-tyrA | HZ-12 with pHY-P12-Bao-tyrA | this study |
| HZ/P43-PylB-Bao-tyrA | HZ-12 with pHY-P43-ylB-Bao-tyrA | this study |
| HZ/PR5-Bao-tyrA | HZ-12 with pHY-PR5-Bao-tyrA | this study |
| HZ/PRBS6-Bao-tyrA | HZ-12 with pHY-PRBS6-Bao-tyrA | this study |
| HZ/P43-UTR1-Bao-tyrA | HZ-12 with pHY-P43-UTR1-Bao-tyrA | this study |
| HZ/P43-UTR2-Bao-tyrA | HZ-12 with pHY-P43-UTR2-Bao-tyrA | this study |
| HZ/P43-UTR3-Bao-tyrA | HZ-12 with pHY-P43-UTR3-Bao-tyrA | this study |
| HZ/P43-UTR4-Bao-tyrA | HZ-12 with pHY-P43-UTR4-Bao-tyrA | this study |
| HZ/P43-UTR5-Bao-tyrA | HZ-12 with pHY-P43-UTR5-Bao-tyrA | this study |
| HZ/P43-UTR6-Bao-tyrA | HZ-12 with pHY-P43-UTR6-Bao-tyrA | this study |
| pHY300PLK | E. coli–Bacillus shuttle vector for gene expression, Apr, Tetr | stored in lab |
| pHY-P43-Eco-tyrA | pHY300PLK + P43 + Eco-tyrA + TamyL | this study |
| pHY-P43-Bao-tyrA | pHY300PLK + P43 + Bao-tyrA + TamyL | this study |
| pHY-P43-Bld-tyrA | pHY300PLK + P43 + Bld-tyrA+ TamyL | this study |
| pHY-P43-Bsu-tyrA | pHY300PLK + P43 + Bsu-tyrA + TamyL | this study |
| pHY-P43-Btb-tyrA | pHY300PLK + P43 + Btb-tyrA + TamyL | this study |
| pHY-P43-Bpum-tyrA | pHY300PLK + P43 + Bpum-tyrA + TamyL | this study |
| pHY-P43-Cgb-tyrA | pHY300PLK + P43 + Cgb-tyrA + TamyL | this study |
| pHY-P43-Sce-tyrA | pHY300PLK + P43 + Sce-tyrA + TamyL | this study |
| pHY-P43-Lpt-tyrA | pHY300PLK + P43 + Lpt-tyrA + TamyL | this study |
| pHY-P43-Bcoa-tyrA | pHY300PLK + P43 + Bcoa-tyrA + TamyL | this study |
| pHY-P43-Bce-tyrA | pHY300PLK + P43 + Bce-tyrA + TamyL | this study |
| pHY-P43-Bmh1-tyrA | pHY300PLK + P43 + Bmh1-tyrA + TamyL | this study |
| pHY-P43-Bmh2-tyrA | pHY300PLK + P43 + Bmh2-tyrA + TamyL | this study |
| pHY-P43-Zmc-tyrA | pHY300PLK + P43 + Zmc-tyrA + TamyL | this study |
| pHY-P43-Pap-tyrA | pHY300PLK + P43 + Pap-tyrA + TamyL | this study |
| pHY-PsrfA-Bao-tyrA | pHY300PLK + PsrfA + Bao-tyrA + TamyL | this study |
| pHY-PytzE-Bao-tyrA | pHY300PLK + PytzE + Bao-tyrA + TamyL | this study |
| pHY-PylB-Bao-tyrA | pHY300PLK + PylB + Bao-tyrA + TamyL | this study |
| pHY-Pbay-Bao-tyrA | pHY300PLK + Pbay + Bao-tyrA + TamyL | this study |
| HZ/PykzA-Bao-tyrA | pHY300PLK + PykzA + Bao-tyrA + TamyL | this study |
| pHY-PykzA-RBS6-Bao-tyrA | pHY300PLK + PykzA-PRBS6 + Bao-tyrA + TamyL | this study |
| pHY-PmmgA-Bao-tyrA | pHY300PLK + PmmgA + Bao-tyrA + TamyL | this study |
| pHY-PabrB-Bao-tyrA | pHY300PLK + PabrB + Bao-tyrA + TamyL | this study |
| pHY-PbacA-Bao-tyrA | pHY300PLK + PbacA + Bao-tyrA + TamyL | this study |
| pHY-P12-Bao-tyrA | pHY300PLK + P12 + Bao-tyrA + TamyL | this study |
| pHY-P43-ylB-Bao-tyrA | pHY300PLK + P43-PylB + Bao-tyrA + TamyL | this study |
| pHY-PR5-Bao-tyrA | pHY300PLK + PR5 + Bao-tyrA + TamyL | this study |
| pHY-PRBS6-Bao-tyrA | pHY300PLK + PRBS6 + Bao-tyrA + TamyL | this study |
| pHY-P43-UTR1-Bao-tyrA | pHY300PLK + P43 + UTR1-Bao-tyrA + TamyL | this study |
| pHY-P43-UTR2-Bao-tyrA | pHY300PLK + P43 + UTR2-Bao-tyrA + TamyL | this study |
| pHY-P43-UTR3-Bao-tyrA | pHY300PLK + P43 + UTR3-Bao-tyrA + TamyL | this study |
| pHY-P43-UTR4-Bao-tyrA | pHY300PLK + P43 + UTR4-Bao-tyrA + TamyL | this study |
| pHY-P43-UTR5-Bao-tyrA | pHY300PLK + P43 + UTR5-Bao-tyrA + TamyL | this study |
| pHY-P43-UTR6-Bao-tyrA | pHY300PLK + P43 + UTR6-Bao-tyrA + TamyL | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, A.; Bao, P.; Ma, A.; Wei, X. An Efficient Prephenate Dehydrogenase Gene for the Biosynthesis of L-tyrosine: Gene Mining, Sequence Analysis, and Expression Optimization. Foods 2023, 12, 3084. https://doi.org/10.3390/foods12163084
Ji A, Bao P, Ma A, Wei X. An Efficient Prephenate Dehydrogenase Gene for the Biosynthesis of L-tyrosine: Gene Mining, Sequence Analysis, and Expression Optimization. Foods. 2023; 12(16):3084. https://doi.org/10.3390/foods12163084
Chicago/Turabian StyleJi, Anying, Pengfei Bao, Aimin Ma, and Xuetuan Wei. 2023. "An Efficient Prephenate Dehydrogenase Gene for the Biosynthesis of L-tyrosine: Gene Mining, Sequence Analysis, and Expression Optimization" Foods 12, no. 16: 3084. https://doi.org/10.3390/foods12163084
APA StyleJi, A., Bao, P., Ma, A., & Wei, X. (2023). An Efficient Prephenate Dehydrogenase Gene for the Biosynthesis of L-tyrosine: Gene Mining, Sequence Analysis, and Expression Optimization. Foods, 12(16), 3084. https://doi.org/10.3390/foods12163084






