Recent Trends in Cereal- and Legume-Based Protein-Mineral Complexes: Formulation Methods, Toxicity, and Food Applications
Abstract
:1. Introduction
2. Cereal and Legume Proteins
2.1. Cereal Proteins
2.2. Legume Proteins
3. Development of Protein-Mineral Complexes
| Mineral | Source of Protein | Type of Interaction | References |
|---|---|---|---|
| Iron | Red Lentil Protein Hydrolysate | Electrostatic interaction | [48] |
| Iron | Mung-Bean Protein Hydrolysate | Hydrophobic interaction | [37] |
| Calcium | Soy Bean Protein Hydrolysate | Electrostatic interaction | [65] |
| Iron | Mung Bean Protein Hydrolysate | Hydrophobic and hydrophilic interaction | [52] |
| Iron | Mung Bean Protein Hydrolysate | Covalent interaction | [32] |
| Iron | Soybean Protein Hydrolysate | Electrostatic interactions | [36] |
| Iron | Soy Protein hydrolysate | Electrostatic interaction | [34] |
| Copper and Iron | Jamapa Protein Hydrolysate | Electrostatic and ionic interaction | [55] |
| Zinc | Peanut Protein Isolate | n-π* interaction | [39] |
| Zinc | Sesame Protein Hydrolysate | Coordination and weaker interactions | [41] |
| Cadmium | Rice Protein | Coordination interaction | [55] |
| Iron, Calcium, Copper, and Zinc | Barley Protein Hydrolysate | Electrostatic interaction and hydrophobic interaction | [43] |
| Calcium | Black Bean Hydrolysate | Electrostatic and hydrophilic interaction | [43] |
| Iron | Oats Protein Hydrolysate | Electrostatic interaction | [34] |
| Calcium | Wheat Germ Protein Hydrolysate | Electrostatic interaction | [48] |
| Copper | Rice Bran Protein | Electrostatic interaction | |
| Calcium | Sunflower seed protein | Ionic interaction | [55] |
| Calcium | Peanut seed protein | Ionic interaction | [55] |
3.1. Binding Efficiency and Characterization of Cereal- and Legume-Based Protein-Mineral Complexes
3.1.1. Binding Efficiency of Complexes
3.1.2. Characterization Using Various Analytical Techniques
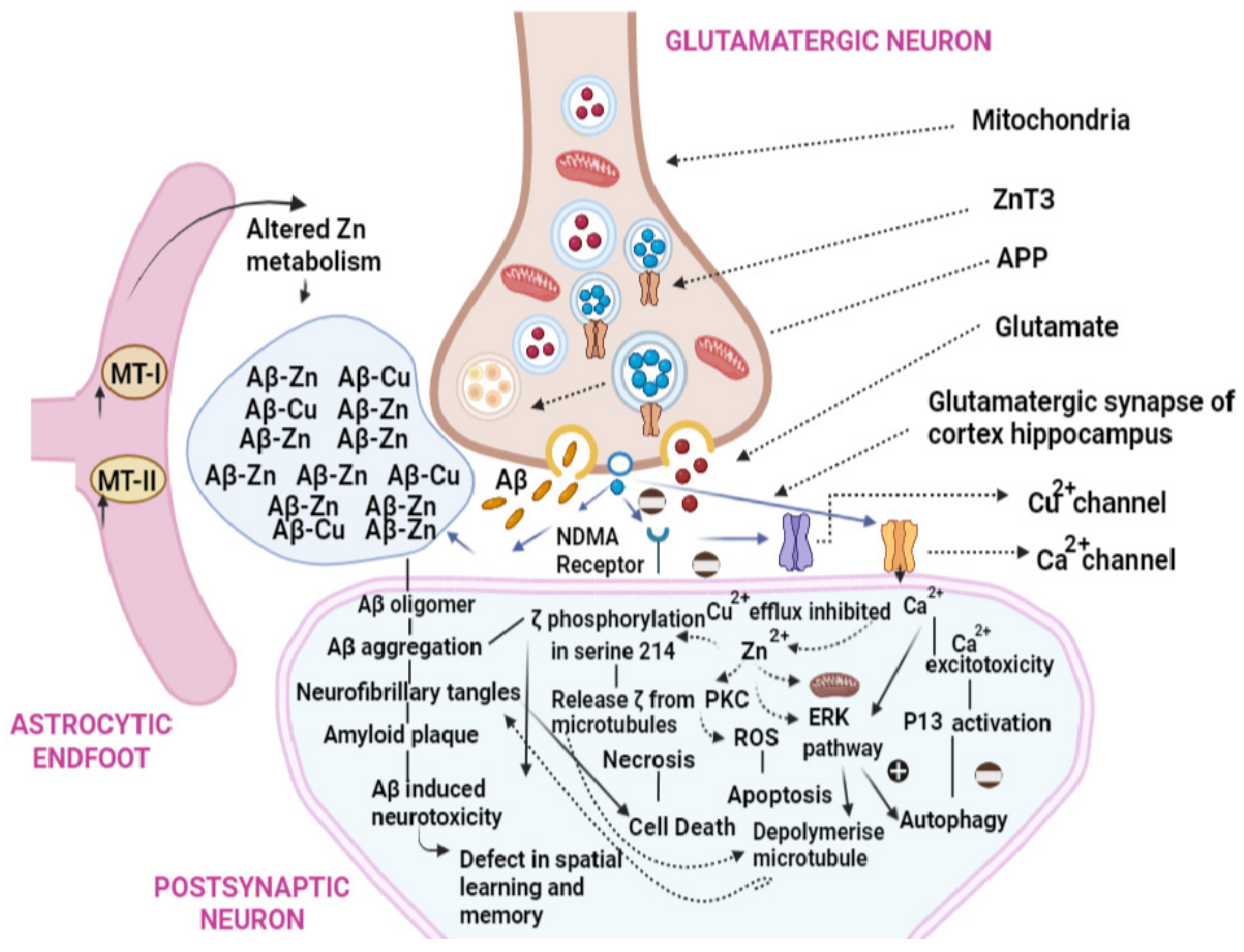
4. Toxic Effects of Inorganic Minerals
4.1. Mechanism of Toxicity
4.2. In Vitro Studies: Toxicity Determination
4.3. In Vivo Studies: Toxicity Determination
4.4. Sensory Challenges of Inorganic Minerals
5. Application of Cereal- and Legume-Derived Protein-Mineral Complexes
5.1. Fortification of Food Products
5.2. Enhancing Nutritional Value
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, G.A.; Beal, T.; Mbuya, M.N.N.; Luo, H.; Neufeld, L.M.; Global Micronutrient Deficiencies Research Group. Micronutrient Deficiencies among Preschool-Aged Children and Women of Reproductive Age Worldwide: A Pooled Analysis of Individual-Level Data from Population-Representative Surveys. Lancet Glob. Health 2022, 10, e1590–e1599. [Google Scholar] [CrossRef] [PubMed]
- Godswill, A.G.; Somtochukwu, I.V.; Ikechukwu, A.O.; Kate, E.C. Health Benefits of Micronutrients (Vitamins and Minerals) and Their Associated Deficiency Diseases: A Systematic Review. Int. J. Food Sci. 2020, 3, 1–32. [Google Scholar] [CrossRef]
- Beal, T.; Gardner, C.D.; Herrero, M.; Iannotti, L.L.; Merbold, L.; Nordhagen, S.; Mottet, A. Friend or Foe? The Role of Animal-Source Foods in Healthy and Environmentally Sustainable Diets. J. Nutr. 2023, 153, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.; Solenthaler, F.; Albrecht, C. Materno-Fetal Iron Transfer and the Emerging Role of Ferroptosis Pathways. Biochem. Pharmacol. 2022, 202, 115141. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.R.; Esquivel, D.F.G.; Ayala, T.B.; Pineda, B.; Manzo, S.G.; Quino, J.M. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Zavitsanou, A.; Drigas, A. Nutrition in Mental and Physical Health. Tech. Soc. Sci. J. 2021, 23, 67–77. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Mauli, S.; Thow, A.-M.; Mulcahy, G.; Andrew, G.; Ride, A.; Tutuo, J. Opportunities to Strengthen Fish Supply Chain Policy to Improve External Food Environments for Nutrition in the Solomon Islands. Foods 2023, 12, 900. [Google Scholar] [CrossRef]
