Influence of Heat Treatment on Tea Polyphenols and Their Impact on Improving Heat Tolerance in Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. TP Concentration Screening Experiment
2.3. Effect of TP on the Heat Resistance of Drosophila
2.4. Analysis of TP Composition
2.5. Transcriptomic Sequencing
2.6. Metabolomics Experiments
2.7. Data Analysis
3. Results and Discussion
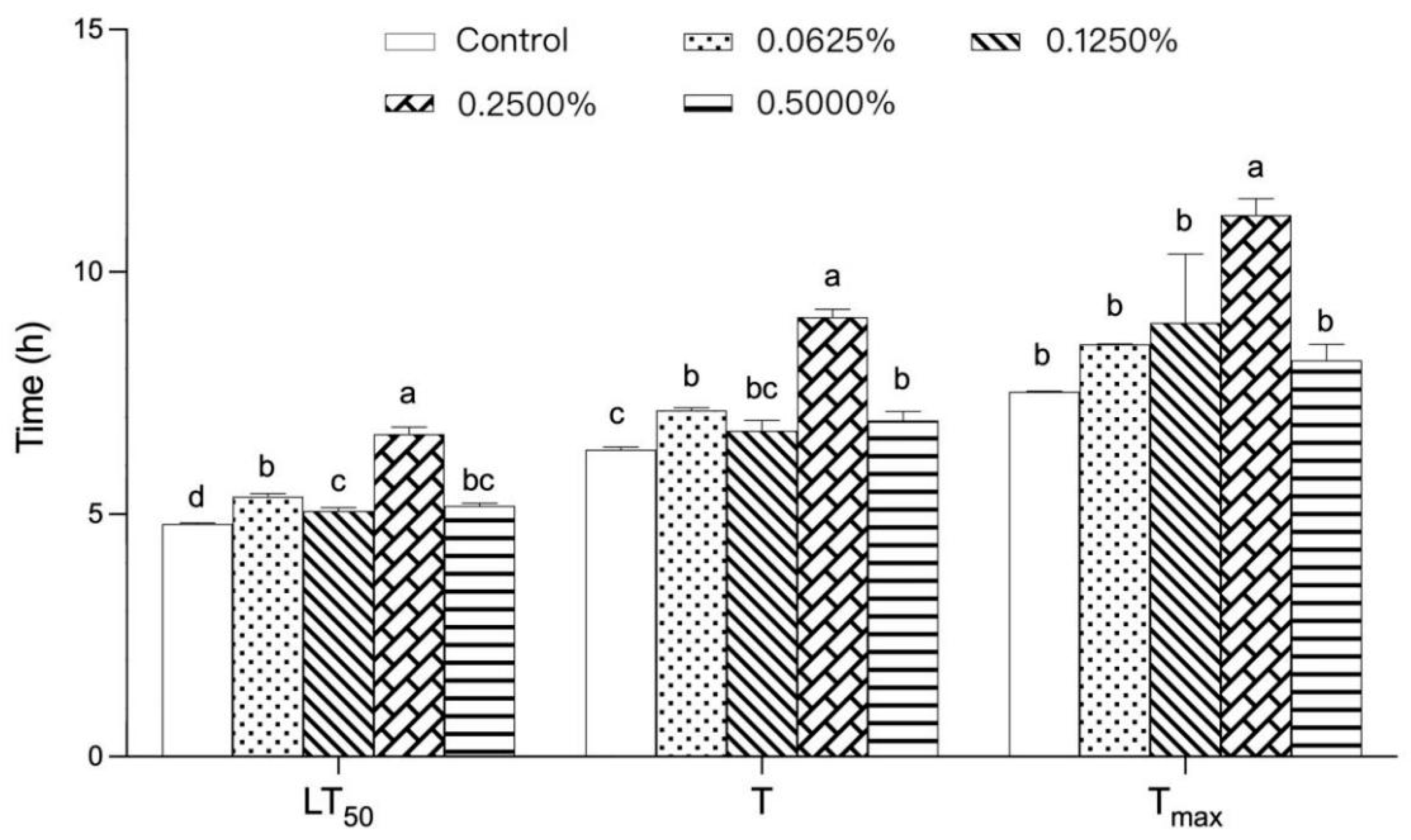
3.1. Effect of Different Concentrations of TP on the Heat Tolerance of Drosophila melanogaster
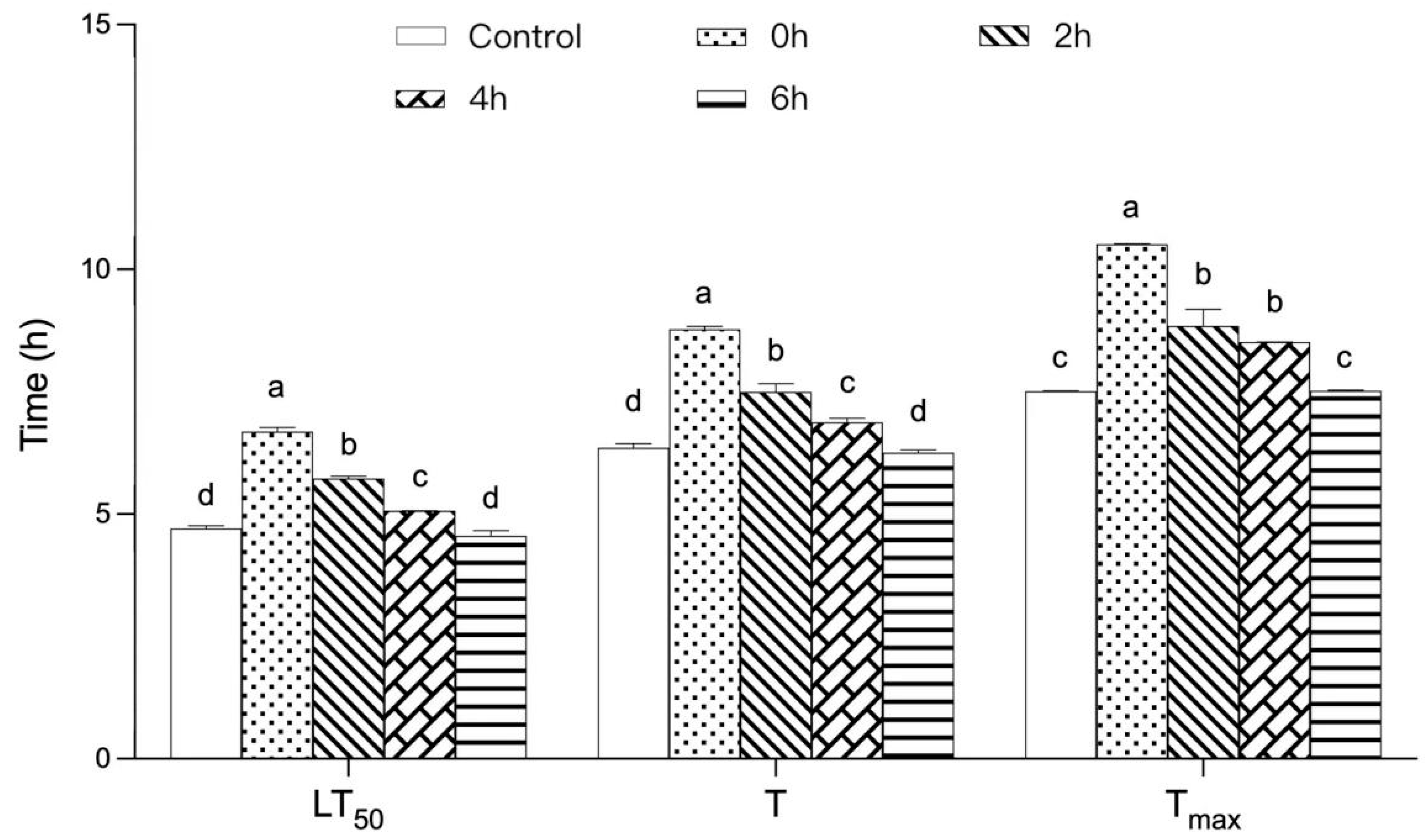
3.2. Effect of TPs on the Thermal Tolerance of Drosophila after High-Temperature Treatment
3.3. Relative Content Analysis of Polyphenols after TP High-Temperature Treatment
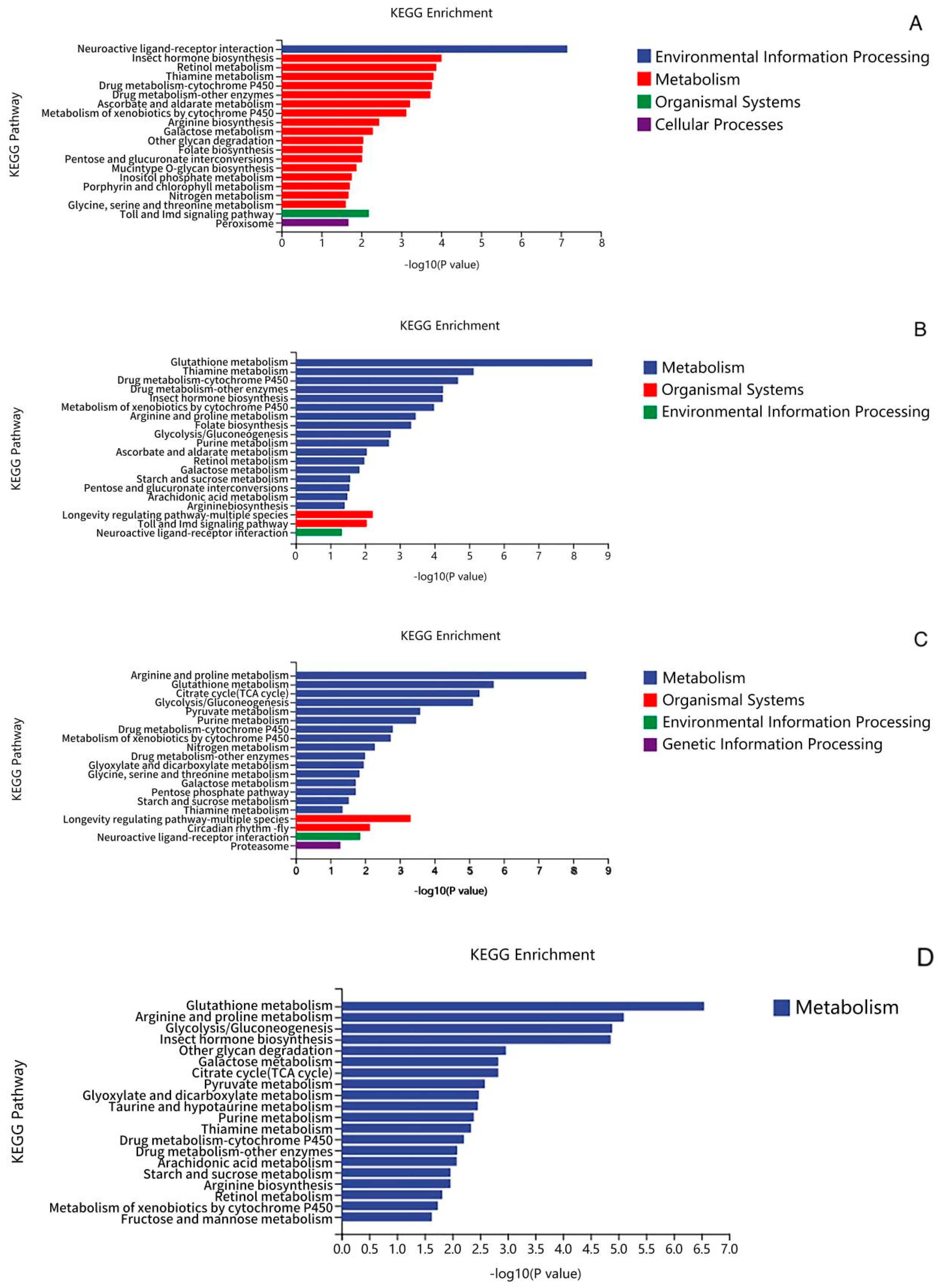
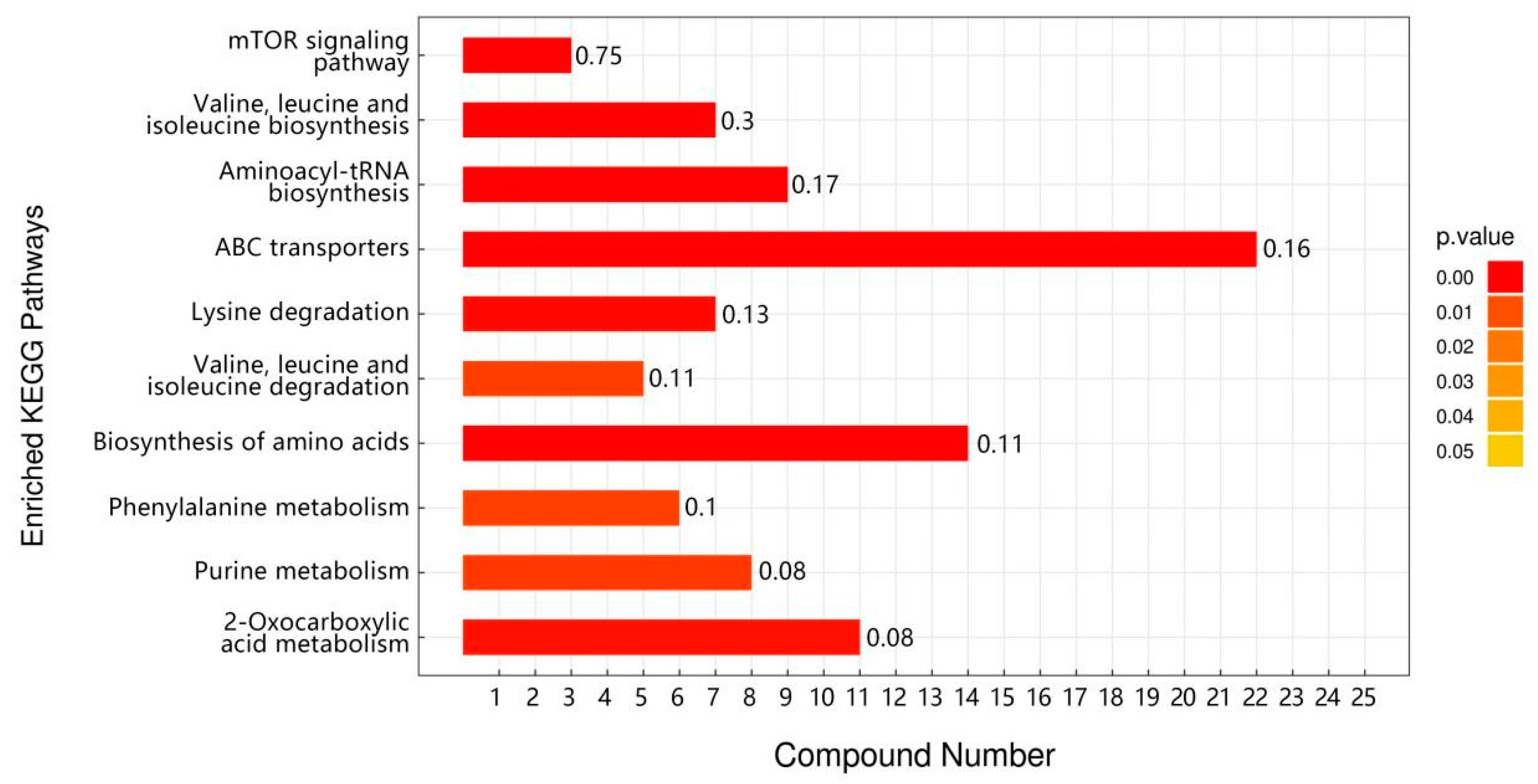
3.4. Transcriptomic Analysis of TPs to Improve Heat Tolerance in Drosophila
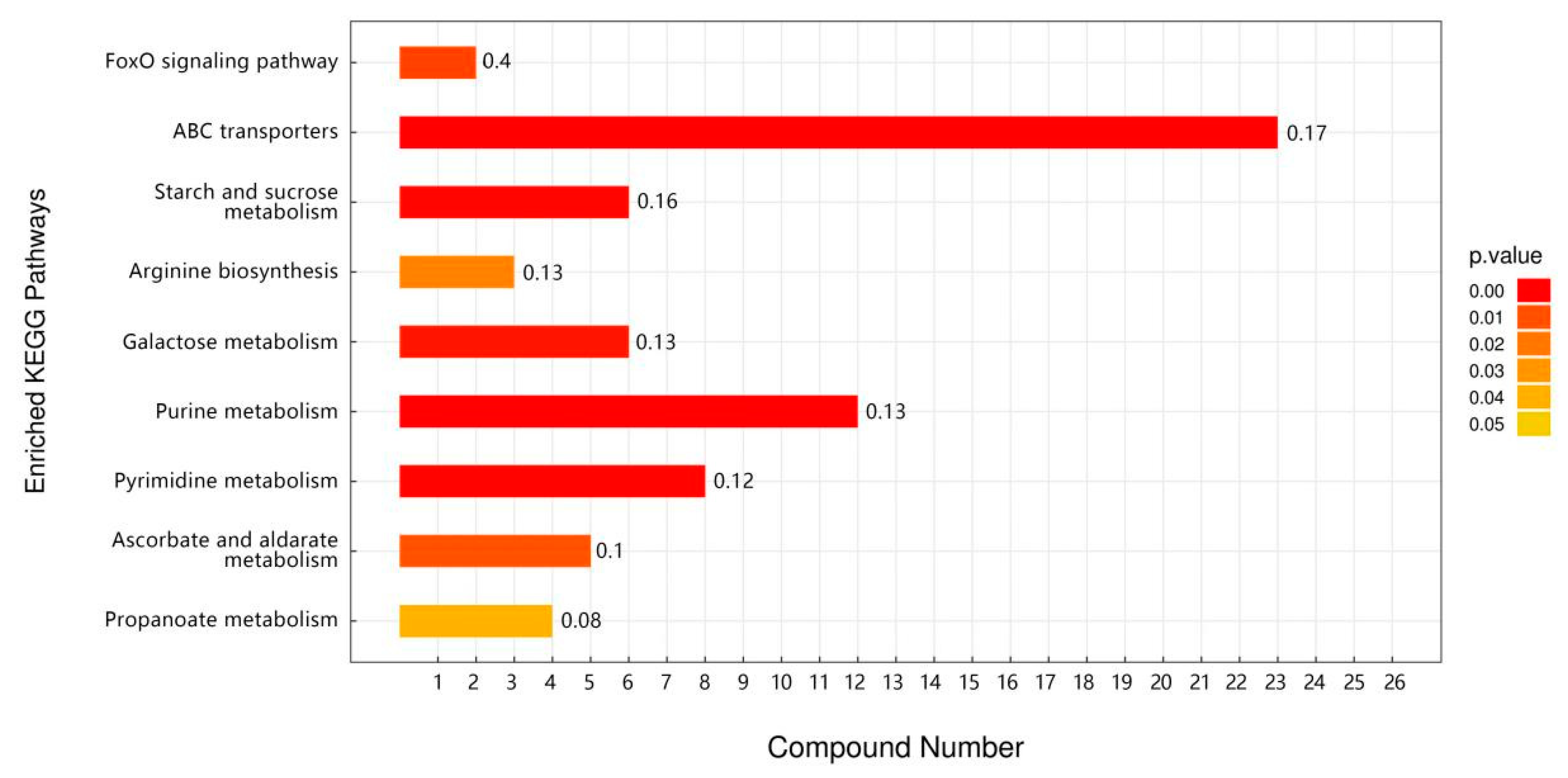
3.5. Metabolomic Analysis of TP for Improving Heat Tolerance in Drosophila
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sher, F.; Curnick, O.; Azizan, M.T. Sustainable conversion of renewable energy sources. Sustainability 2021, 13, 2940. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, P.; Su, X.; Zheng, M.; Chi, W.; Lin, S.; Huang, Z.; Qin, S.; Zeng, S. Hsian-Tsao (Mesona chinensis Benth.) Extract improves the thermal tolerance of Drosophila melanogaster. Front. Nutr. 2022, 9, 819319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X. Interactions of tea polyphenols with intestinal microbiota and their implication for anti-obesity. J. Sci. Food Agric. 2020, 100, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, H.; Luo, Q.; Wu, D.T.; Zou, L.; Liu, Y.; Li, H.B.; Gan, R.Y. Current extraction, purification, and identification techniques of tea polyphenols: An updated review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3912–3930. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Lin, Z.; Chen, C.; Chen, Z.; You, X.; Chen, R. Effects of baking temperature on quality and chemical components of jiulongpao oolong tea. J. Tea Sci. 2014, 34, 9–20. [Google Scholar]
- Huang, Y.; Zhang, J.; Ma, C.; Zhao, J.; Liu, J.; Jiang, J.; Zeng, S. Effects of water extracts from different tea on heat tolerance of Drosophila melanogaster. J. Tea Commun. 2020, 47, 630–639. [Google Scholar]
- Hidehiro, T.; Kenjiro, S.; Fumihito, M.; Naoki, K.; Kenji, I.; Katsuhiko, H.; Mitinori, S.; Mikita, S.; Takashi, I.; Hiroyuki, S. Software updates in the Illumina HiSeq platform affect whole-genome bisulfite sequencing. BMC Genom. 2017, 18, 31. [Google Scholar]
