1. Introduction
In recent years, there is a growing trend to find alternative protein sources to animal proteins. Legumes are one of the main sources of plant-based protein and have historically been an important protein source for the human diet [
1]. Legumes also present other benefits, since they are adapted to a wide range of climatic conditions and fix atmospheric nitrogen. Among them, lupins have a high proportion of proteins and they have emerged as a cheap functional food [
2,
3]. In fact, protein contents ranged between 30 and 50%, depending on the lupin specie, with an excellent amino acid profile, particularly rich in lysine in contrast to other plant proteins [
3]. However, taking into account their potential use in the food industry and in human nutrition, lupins are underused legumes [
4]. The most important lupin species include white lupin (
Lupinus albus), narrow-leaved lupin (
Lupinus angustifolius), pearl lupin (
Lupinus mutabilis), and yellow lupin (
Lupinus luteus) [
2]. Moreover, in addition to nutritional aspects, the consumption of lupin proteins was related to hypolipidemic, hypoglycemic, hypotensive, anticarcinogenic, and antiobesity properties [
5].
There is increasing interest in the production of protein isolates and concentrates since they are vital for food processing and are used in several applications in the food industry [
2,
6]. Among lupin species, yellow lupin (
L. luteus) normally presented the highest protein and the lowest fat contents, which are two essential qualities to produce protein isolates [
2]. Generally, two main procedures exist to extract proteins from lupin. These include dry-fractionation, in which proteins are separated from the other constituents according to their size, density, and electrostatic properties [
7], and wet-extraction, in which proteins are solubilized at alkaline pH, and they are recovered by precipitation at isoelectric point. Other extractions (wet extractions) consist of micellization (salt extraction) and selective fractionation (acid extractions) [
4]. Among both procedures, wet-extraction is better, since protein isolates that are produced have higher purity, digestibility, and quality, while protein concentrates obtained with dry-fractionation presented low purity (~50%) [
8], with high amounts of other constituents (carbohydrates, lipids, etc.), and thus should be further processed for concentration [
6]. Moreover, among the wet-extraction procedures, alkaline solubilization-isoelectric precipitation is the most used and resulted in LPI with high purity (i.e., in comparison with micellization), but it is important to highlight that this procedure promotes protein denaturation, which can affect the lupin protein isolate’s (LPI) technofunctional properties [
9].
Due to the aforementioned advantages, many studies use wet-extraction as the main method to obtain protein isolates from lupin or from other legumes [
2]. Most focus on the first stage of solubilization at alkaline pH, followed by a separation of the insoluble fraction, and a precipitation of the proteins at the isoelectric point. There are countless procedures, which include steps in which moderate temperatures are applied, which are left to rest overnight, or undergo applications of different pH (between 8 and 11 for protein solubilization and between 4 and 6 for precipitation). It is well known that temperature can significantly affect the conformation of proteins, producing protein denaturalization and insolubilization, and therefore it could compromise their technological properties [
10]. Moreover, the fact of carrying out long procedures, and with stages of rest overnight, means that they are not the most neither suitable nor efficient for the industry, which would make it difficult to be able to scale these protocols. Additionally, some compounds such as oil and carotenoids are present in lupin seeds, and the defatted step is a typical procedure before protein extraction [
2]. The defatted procedure increases purity and yield, and also affects the techno-functional properties [
6]; however, it is also a previous step that adds complexity to the process and the application of unwanted solvents to the sample. Moreover, the use of some solvents during the defatted phase increased protein denaturation, resulting in decreased solubility and lower protein recovery [
4]. Protein extraction is a very complex procedure, which includes the penetration of the solvent into the cells and the correct solubilization of the proteins [
11]. Thus, several authors conclude that optimization of the extraction protocol is essential to be able to obtain high yields of lupin protein isolates of high purity [
4]. Moreover, the optimum pH for alkali solubilization needs to be explored further to increase yield and LPI quality [
9]. For all these reasons, in this study, an extraction based on wet-extraction has been proposed, but without a defatted step, the application of temperature or long stages, which optimize the pH values, allow to obtain the highest yield and highest purity.
Therefore, the main objective of the present study was to design an efficient, fast, simple, and safe process to obtain the lupin protein isolate. For this, a simple protocol was employed and the main extraction conditions, which include solubilization pH, precipitation pH, and extraction time were optimized.