- Pathaw, N.; Devi, K.S.; Sapam, R.; Sanasam, J.; Monteshori, S.; Phurailatpam, S.; Devi, H.C.; Chanu, W.T.; Wangkhem, B.; Mangang, N.L. A Comparative Review on the Anti-Nutritional Factors of Herbal Tea Concoctions and Their Reduction Strategies. Front. Nutr. 2022, 9, 988964. [Google Scholar] [CrossRef]
- Olson, R.; Gavin-Smith, B.; Ferraboschi, C.; Kraemer, K. Food Fortification: The Advantages, Disadvantages and Lessons from Sight and Life Programs. Nutrients 2021, 13, 1118. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Chauhan, A.K. Iron Nanoparticles as a Promising Compound for Food Fortification in Iron Deficiency Anemia: A Review. J. Food Sci. Technol. 2022, 59, 3319–3335. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Sridhar, K.; Kanda, Y.; Yamanaka, S. Pure hydroxyapatite synthesis originating from amorphous calcium carbonate. Sci. Rep. 2021, 11, 11546. [Google Scholar] [CrossRef] [PubMed]
- Olga, J.R.; Ares, M. Environmentally Friendly Thermoelectric Materials: High Performance from Inorganic Components with Low Toxicity and Abundance in the Earth. Adv. Sustain. Syst. 2021, 5, 2100095. [Google Scholar]
- Squires, J.E.; Alonso, E.M.; Ibrahim, S.H.; Kasper, V.; Kehar, M.; Martinez, M.; Squires, R.H. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper on the Diagnosis and Management of Pediatric Acute Liver Failure. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Management of Metabolic Disorders (Including Metabolic Diseases) in Ruminant and Nonruminant Animals. In Animal Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 471–491. ISBN 9780128170526. [Google Scholar]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the Impact of Anthropogenic Aspects and Climatic Factors on Long-Term Soil Monitoring and Management. Environ. Sci. Pollut. Res. Int. 2021, 28, 30528–30550. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The Role of Zinc, Copper, Manganese and Iron in Neurodegenerative Diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Apata, I.W.; Bailey, J.L.; Franch, H.A. Metabolic and Nutritional Responses to Acidemia and Alkalemia. In Nutritional Management of Renal Disease; Elsevier: Amsterdam, The Netherlands, 2022; pp. 127–145. ISBN 9780128185407. [Google Scholar]
- Wang, R.; Guo, S. Phytic Acid and Its Interactions: Contributions to Protein Functionality, Food Processing, and Safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2081–2105. [Google Scholar] [CrossRef]
- Ying, R.; Li, T.; Wu, C.; Huang, M. Role of Aleurone Cell Walls in Water Diffusion and Distribution within Cereal Grains. J. Cereal Sci. 2020, 93, 102952. [Google Scholar] [CrossRef]
- Golfam, R.; Kiarostami, K.; Lohrasebi, T.; Hasrak, S.; Razavi, K. A Review of Drought Stress on Wheat (Triticum aestivum L.) Starch. Farming Manag. 2021, 6, 47–57. [Google Scholar]
- Azizi, K.; Chehregani Rad, A.; Soltani, J. Novel Cytological Findings on Gametophyte Development and Embryogenesis in Wheat (Triticum aestivum L.). J. Genet. Resour. 2022, 8, 218–235. [Google Scholar]
- Abamecha, N. Research Review on Formulation and Sensory Evaluation of Complementary Foods from Cereals and Legumes in Ethiopia. Food Sci. Nutr. Technol. 2020, 5, 1–7. [Google Scholar]
- Jayaprakash, G.; Bains, A.; Chawla, P.; Fogarasi, M.; Fogarasi, S. A Narrative Review on Rice Proteins: Current Scenario and Food Industrial Application. Polymers 2022, 14, 3003. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.M.; De Majo, C. The Age of the Soybean: An Environmental History of Soy During the Great Acceleration; The White Horse Press: New Milford, CT, USA, 2022. [Google Scholar]