- Luo, J.; Zhong, Y.; Zhu, J.; Zhou, G.; Huang, H.; Wu, Y. Comparative transcriptomics of Buzura suppressaria (lepidoptera: Geometridae) assembled de novo yield insights into response after Buzura suppressaria nuclear polyhedrosis virus infection. J. Econ. Entomol. 2017, 110, 1259–1268. [Google Scholar]
- Zhao, Y.; Zhou, M.; Xu, K.; Li, J.; Yang, X. Integrated transcriptomics and metabolomics analyses provide insights into cold stress response in wheat. Crop. J. 2019, 7, 857–866. [Google Scholar] [CrossRef]
- Vonesch, S.C.; Lamparter, D.; Mackay, T.F.; Bergmann, S.; Hafen, E. Genome-wide analysis reveals novel regulators of growth in Drosophila melanogaster. PLoS Genet. 2016, 12, e1005616. [Google Scholar] [CrossRef]
- Gallio, M.; Ofstad, T.A.; Macpherson, L.J.; Wang, J.W.; Zuker, C.S. The coding of temperature in the drosophila brain. Cell 2011, 144, 614–624. [Google Scholar] [CrossRef]
- Parkash, R.; Ranga, P.; Aggarwal, D.D. Developmental acclimation to low or high humidity conditions affect starvation and heat resistance of Drosophila melanogaster. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2014, 175, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Nan, S.; Wang, P.; Zhang, Y.; Fan, J. Epigallocatechin-3-gallate provides protection against alzheimer’s disease-induced learning and memory impairments in rats. Drug Des. Dev. Ther. 2021, 15, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Auger, B.; Marnet, N.; Gautier, V.; Maia-Grondard, A.; Leprince, F.; Renard, M.; Guyot, S.; Nesi, N.; Routaboul, J.M. A