2. Materials and Methods
2.1. Raw Material
Lupin seeds (Lupinus luteus L. [Tremosilla]) were purchased from Semillas Batlle S.A. (Barcelona, Spain). They were ground to obtain homogeneous lupin flour.
2.2. Preparation of Lupin Protein Isolate
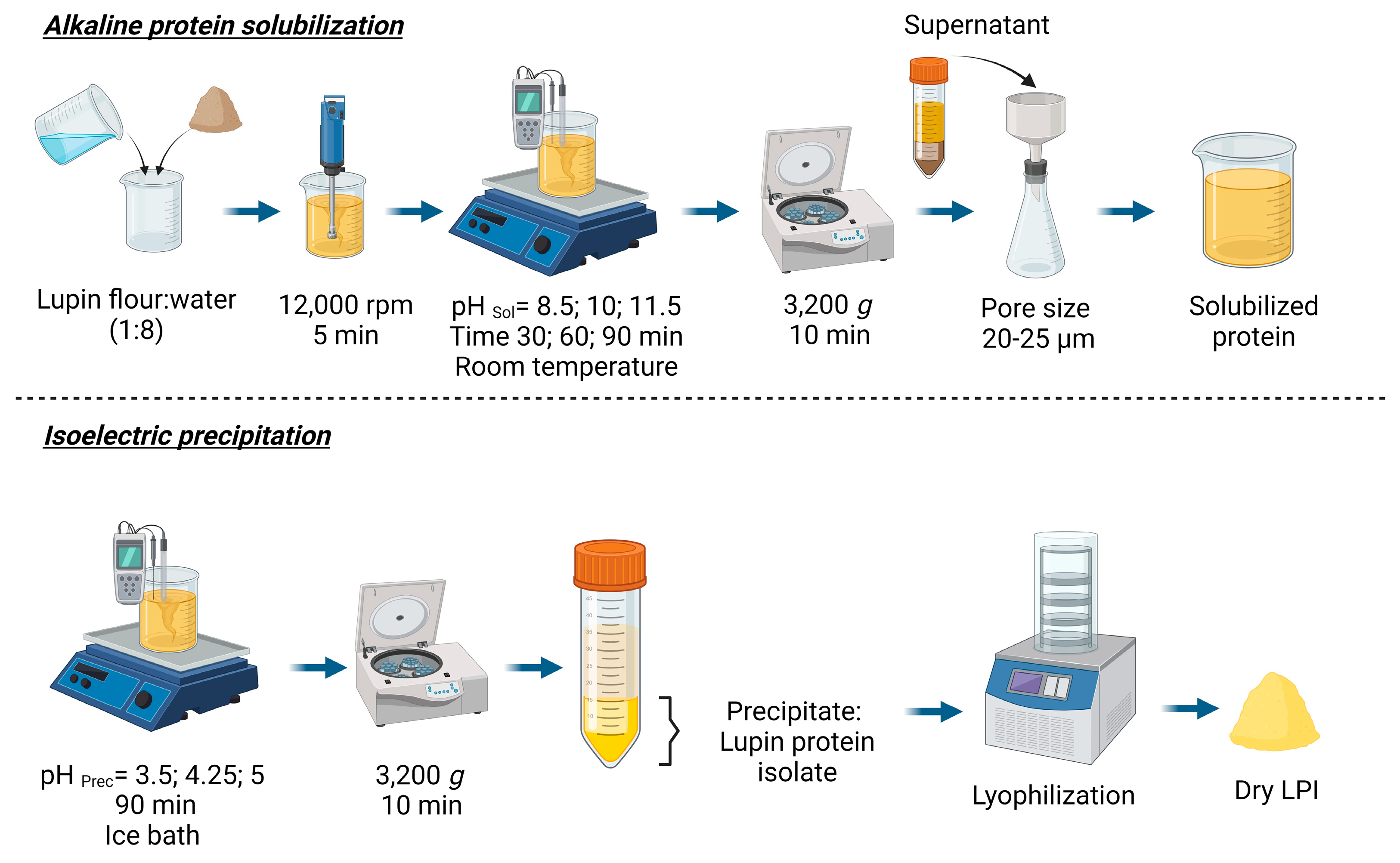
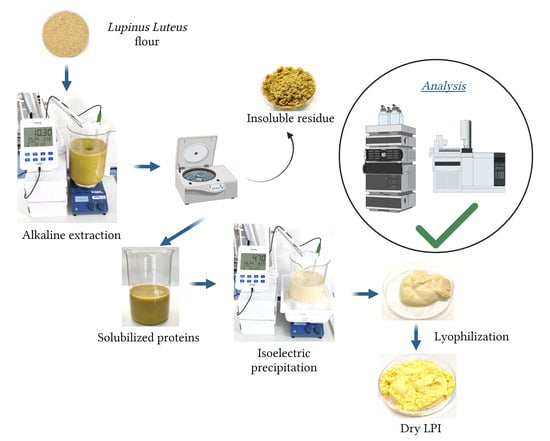
The lupin protein isolates (LPI) were prepared from lupin flour (
Figure 1), using the pH and time conditions specified in the experimental design section. Briefly, flour was dispersed with distilled water (1:8
w/
w) and homogenized with UltraTurrax (IKA, model T18; Staufen, Germany) for 5 min at 12,000 rpm. The protein was solubilized by adjustment of pH at 8.5–11.5 with 2M NaOH. The mixture was stirred (magnetic stirred) and protein extraction was tested at three different times (30, 60, and 90 min) and at room temperature. Then, the mixture was centrifuged (3200×
g for 10 min; Beckman Coulter (Brea, CA, USA), model Allegra X-22R, rotor SX4250) to separate the residual starch and insoluble fibers, and the supernatant was filtered through a paper filter (pore size 20–25 µm; Filterlab 1238, Barcelona, Spain). The pellet was washed with distilled water with the pH adjusted at the desired extraction pH, centrifuged, and filtered again. Both supernatants were combined.
For protein precipitation (isoelectric point), the supernatant was placed in an ice bath, the pH was adjusted to pH 3.5–5.0 with 4M HCl and magnetically stirred for 30 min. Then, the mixture was left to rest for an hour. The protein isolate was recovered by centrifugation (3200× g for 10 min; Beckman Coulter (Brea, CA, USA), model Allegra X-22R, rotor SX4250), and the LPI was washed with distilled water (1:8 w/v), and centrifuged again (3200× g for 10 min; Beckman Coulter (Brea, CA, USA), model Allegra X-22R, rotor SX4250). The precipitate was lyophilized (Lyovapor L300, Büchi; Barcelona, Spain; Primary drying pressure limit: 0.500 mbar, time 240 min; Secondary drying pressure limit: 0.400 mbar, time 120 min) and stored at −20 °C until further analysis.
2.3. Experimental Design and Optimized Responses
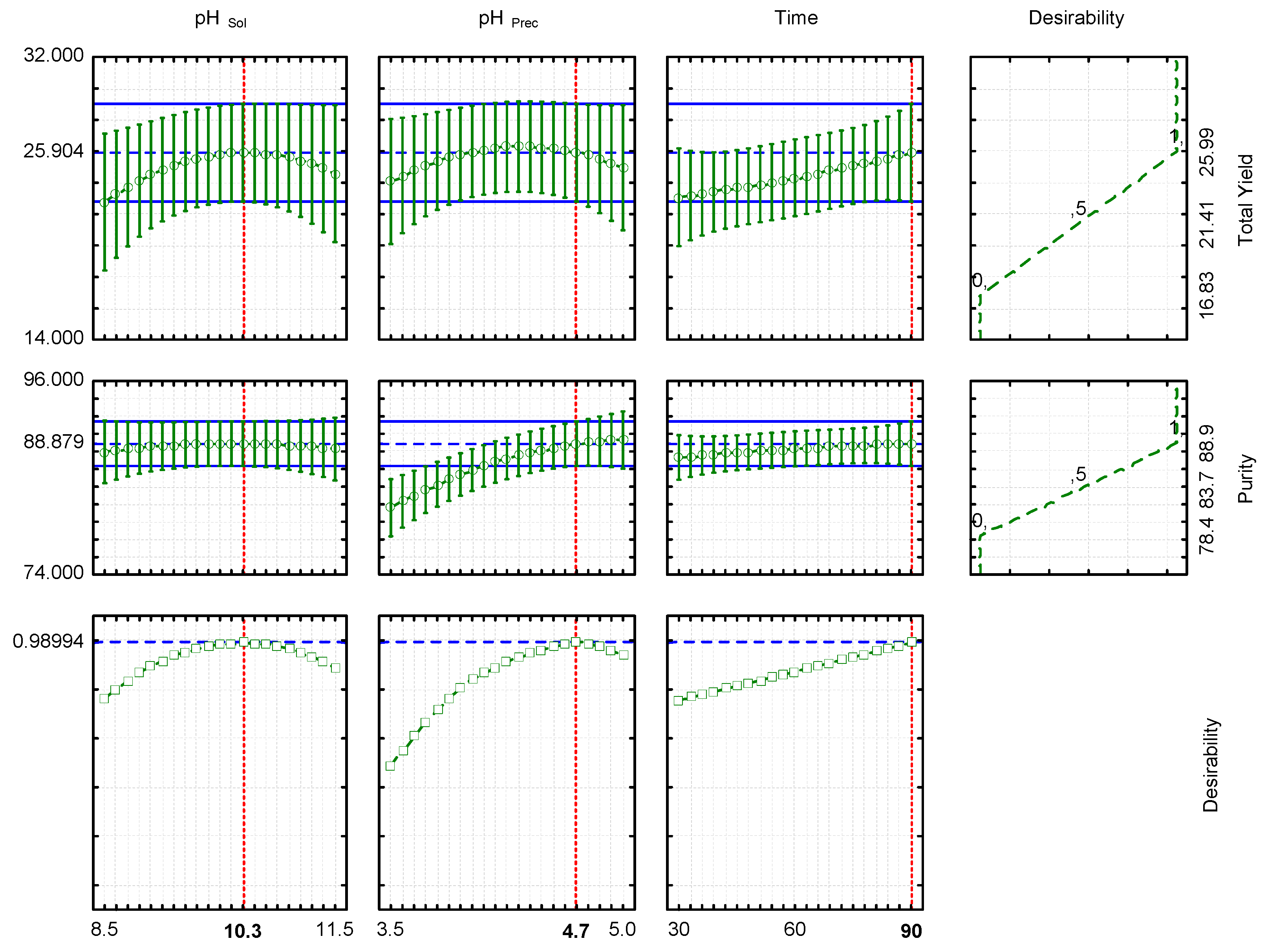
This study was conducted using an independent quadratic Box–Behnken experimental design (3-factor and 3-level) with 15 experimental runs and three center points (3 × 1 × 15) (
Table 1). The response surface methodology (RSM) was used to identify the optimal levels of the independent variables for maximize the responses. The effect of the solubilization pH (pH
sol; x
1), extraction time (x
2; minutes), and precipitation pH (pH
prec; x
3) (independent variables) on the protein extraction yield (y
1) and LPI purity (y
2) (dependent variables) were studied. The experimental data were adjusted to the second-order polynomial model, which describes the interaction between the factors and response variables obtained through RSM, according to Equation (1).
where the
Y is the predicted result;
β0 is a coefficient of the models;
βi,
βii, and
βij are the coefficients of the equations representing linear, quadratic, and interaction models, respectively; and
Xi and
Xj are the independent variables that determine changes in the response variable.