- Hadidi, M.; Garcia, S.R.; Ziogkas, D.; McClements, D.J.; Moreno, A. Cereal Bran Proteins: Recent Advances in Extraction, Properties, and Applications. Crit. Rev. Food Sci. Nutr. 2023, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of Wheat Gluten Proteins: Qualitative Composition. Cereal Chem. 2023, 100, 23–35. [Google Scholar] [CrossRef]
- Lama, S. Bread-Making Quality in a Changing Climate: In Search of Climate Stable Genotypes and Robust Screening Methods for Wheat. Hortic. Crop Prod. Sci. 2020, 2020, 3. [Google Scholar]
- Ghanghas, N.; Mt, M.; Sharma, S.; Prabhakar, P.K. Classification, Composition, Extraction, Functional Modification and Application of Rice (Oryza sativa) Seed Protein: A Comprehensive Review. Food Rev. Int. 2022, 38, 354–383. [Google Scholar] [CrossRef]
- Sethi, M.; Singh, A.; Kaur, H.; Phagna, R.K.; Rakshit, S.; Chaudhary, D.P. Expression Profile of Protein Fractions in the Developing Kernel of Normal, Opaque-2 and Quality Protein Maize. Sci. Rep. 2021, 11, 2469. [Google Scholar] [CrossRef]
- Utpal, J.A.; Broadbent, K.; Byrne, M.J.; Blundell, C.A.; Howitt, M.L. Proteome Analysis of Hordein-Null Barley Lines Reveals Storage Protein Synthesis and Compensation Mechanisms. J. Agric. Food Chem. 2020, 68, 5763–5775. [Google Scholar]
- Avezum, L.; Rondet, E.; Mestres, C.; Achir, N.; Madode, Y.; Gibert, O.; Lefevre, C.; Hemery, Y.; Verdeil, J.-L.; Rajjou, L. Improving the Nutritional Quality of Pulses via Germination. Food Rev. Int. 2022, 39, 6011–6044. [Google Scholar] [CrossRef]
- Gokhisar, O.K.; Turhan, M. Cereals and Pulses: A Duet of the Mediterranean Diet for a Healthier Future. In Cereal-Based Foodstuffs: The Backbone of Mediterranean Cuisine; Springer International Publishing: Cham, Switzerland, 2021; pp. 151–165. ISBN 9783030692278. [Google Scholar]
- Doyle, J.J. Legume Phylogeny: Context for the Family, the Major Groups, and the Relationships of Crop and Model Species; AOCS Press: Champaign, IL, USA, 2019. [Google Scholar]
- Gravel, A.; Doyen, A. Pulse Globulins 11S and 7S: Origins, Purification Methods, and Techno-Functional Properties. J. Agric. Food Chem. 2023, 71, 2704–2717. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wang, T.; Luo, Y. A Review on Plant-Based Proteins from Soybean: Health Benefits and Soy Product Development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Pilco, C.; Jácome, D.N.; Torres, R.; Ramón, N.J.; Guerrero, K.B.; Moso, N.; Monar, B.; Herrera, M.I.; García, F. Analysis of Protein, Fiber Content and Amino Acid Profiles in Tempeh Obtained by Fermentation of Beans (Phaseolus vulgaris L.) and Quinoa (Chenopodium quinoa) with Rhizopus Oligosporus. EurAsian J. BioSci. 2019, 13, 1195–1199. [Google Scholar]
- Webb, D.; Li, Y.; Alavi, S. Chemical and Physicochemical Features of Common Plant Proteins and Their Extrudates for Use in Plant-Based Meat. Trends Food Sci. Technol. 2023, 131, 129–138. [Google Scholar] [CrossRef]
- Liu, S.; Cui, S.; Zhang, X.; Wang, Y.; Mi, G.; Gao, Q. Synergistic Regulation of Nitrogen and Sulfur on Redox Balance of Maize Leaves and Amino Acids Balance of Grains. Front. Plant Sci. 2020, 11, 576718. [Google Scholar] [CrossRef]
- Zahra, N.; Raza, Z.A.; Mahmood, S. Effect of Salinity Stress on Various Growth and Physiological Attributes of Two Contrasting Maize Genotypes. Braz. Arch. Biol. Technol. 2020, 63, e20200072. [Google Scholar] [CrossRef]
- Grewal, S.; Kanu, P.; Sharma, R.D.; Bharadwaj, V.; Sukhpreet, K.; Sidhu, S.; Singh, P. Characterization of Chickpea Cultivars and Trait Specific Germplasm for Grain Protein Content and Amino Acids Composition and Identification of Potential Donors for Genetic Improvement of Its Nutritional Quality. Plant Genet. Resour. 2022, 20, 383–393. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Netto, F.M.; Bertoldo-Pacheco, M.T.; Alegría, A.; Cilla, A. Peptide-Metal Complexes: Obtention and Role in Increasing Bioavailability and Decreasing the pro-Oxidant Effect of Minerals. Crit. Rev. Food Sci. Nutr. 2021, 61, 1470–1489. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Büscher, A.; Köppert, S.; Heiss, A.; Kuro, O.M.; Smith, E.R. Mud in the Blood: The Role of Protein-Mineral Complexes and Extracellular Vesicles in Biomineralisation and Calcification. J. Struct. Biol. 2020, 212, 107577. [Google Scholar] [CrossRef]