detailed survey of seed coat flavonoids in developing seeds of Brassica napus L. J. Agric. Food Chem. 2010, 58, 6246–6256. [Google Scholar] [CrossRef] [PubMed]
- Dobrini, A.; Repaji, M.; Garofuli, I.E.; Tuen, L.; Levaj, B. Comparison of different extraction methods for the recovery of olive leaves polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, L.; Wang, Y.; Deng, Q.; Fang, Z.; Zhao, L.; Zhao, J. Protective mechanism of tea polyphenols against muscle quality deterioration of shrimp (Penaeus vannamei) induced by aflatoxin B1. Aquaculture 2021, 532, 736093. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, Y.; Chen, H.; Lu, H.; Lin, X.; Ma, L. Inhibitive action of tea-polyphenol on some plant pathogenic fungi. Nat. Prod. Res. Dev. 2008, 20, 690–694. [Google Scholar]
- Donlao, N.; Ogawa, Y. The influence of processing conditions on catechin, caffeine and chlorophyll contents of green tea (Camelia sinensis) leaves and infusions. LWT 2019, 116, 108567. [Google Scholar] [CrossRef]
- Kothe, L.; Zimmermann, B.F.; Galensa, R. Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem. 2013, 141, 3656–3663. [Google Scholar] [CrossRef]
- Lee, J.; Chambers, D.; Chambers, E.I. Sensory and instrumental flavor changes in green tea brewed multiple times. Foods 2013, 2, 554–571. [Google Scholar] [CrossRef]