The adjustment of the model and statistical values was determined using an ANOVA test. The dependent variables were analyzed to obtain the optimal conditions using a multi-response surface optimization, and the optimal extraction conditions were estimated with the response desirability profiling function. For the model validation, four independent extractions were carried out at the optimal conditions, and predicted and experimental values were compared.
2.4. Lupin Flour and Optimized LPI Characterization
2.4.1. Chemical Composition and Color Determination
The chemical composition of lupin flour and lyophilized LPI obtained at the optimal conditions was determined according to ISO procedures for protein [
12] (N × 6.25) and ash [
13], while lipid content was determined according to Procedure Am 5–04 [
14]. Color parameters were measured using a portable CR-400 colorimeter (Konica Minolta Sensing Inc., Osaka, Japan).
2.4.2. Fatty Acid Analysis
The fatty acid determination was carried out with gas chromatography. Briefly, lupin oil was extracted using the Bligh and Dyer [
15] procedure. Then, fatty acids were trans-esterified with sodium methoxide and sulfuric acid-methanol solutions [
16]. The fatty acids methyl esters were separated, identified, and quantified using GC-FID (GC-Agilent 7890B, Agilent Technologies, Santa Clara, CA, USA), equipped with a capillary column DB-23 (60 m, 0.25 mm i.d., 0.25 µm film thickness; Agilent Technologies). The procedure and the chromatographic conditions were previously described by Barros et al. [
16]. The fatty acids results were expressed as g/100 g of total fatty acids.