- Brokesh, A.M.; Gaharwar, A.K. Inorganic Biomaterials for Regenerative Medicine. ACS Appl. Mater. Interfaces 2020, 12, 5319–5344. [Google Scholar] [CrossRef]
- Imberti, C.; Sadler, P.J. 150 Years of the Periodic Table: New Medicines and Diagnostic Agents. In Medicinal Chemistry; Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–56. ISBN 9780128191965. [Google Scholar]
- Lückemeier, L.; Pierau, M.; Glorius, F. Asymmetric Arene Hydrogenation: Towards Sustainability and Application. Chem. Soc. Rev. 2023, 52, 4996–5012. [Google Scholar] [CrossRef] [PubMed]
- Krężel, A.; Maret, W. The Biological Inorganic Chemistry of Zinc Ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Lemloh, M.-L.; Altintoprak, K.; Wege, C.; Weiss, I.M.; Rothenstein, D. Biogenic and Synthetic Peptides with Oppositely Charged Amino Acids as Binding Sites for Mineralization. Materials 2017, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sarteshnizi, R.A.; Boachie, R.T.; Okagu, O.D.; Abioye, R.O.; Pfeilsticker Neves, R.; Ohanenye, I.C.; Udenigwe, C.C. Peptide-Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency. Foods 2020, 9, 1402. [Google Scholar] [CrossRef] [PubMed]
- Azizi, D.; Larachi, F.; Garnier, A.; Lagüe, P.; Levasseur, B. Sorption of Aqueous Amino Acid Species on Sulphidic Mineral Surfaces—DFT Study and Insights on Biosourced-reagent Mineral Flotation. Can. J. Chem. Eng. 2021, 99, 1758–1779. [Google Scholar] [CrossRef]
- El-Aziz, A.; Morsi, S.M.M.; Salama, D.M.; Abdel-Aziz, M.S.; Abd Elwahed, M.S.; Shaaban, E.A.; Youssef, A.M. Preparation and Characterization of Chitosan/Polyacrylic Acid/Copper Nanocomposites and Their Impact on Onion Production. Int. J. Biol. Macromol. 2019, 123, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.; Bamdad, F.; Chen, L. Metal Solubility Enhancing Peptides Derived from Barley Protein. Food Chem. 2014, 159, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.E.; Esfandi, R.; Tsopmo, A. Potential of Food Hydrolyzed Proteins and Peptides to Chelate Iron or Calcium and Enhance Their Absorption. Foods 2018, 7, 172. [Google Scholar] [CrossRef]
- Ashaolu, T.; Joshua, C.C.; Lee, J.O.; Ashaolu, H.; Pourjafar, S.M. Metal-Binding Peptides and Their Potential to Enhance the Absorption and Bioavailability of Minerals. Food Chem. 2023, 428, 136678. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Ao, J. Separation and Identification of Zinc-Chelating Peptides from Sesame Protein Hydrolysate Using IMAC-Zn2+ and LC-MS/MS. Food Chem. 2012, 134, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, X.; Liu, Y.; Luo, C.; Lu, X.; Cai, Y. Polymerization-Induced Electrostatic Self-Assembly Governed by Guanidinium Ionic Hydrogen Bonds. Macromolecules 2022, 55, 7003–7012. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of Enhancing Dietary Zinc Bioavailability with Food-Derived Zinc-Chelating Peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, Y.; Ying, Z.; Li, H.; Liu, W.; Wang, J.; Liu, X. The Formation, Structural Characteristics, Absorption Pathways and Bioavailability of Calcium-Peptide Chelates. Foods 2022, 11, 2762. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Haes, A.J. Elucidation of PH Impacts on Monosubstituted Benzene Derivatives Using Normal Raman and Surface-Enhanced Raman Scattering. J. Chem. Phys. 2020, 153, 184707. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, B.; Li, H. Zn (II) Chelating with Peptides Found in Sesame Protein Hydrolysates: Identification of the Binding Sites of Complexes. Food Chem. 2014, 165, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Revisiting Phytate-Element Interactions: Implications for Iron, Zinc and Calcium Bioavailability, with Emphasis on Legumes. Crit. Rev. Food Sci. Nutr. 2022, 62, 1696–1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Z.; Liu, C.; Sun, H.; Liu, Y. Investigating the Calcium Binding Characteristics of Black Bean Protein Hydrolysate. Food Funct. 2020, 11, 8724–8734. [Google Scholar] [CrossRef]