- Kawasaki, F.; Koonce, N.L.; Guo, L.; Fatima, S.; Qiu, C.; Moon, M.T.; Zheng, Y.; Ordway, R.W. Small heat shock proteins mediate cell-autonomous and -nonautonomous protection in a drosophila model for environmental-stress-induced degeneration. Dis. Models Mech. 2016, 9, 953–964. [Google Scholar]
- Stetina, T.; Kostal, V.; Korbelova, J. The role of inducible hsp70, and other heat shock proteins, in adaptive complex of cold tolerance of the fruit fly (Drosophila melanogaster). PLoS ONE 2015, 10, e128976. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhao, Y.; Su, Y.; Wu, J.; Wang, Z.; Hu, J.; Liu, L.; Zhao, Z.; Hoffmann, A.A.; Chen, B.; et al. A transcriptional and functional analysis of heat hardening in two invasive fruit fly species, Bactrocera dorsalis and Bactrocera correcta. Evol. Appl. 2019, 12, 1147–1163. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.T.; Chen, B.; Li, Z.H. Thermal plasticity is related to the hardening response of heat shock protein expression in two Bactrocera fruit flies. J. Insect Physiol. 2014, 67, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Chen, X.; Yan, Y.; Liu, F.; Zhang, Y.; Guo, X.; Xu, B. Glutaredoxin 1, glutaredoxin 2, thioredoxin 1, and thioredoxin peroxidase 3 play important roles in antioxidant defense in apis cerana cerana. Free Radic. Biol. Med. 2014, 68, 335–346. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (sods) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben, M.M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Ringers, C.; Olstad, E.W.; Jurisch-Yaksi, N. The role of motile cilia in the development and physiology of the nervous system. Philos. Trans. R. Soc. B-Biol. Sci. 2020, 375, 20190156. [Google Scholar] [CrossRef]
- Bae, J.E.; Kang, G.M.; Min, S.H.; Jo, D.S.; Jung, Y.K.; Kim, K.; Kim, M.S.; Cho, D.H. Primary cilia mediate mitochondrial stress responses to promote dopamine neuron survival in a parkinson’s disease model. Cell Death Dis. 2019, 10, 952. [Google Scholar] [CrossRef]
- Jiang, Y.; Gaur, U.; Cao, Z.; Hou, S.T.; Zheng, W. Dopamine d1- and d2-like receptors oppositely regulate lifespan via a dietary restriction mechanism in Caenorhabditis elegans. BMC Biol. 2022, 20, 71. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Zhang, X.; Cheng, J.; Liu, D.; Yan, Y.; Wang, H. Transcriptomic analysis of the life-extending effect exerted by black rice anthocyanin extract in D. melanogaster through regulation of aging pathways. Exp. Gerontol. 2019, 119, 33–39. [Google Scholar] [CrossRef]
- Syed, K.; Shale, K.; Nazir KH, M.N.; Krasevec, N.; Mashele, S.S.; Pagadala, N.S. Genome-wide identification, annotation and characterization of novel thermostable cytochrome p450 monooxygenases from the thermophilic biomass-degrading fungi Thielavia terrestris and Myceliophthora thermophi. Genes Genom. 2014, 36, 321–333. [Google Scholar] [CrossRef]
- Zhao, H.; Ke, H.; Zhang, L.; Zhao, Z.; Lai, J.; Zhou, J.; Huang, Z.; Li, H.; Du, J.; Li, Q. Integrated analysis about the effects of heat stress on physiological responses and energy metabolism in Gymnocypris chilianensis. Sci. Total Environ. 2022, 806, 151252. [Google Scholar] [CrossRef] [PubMed]
- Swanson, R.; Hoben, P.; Sumner-Smith, M.; Uemura, H.; Watson, L.; Söll, D. Accuracy of in vivo aminoacylation requires proper balance of trna and aminoacyl-trna synthetase. Science 1988, 242, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- González, J.A.; Roldán, A.; Gallardo, M.; Escudero, T.; Prado, F.E. Quantitative determinations of chemical compounds with nutritional value from inca crops: Chenopodium quinoa (‘quinoa’). Plant Foods Hum. Nutr. 1989, 39, 331–337. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, A.; Yang, S.; Huang, Z. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 102937. [Google Scholar] [CrossRef]
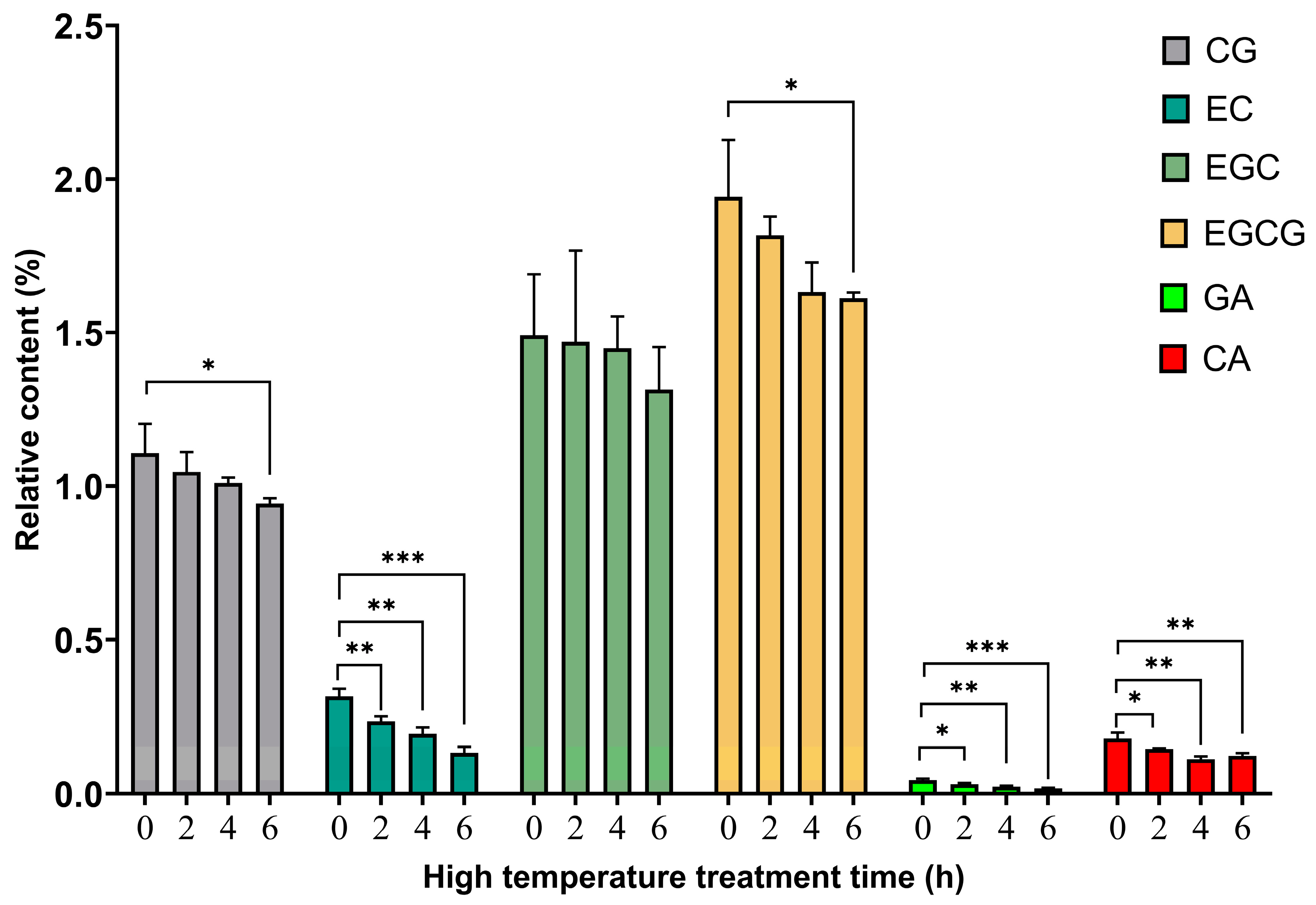



| Compound Name | Relative Percentage (%) | |||
|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | |
| catechin (C) | 0.0611 | 0.0688 | 0.0786 | 0.0763 |