2.4.3. Amino Acid Analysis and Chemical Score
The amino acid content of the lupin flour and LPI was determined using liquid chromatography, following the sample treatment, derivation, and chromatographic conditions described by Munekata et al. [
17]. Briefly, the LPI samples (0.1 g) were hydrolyzed with HCl (6N) for 24 h at 110 °C. Then, the extracts were derivatized using the AccQ-Tag method (Waters, Milford, MA, USA), and the separation, identification, and quantification were performed in a high-performance liquid chromatography (Alliance 2695 model, Waters, Milford, MA, USA) using a scanning fluorescence detector (model 2475, Waters). All results were expressed as mg/g protein.
The amino acid composition of LPI was used for the determination of chemical score, considering the values of essential amino acids of the sample (EAAs) and the pattern concentration (EAAp) according to FAO/WHO/UNU [
18] for adults:
2.5. Technofunctional Properties of Optimized LPI
2.5.1. Water and Oil Absorption Capacity
For the water and oil absorption capacity measurement, 0.5 g of LPI was weighed and mixed with 5 mL of water or oil. The mixture was vortexed for 1 min and left to settle for 30 min at room temperature. Then, samples were centrifuged (1600× g for 25 min; Beckman Coulter, model Allegra X-22R, rotor SX4250), and the supernatant was discarded. The sample was then weighted again, and the water or oil absorbed was expressed as g oil or water/g LPI.
2.5.2. Foam Properties
Foam properties were evaluated according to the procedure described by Liang et al. [
19] with modifications. In total, 1 g of LPI was dispersed in 100 mL of distilled water, and the pH was adjusted to pH 7. The solution was magnetically stirred for 1 h. Then, this solution (