- Tian, Q.; Fan, Y.; Hao, L.; Wang, J.; Xia, C.; Wang, J.; Hou, H. A Comprehensive Review of Calcium and Ferrous Ions Chelating Peptides: Preparation, Structure and Transport Pathways. Crit. Rev. Food Sci. Nutr. 2023, 63, 4418–4430. [Google Scholar] [CrossRef]
- Yuan, X.; Bao, X.; Feng, G.; Zhang, M.; Ma, S. Effects of Peptide-Calcium Complexes from Sunflower Seeds and Peanuts on Enhancing Bone Mineral Density. Int. J. Food Sci. Technol. 2020, 55, 2942–2953. [Google Scholar] [CrossRef]
- Chunkao, S.; Youravong, W.; Yupanqui, C.T.; Alashi, A.M.; Aluko, R.E. Structure and Function of Mung Bean Protein-Derived Iron-Binding Antioxidant Peptides. Foods 2020, 9, 1406. [Google Scholar] [CrossRef]
- Hendrickson, T.L.; Wood, W.N.; Rathnayake, U.M. Did Amino Acid Side Chain Reactivity Dictate the Composition and Timing of Aminoacyl-TRNA Synthetase Evolution? Genes 2021, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, Y.; Zhang, X.; Li, Y.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Chen, Z. Isolation of a Novel Calcium-Binding Peptide from Wheat Germ Protein Hydrolysates and the Prediction for Its Mechanism of Combination. Food Chem. 2018, 239, 416–426. [Google Scholar] [CrossRef]
- Yu, X.; Wei, S.; Yang, Y.; Ding, Z.; Wang, Q.; Zhao, J.; Liu, X.; Chu, X.; Tian, J.; Wu, N.; et al. Identification of Cadmium-Binding Proteins from Rice (Oryza sativa L.). Int. J. Biol. Macromol. 2018, 119, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Sinha, A.S.K.; Nigam, K.D.P.; Dwivedi, D.; Sangwai, J.S. Functionalized Nanoparticles: Tailoring Properties through Surface Energetics and Coordination Chemistry for Advanced Biomedical Applications. Nanoscale 2023, 15, 6075–6104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, R.; Xu, Z.; Jiang, L.; Sui, X. Recent Advances in Soy Protein Extraction Technology. J. Am. Oil Chem. Soc. 2023, 100, 187–195. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Hsu, C.-C.; Hung, J.-N.; Wang, Y.-T.; Choong, W.-K.; Zeng, M.-Y.; Lin, P.-Y.; Hong, R.-W.; Sung, T.-Y.; Chen, Y.-J. Sequential Phosphoproteomic Enrichment through Complementary Metal-Directed Immobilized Metal Ion Affinity Chromatography. Anal. Chem. 2014, 86, 685–693. [Google Scholar] [CrossRef]
- Dhumal, V.; Chanda, K.; Pal, P. Synthesis, Characterization, and Antimicrobial Efficacy of Composite Films from Guar Gum/Sago Starch/Whey Protein Isolate Loaded with Carvacrol, Citral and Carvacrol-Citral Mixture. J. Mater. Sci. Mater. Med. 2019, 30, 117. [Google Scholar] [CrossRef]
- Lv, Y.; Bao, X.; Liu, H.; Ren, J.; Guo, S. Purification and Characterization of Caclium-binding Soybean Protein Hydrolysates by Ca2+/Fe3+ Immobilized Metal Affinity Chromatography (IMAC). Food Chem. 2013, 141, 1645–1650. [Google Scholar] [CrossRef]
- Kubglomsong, S.; Theerakulkait, C.; Reed, R.L.; Yang, L.; Maier, C.S.; Stevens, J.F. Isolation and Identification of Tyrosinase-Inhibitory and Copper-Chelating Peptides from Hydrolyzed Rice-Bran-Derived Albumin. J. Agric. Food Chem. 2018, 66, 8346–8354. [Google Scholar] [CrossRef]
- Feng, W.; Dong, T.; Li, K.; Wang, T.; Chen, Z.; Wang, R. Characterization of Binding Behaviors of Cd2+ to Rice Proteins. Food Chem. 2019, 275, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Ke-Xue, X.-P.; Wang, X.-N. Isolation and Characterization of Zinc-Chelating Peptides from Wheat Germ Protein Hydrolysates. J. Funct. Foods 2015, 12, 23–32. [Google Scholar]