Vl = 100 mL) was placed in a 250 mL graduated cylinder and homogenized using an UltraTurrax disperser (17,500 rpm for 2 min). The foam volume was recorded after homogenization (
V0) and after 30 min (
V30). The foam capacity and foam stability were calculated using the following equations.
2.5.3. Emulsifying Properties
Emulsifying properties were assessed using the protocol described by Zhao et al. [
20], with modifications. In total, 15 mL of LPI solution (1%) at pH 7 was placed in a 50 mL volume falcon tube, and homogenized for 15 s (UltraTurrax, 12,000 rpm). Then, 15 mL of soybean oil were added slowly, and the mixture was homogenized again for 1 min. The emulsion was centrifuged at 1300×
g for 5 min (Beckman Coulter, model Allegra X-22R, rotor SX4250) and at room temperature. The emulsifying capacity was calculated according to the next equation.
For the determination of emulsifying stability, the emulsion obtained after homogenization was heated for 30 min at 80 °C. Then, they were cooled at room temperature and centrifuged (1300×
g for 5 min; Beckman Coulter, model Allegra X-22R, rotor SX4250), and the emulsifying stability was calculated according to the next equation.
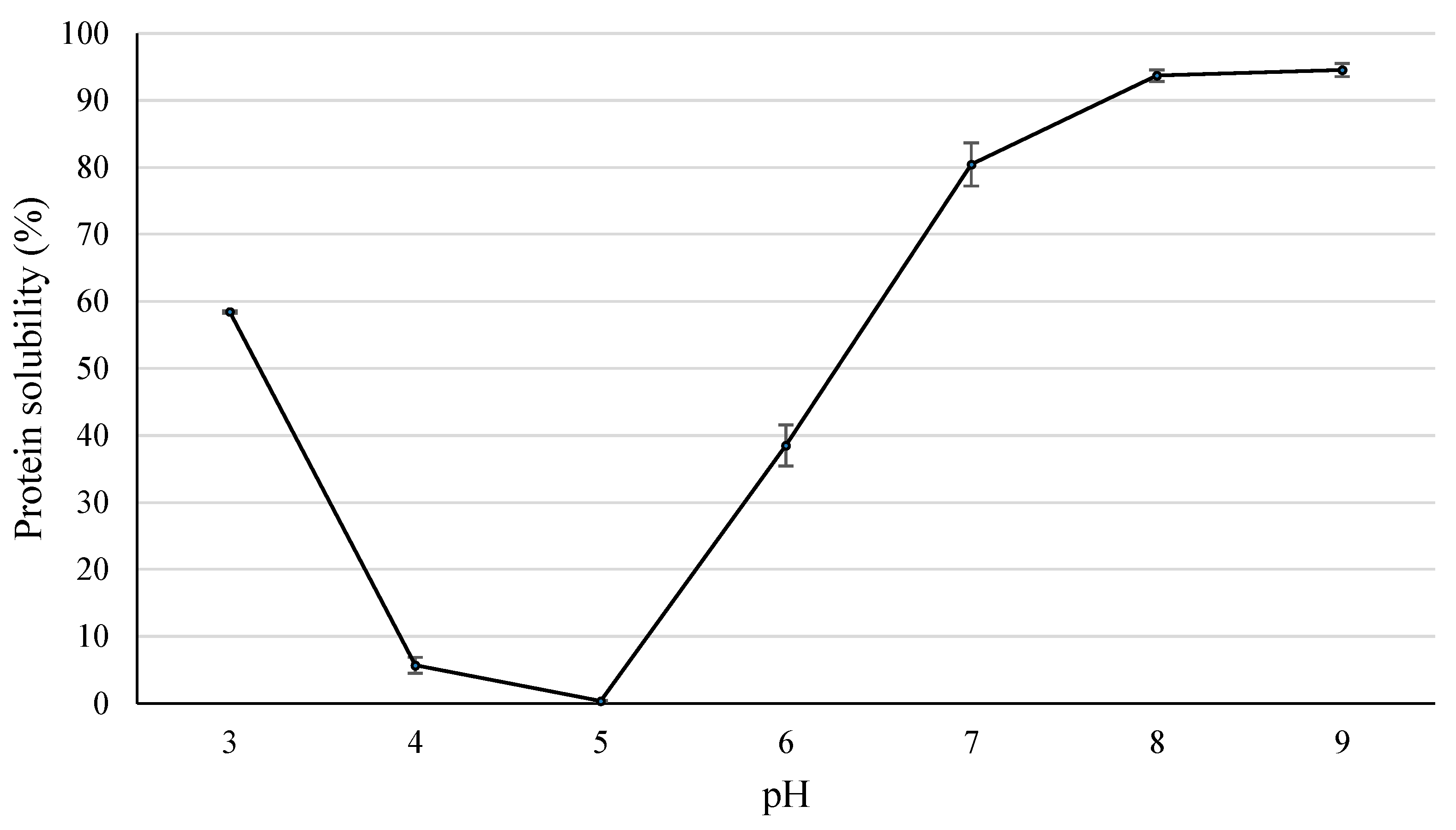
2.5.4. Protein Solubility
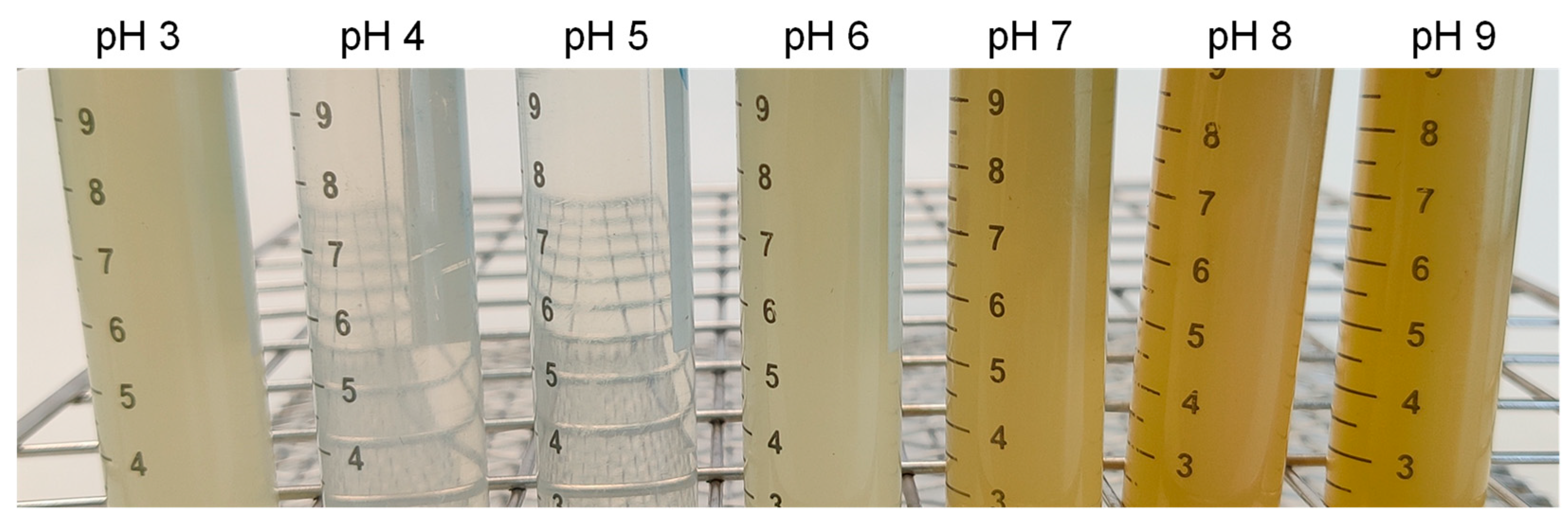
The LPI solubility (%) was determined over the pH range of 3–9 following the procedure described by Vogelsang-O’Dwyer [
21] with modifications. For the measurement, 0.75 g of LPI was mixed with 25 mL of distilled water. The pH of each solution was adjusted using 1M NaOH or 1M HCl, and the suspension was magnetically stirred at room temperature for 1 h. Then, the suspensions were centrifuged (10,000×
g for 15 min), and 5 mL of the supernatant was used for the nitrogen determination using the Kjeldahl method [
12]. The protein solubility was calculated according to the following equation.