- Legcharoen, T.; Kubglomsong, S.; Theerakulkait, C. Inhibitory Effect of Rice Bran Protein and Its Fractions on Enzymatic Browning in Potato Puree. Agric. Nat. Resour. 2020, 54, 301–308. [Google Scholar]
- Kong, F.; Kang, S.; Zhang, J.; Jiang, L.; Liu, Y.; Yang, M.; Cao, X.; Zheng, Y.; Shao, J.; Yue, X. The Non-Covalent Interactions between Whey Protein and Various Food Functional Ingredients. Food Chem. 2022, 394, 133455. [Google Scholar] [CrossRef] [PubMed]
- Bist, P.; Choudhary, S. Impact of Heavy Metal Toxicity on the Gut Microbiota and Its Relationship with Metabolites and Future Probiotics Strategy: A Review. Biol. Trace Elem. Res. 2022, 200, 5328–5350. [Google Scholar] [CrossRef] [PubMed]
- Bloor, S.R.; Schutte, R.; Hobson, A.R. Oral Iron Supplementation—Gastrointestinal Side Effects and the Impact on the Gut Microbiota. Microbiol. Res. 2021, 12, 491–502. [Google Scholar] [CrossRef]
- Ribeiro, M.; Fonseca, L.; Anjos, J.S.; Capo-Chichi, J.C.C.; Borges, N.A.; Burrowes, J.; Mafra, D. Oral Iron Supplementation in Patients with Chronic Kidney Disease: Can It Be Harmful to the Gut Microbiota? Nutr. Clin. Pract. 2022, 37, 81–93. [Google Scholar] [CrossRef]
- Amrousy, E.; Doaa, D.; El-Afify, A.; Elsawy, M. Lactoferrin for Iron-Deficiency Anemia in Children with Inflammatory Bowel Disease: A Clinical Trial. Pediatr. Res. 2022, 92, 762–766. [Google Scholar] [CrossRef]
- Davison, K.M. Nutrition Guide for Physicians and Related Healthcare Professionals; Springer: Berlin/Heidelberg, Germany, 2017; pp. 261–272. [Google Scholar]
- Du, W.; Wang, X.; Hu, M.; Hou, J.; Du, Y.; Si, W.; Yang, L.; Xu, L.; Xu, Q. Modulating Gastrointestinal Microbiota to Alleviate Diarrhea in Calves. Front. Microbiol. 2023, 14, 1181545. [Google Scholar] [CrossRef]
- Venkataramani, V. Iron Irons Homeostasis and Metabolism: Two Sides of a Coin. In Ferroptosis: Mechanism and Diseases; Springer: Berlin/Heidelberg, Germany, 2021; pp. 25–40. [Google Scholar]
- Bhadra, P.; Deb, A. A Review on Nutritional Anemia. Indian J. Nat. Sci. 2020, 10, 18466–18474. [Google Scholar]
- Islam, S.; Hoque, N.; Nasrin, N.; Hossain, M.; Rizwan, F.; Biswas, K.; Asaduzzaman, M.; Rahman, S.; Hoskin, D.W.; Sultana, S.; et al. Iron Overload and Breast Cancer: Iron Chelation as a Potential Therapeutic Approach. Life 2022, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.M.; Halliday, J.W.; Powell, L.W. Interrelationships of Alcohol and Iron in Liver Disease with Particular Reference to the Iron-Binding Proteins, Ferritin and Transferrin. J. Gastroenterol. Hepatol. 1999, 14, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Z.; Cheng, P.; She, Y.; Wang, W.; Tian, Y.; Ma, J.; Sun, Z. Efficient Activation of Peracetic Acid by Mixed Sludge Derived Biochar: Critical Role of Persistent Free Radicals. Water Res. 2022, 223, 119013. [Google Scholar] [CrossRef] [PubMed]
- Rani, I.; Goyal, A.; Bhatnagar, M.; Manhas, S.; Goel, P. Potential Molecular Mechanisms of Zinc-and Copper-Mediated Antiviral Activity on COVID-19. Nutr. Res. 2021, 92, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative Stress in the Aging Substantia Nigra and the Etiology of Parkinson’s Disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Opportunities for Plant-Derived Enhancers for Iron, Zinc, and Calcium Bioavailability: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 652–685. [Google Scholar] [CrossRef] [PubMed]
- Liberal, Â.; Pinela, J.; Vívar-Quintana, A.M.; Ferreira, I.C.F.R.; Barros, L. Fighting Iron-Deficiency Anemia: Innovations in Food Fortificants and Biofortification Strategies. Foods 2020, 9, 1871. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Chen, D.; Chen, M.; Xiang, A.; Xie, B.; Sun, Z. Effect of High Pressure Processing on Gastrointestinal Fate of Carotenoids in Mango Juice: Insights Obtained from Macroscopic to Microscopic Scales. Innov. Food Sci. Emerg. Technol. 2023, 85, 103325. [Google Scholar] [CrossRef]