2.5.5. Gelling Capacity
Gelling capacity determination was based on the procedure described by Lqari et al. [
22] with modifications. LPI suspensions between 2% and 20% were prepared in 5 mL phosphate buffer (50 mM; pH 7) at room temperature. The solutions were vortexed for 30 s, rest for 5 min, and vortexed again for another 30 s. The tubes containing the suspensions were heated in a bath (100 °C) for 1 h. After that, the tubes were cooled in an ice bath (1.5 h). Finally, the tubes are inverted, and the gelling concentration is considered as the minimum percentage of LPI necessary to achieve gelling of the sample (sample which did not fall out of or slip from the test tube).
2.6. Statistical Analysis
The software Statistics V8.0 (Statsoft Inc., Tulsa, OK, USA) was used to analyze the results, calculate the regression coefficients and optimize the conditions of all responses. The adequacy of the model was determined by the coefficient of determination (R2). SPSS software (version 25.0, SPSS Inc., Chicago, IL, USA) was used to analyze the data from lupin flour and LPI characterization using one-way analysis of variance (ANOVA), and significant differences were considered at 5% significance level (p < 0.05). The results were presented as mean and standard deviation.
4. Conclusions
The alkaline solubilization following isoelectric precipitation is the main procedure to obtain protein isolates. However, several factors can affect both, the yield and purity of these isolates. With this in mind, the effect of different parameters, such as the pH and the extraction time was studied in the present research. The solubilization and precipitation pHs were the most important parameters affecting the lupin purity. After the Box–Behnken design, the optimal conditions were at pH Sol = 10.3, pH Prec = 4.7, and extraction for 90 min. The model was a good tool to predict the yield and purity values, and under optimal conditions, the experimental results were 23.19 g LPI/100 g flour for yield and 87.74 g protein/100 g LPI for purity.
The optimized LPI had important content of high-quality proteins. The major amino acids were glutamic, aspartic acid, and arginine. The protein chemical scores showed that the protein isolate was only deficient in methionine and valine. Therefore, LPI protein contains high amounts of essential amino acids, and it would cover the nutritional requirements for humans, following the criteria of the FAO. Similarly, the fatty acids composition showed that lupin oil had important amounts of healthy unsaturated fatty acids.
On the other hand, the optimized LPI showed interesting technofunctional properties, such as foaming or emulsifying capacity and stability, oil and water absorption capacity, and gelling capacity. Additionally, the protein solubility was >90% at pH 8 or higher. However, the technofunctional properties of LPI are lower than those of other protein isolates.
Therefore, in view of these results, it can be affirmed that lupin protein isolates can constitute an ingredient or an agent for the formulation of the food, with excellent nutritional composition, protein quality, and adequate properties similar to those of the protein isolates currently used. For this reason, it can be concluded that the use of LPI by the food industry would allow the development of fortified foods (with a higher content of high-value proteins, or peptides with health benefits) at the same time that it would serve to improve the food properties (gelling, foaming, emulsifying capacity, etc.).
Further studies must be carried out to fully characterize the isolates, as well as to propose alternative and clean technologies in order to increase the purity and/or improve the technofunctional properties of the isolate obtained in this study. These studies are necessary to valorize the lupin protein isolate for their incorporation into the human diet.