- Kitala, K.; Tanski, D.; Godlewski, J.; Krajewska-Włodarczyk, M.; Gromadziński, L.; Majewski, M. Copper and Zinc Particles as Regulators of Cardiovascular System Function—A Review. Nutrients 2023, 15, 3040. [Google Scholar] [CrossRef]
- Eto, K.; Suemoto, T. Identification of Reactive Oxygen Species That Induce Spoptosis, a Novel and Distinctive Mode of Regulated Cell Death. Exp. Cell Res. 2023, 430, 113713. [Google Scholar] [CrossRef]
- Kozlova, E.; Sherstyukova, E.; Sergunova, V.; Kozlov, A.; Gudkova, O.; Inozemtsev, V.; Chernysh, A. The Toxic Influence of Excess Free Iron on Red Blood Cells in the Biophysical Experiment: An in Vitro Study. J. Toxicol. 2022, 2022, 7113958. [Google Scholar] [CrossRef] [PubMed]
- Brazel, E. Understanding and Exploiting Zinc Toxicity in Streptococcus pneumoniae. Ph.D. Thesis, School of Biological Sciences, Cold Spring Harbor, NY, USA, 2020. [Google Scholar]
- Husain, N.; Mahmood, R. Copper (II) Generates ROS and RNS, Impairs Antioxidant System and Damages Membrane and DNA in Human Blood Cells. Environ. Sci. Pollut. Res. 2019, 26, 20654–20668. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Huo, Y.; Li, H.; Yang, F.; Liao, J.; Han, Q.; Li, Y.; Pan, J.; Hu, L.; Guo, J.; et al. New Insights into the Interaction between Duodenal Toxicity and Microbiota Disorder under Copper Exposure in Chicken: Involving in Endoplasmic Reticulum Stress and Mitochondrial Toxicity. Chem. Biol. Interact. 2022, 366, 110132. [Google Scholar] [CrossRef] [PubMed]
- Rab, M.; Ae, B.A.; Van Oirschot, P.A.; Kosinski, J.; Hixon, K.; Johnson, V.; Chubukov, L. AG-348 (Mitapivat), an Allosteric Activator of Red Blood Cell Pyruvate Kinase, Increases Enzymatic Activity, Protein Stability, and Adenosine Triphosphate Levels over a Broad Range of PKLR Genotypes. Haematologica 2021, 106, 238. [Google Scholar] [CrossRef] [PubMed]
- Kaziród, K.; Myszka, M.; Dulak, J.; Łoboda, A. Hydrogen Sulfide as a Therapeutic Option for the Treatment of Duchenne Muscular Dystrophy and Other Muscle-Related Diseases. Cell. Mol. Life Sci. 2022, 79, 608. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Cormick, G.; Hofmeyr, G.J.; Garcia-Casal, M.N.; Peña-Rosas, J.P.; Betrán, A.P. Calcium-Fortified Foods in Public Health Programs: Considerations for Implementation. Ann. N. Y. Acad. Sci. 2021, 1485, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Patil, P.J.; Manzoor, M.F.; Bilal, M.; Ahmed, S.; Murtaza, M.A.; Shah, H.; Nawaz, N.; Amjad, S.; Abrar, M. Dough Rheology and the Impact of Zinc Sulfate on the Quality of Cookies. Food Sci. Technol. 2021, 41, 646–653. [Google Scholar] [CrossRef]
- Sethi, S.; Joshi, A.; Arora, B.; Chauhan, O.P. Chemical Composition of Foods. In Advances in Food Chemistry; Springer Nature: Singapore, 2022; pp. 1–37. ISBN 9789811947957. [Google Scholar]
- Szajnar, K.; Znamirowska, A.; Kalicka, D. Effects of Various Magnesium Salts for the Production of Milk Fermented by Bifidobacterium Animalis Ssp. Lactis Bb-12. Int. J. Food Prop. 2019, 22, 1087–1099. [Google Scholar] [CrossRef]
- Stantiall, S.E.; Serventi, L. Nutritional and Sensory Challenges of Gluten-Free Bakery Products: A Review. Int. J. Food Sci. Nutr. 2018, 69, 427–436. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition Facts and Functional Potential of Quinoa (Chenopodium quinoa Willd.), an Ancient Andean Grain: A Review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Genu Dalbhagat, C.; Mandliya, S.; Niwas Mishra, H. Investigation of Natural Food Fortificants for Improving Various Properties of Fortified Foods: A Review. Food Res. Int. 2022, 156, 111186. [Google Scholar] [CrossRef]
- Prieto-Patron, A.; Detzel, P.; Ramayulis, R.; Sudikno; Irene; Wibowo, Y. Impact of Fortified Infant Cereals on the Burden of Iron Deficiency Anemia in 6- to 23-Month-Old Indonesian Infants and Young Children: A Health Economic Simulation Model. Int. J. Environ. Res. Public Health 2022, 19, 5416. [Google Scholar] [CrossRef]
- Evcan, E.; Gulec, S. The Development of Lentil Derived Protein-Iron Complexes and Their Effects on Iron Deficiency Anemia in Vitro. Food Funct. 2020, 11, 4185–4192. [Google Scholar] [CrossRef]
- Garcés-Rimón, M.; Morales, D.; Miguel-Castro, M. Potential Role of Bioactive Proteins and Peptides Derived from Legumes towards Metabolic Syndrome. Nutrients 2022, 14, 5271. [Google Scholar] [CrossRef]
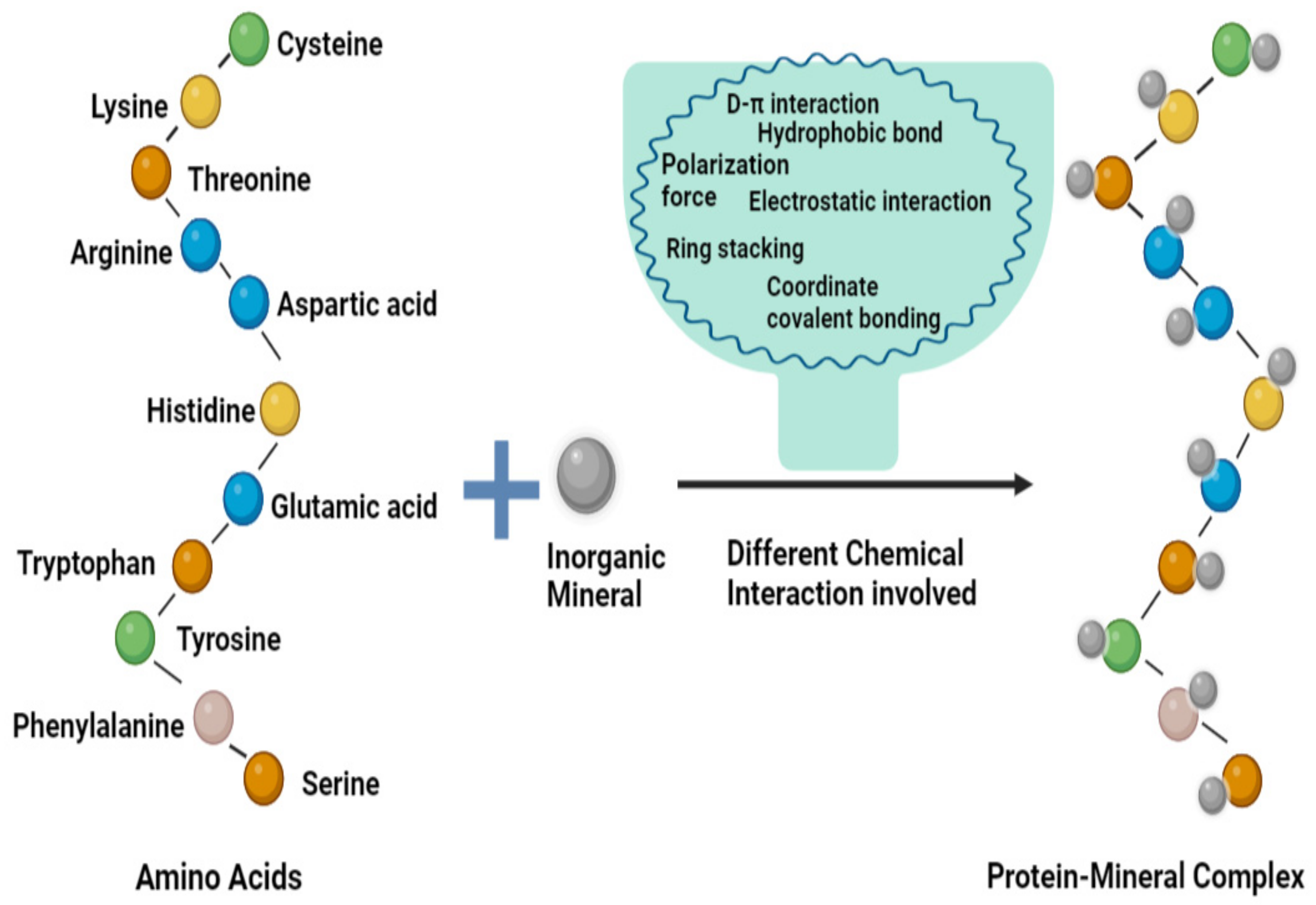
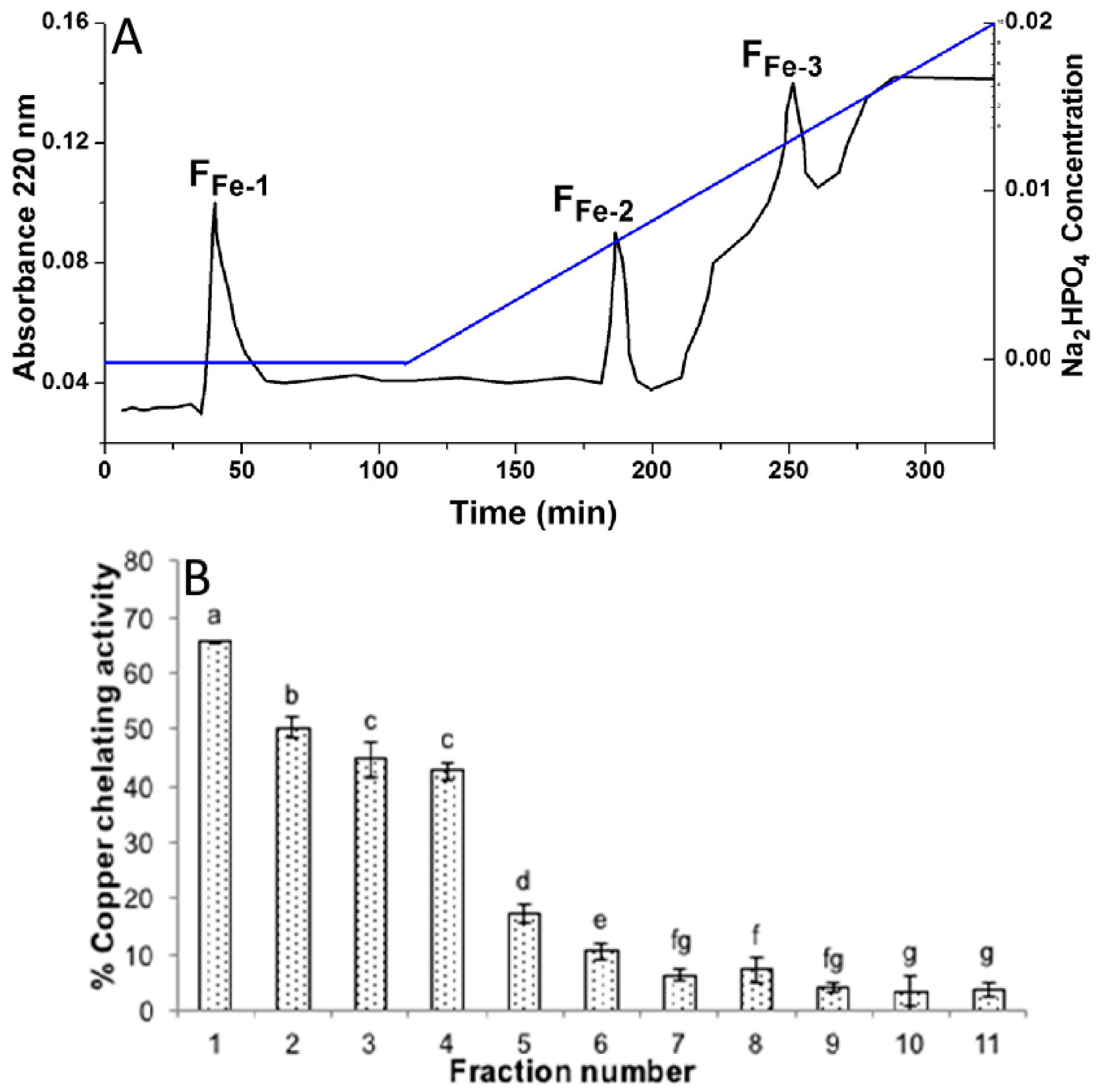
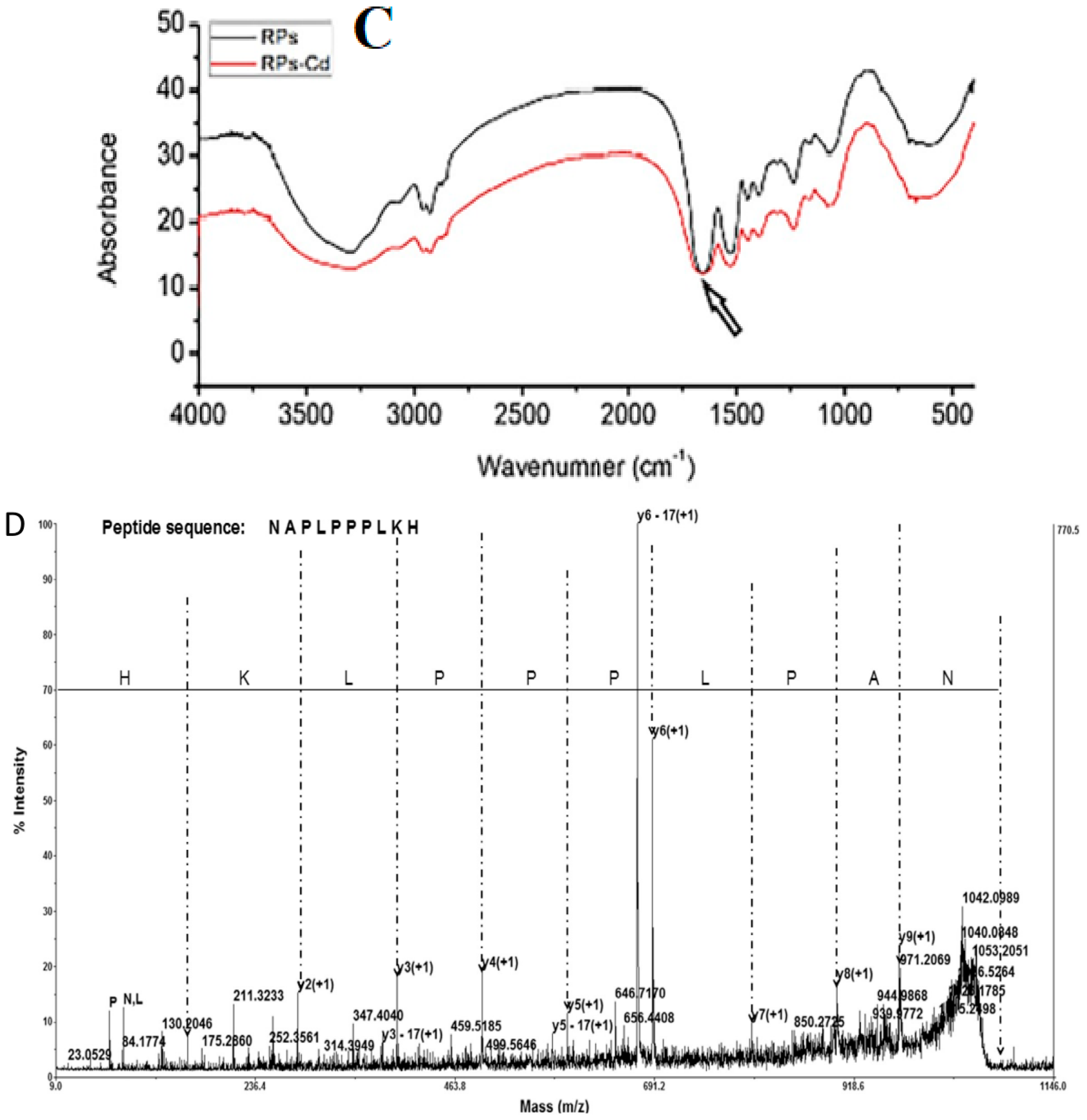
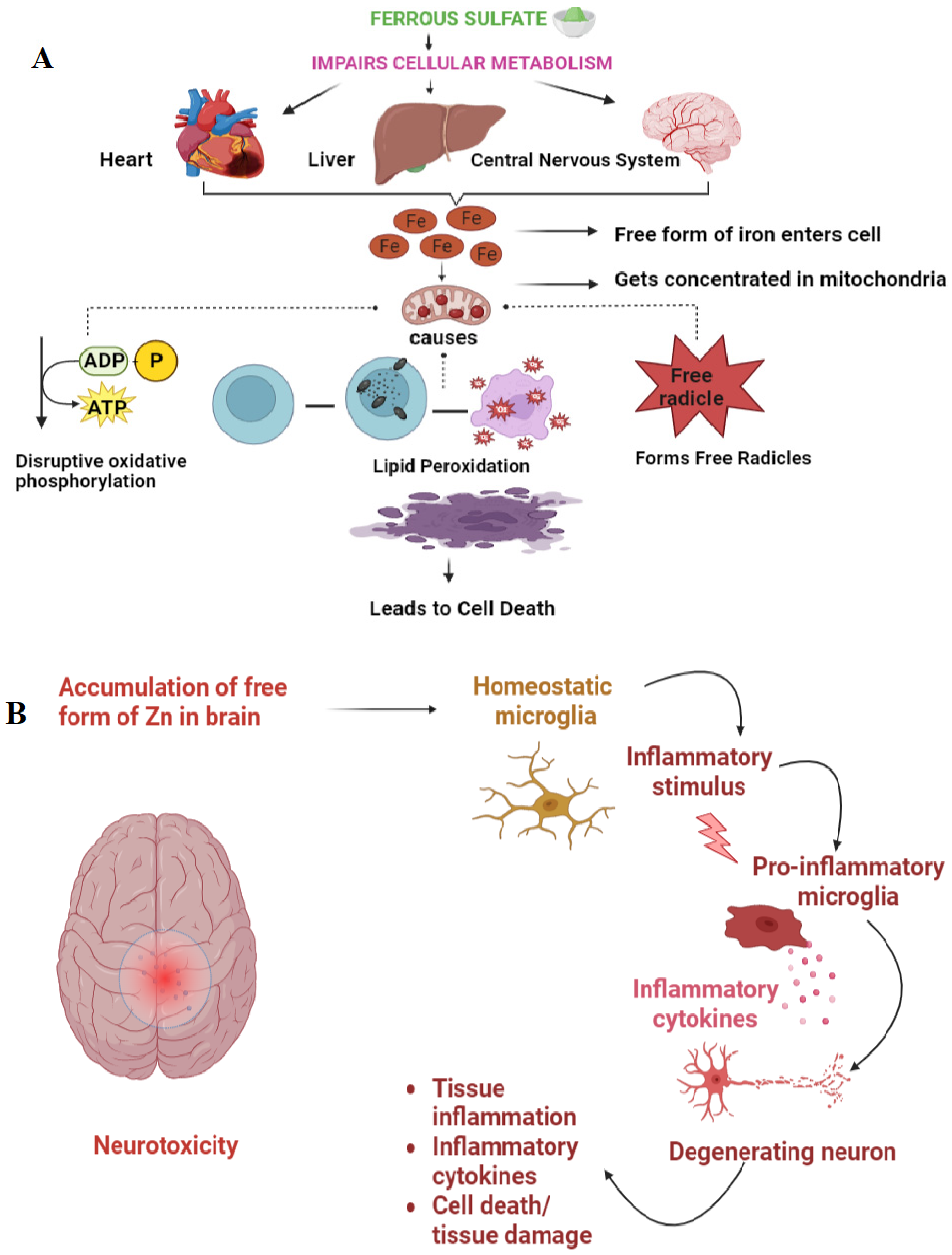
| Protein and Mineral Interaction | Mineral Salt | Binding Efficiency | References |
|---|---|---|---|
| Peanut protein isolate + Zinc | Zinc Sulfate | 124.7 mg/g | |
| Barley protein hydrolysate + Iron | Ferrous Chloride | 188 µg/mL | [39] |
| Barley protein hydrolysate+ Calcium | Calcium Chloride | 141 µg/mL | [39] |
| Barley protein hydrolysate+ Copper | Copper Sulfate | 134 µg/mL | [39] |
| Barley protein hydrolysate+ Zinc | Zinc acetate | 125 µg/mL | [39] |
| Soybean protein hydrolysate + Iron | Ferrous Chloride | 3.87 mg/g | [43] |
| Soybean protein hydrolysate + calcium | Calcium chloride | 66.9 mg/g | [45] |
| Jamapa protein hydrolysate + Iron | Ferrous Chloride | 15.68% | [45] |
| Jamapa protein hydrolysate + Copper | Copper Sulfate | 81.63% | [45] |
| Black bean protein hydrolysate + Calcium | Calcium chloride | 77.54 µg/mg | [43] |
| Red lentil protein hydrolysate + Iron | Ferrous sulfate heptahydrate | 98% | [48] |
| Mung bean protein hydrolysate + Iron | Ferrous chloride tetrahydrate | 153.59 mg/g | [41] |
| Oat protein hydrolysate + Iron | Ferric chloride | 39.7% | [34] |
| Mung Bean protein hydrolysate + Iron | Ferrous sulfate heptahydrate | 61.25 µg/mg | [44] |
| Soybean protein hydrolysate + Iron | Ferric chloride | 12.42 mg/g | [37] |
| Sunflower seed protein + Calcium | Calcium chloride | 124.7 mg/g | [43] |
| Mung bean protein + Calcium | Calcium chloride | 196 mg/g | [41] |
| Mung bean protein + Iron | Ferrous chloride tetrahydrate | 19.24 mg/g | [41] |
| Rice Protein + Cadmium | Cadmium chloride | 15.26 mg/g | [55] |
| Wheat Germ Protein Hydrolysate + Calcium | Calcium chloride | 67.5 mg/g | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jindal, A.; Patil, N.; Bains, A.; Sridhar, K.; Stephen Inbaraj, B.; Tripathi, M.; Chawla, P.; Sharma, M. Recent Trends in Cereal- and Legume-Based Protein-Mineral Complexes: Formulation Methods, Toxicity, and Food Applications. Foods 2023, 12, 3898. https://doi.org/10.3390/foods12213898
Jindal A, Patil N, Bains A, Sridhar K, Stephen Inbaraj B, Tripathi M, Chawla P, Sharma M. Recent Trends in Cereal- and Legume-Based Protein-Mineral Complexes: Formulation Methods, Toxicity, and Food Applications. Foods. 2023; 12(21):3898. https://doi.org/10.3390/foods12213898
Chicago/Turabian StyleJindal, Aprajita, Nikhil Patil, Aarti Bains, Kandi Sridhar, Baskaran Stephen Inbaraj, Manikant Tripathi, Prince Chawla, and Minaxi Sharma. 2023. "Recent Trends in Cereal- and Legume-Based Protein-Mineral Complexes: Formulation Methods, Toxicity, and Food Applications" Foods 12, no. 21: 3898. https://doi.org/10.3390/foods12213898
APA StyleJindal, A., Patil, N., Bains, A., Sridhar, K., Stephen Inbaraj, B., Tripathi, M., Chawla, P., & Sharma, M. (2023). Recent Trends in Cereal- and Legume-Based Protein-Mineral Complexes: Formulation Methods, Toxicity, and Food Applications. Foods, 12(21), 3898. https://doi.org/10.3390/foods12213898