| catechin gallate (CG) | 1.1111 | 1.0343 | 1.0132 | 0.9417 |
| epicatechin (EC) | 0.3162 | 0.2337 | 0.1992 | 0.1376 |
| epigallocatechin (EGC) | 1.5142 | 1.4809 | 1.4510 | 1.3109 |
| epigallocatechin gallate (EGCG) | 1.9141 | 1.7874 | 1.6762 | 1.6163 |
| gallocatechin (GC) | 0.0498 | 0.0606 | 0.0501 | 0.7000 |
| gallic acid (GA) | 0.0440 | 0.0302 | 0.0236 | 0.0173 |
| caffeic acid (CA) | 0.1763 | 0.1488 | 0.1111 | 0.1256 |
| caffeine (CAF) | 5.1471 | 8.3875 | 11.1307 | 12.5517 |
| laccasein | 0.7544 | 3.5787 | 4.7548 | 5.0746 |
| galangal | 0.3487 | 1.6595 | 2.0904 | 2.6214 |
| Control Group vs. Experimental Group | Upregulated | Downregulated | Total |
|---|---|---|---|
| C vs. C_H | 358 | 654 | 1012 |
| TP vs. TP_H | 659 | 744 | 1403 |
| TP6 vs. TP6_H | 203 | 1677 | 1880 |
| C vs. TP | 835 | 545 | 1380 |
| C vs. TP6 | 1428 | 297 | 1725 |
| TP vs. TP6 | 540 | 50 | 590 |
| Gene.ID | Group | log2FC | Regulated |
|---|---|---|---|
| Hsp23 | C vs. C_H | 10.39543125 | up |
| TP vs. TP_H | 11.29768882 | up | |
| TP6 vs. TP6_H | 7.10522365 | up | |
| Hsp26 | C vs. C_H | 6.179345334 | up |
| TP vs. TP_H | 7.037563739 | up | |
| TP6 vs. TP6_H | 5.041077323 | up | |
| Hsp68 | C vs. C_H | 5.359082364 | up |
| TP vs. TP_H | 4.551601646 | up | |
| TP6 vs. TP6_H | 4.133381398 | up | |
| Hsp70Ab | C vs. C_H | 3.565616719 | up |
| TP vs. TP_H | 5.622981232 | up | |
| TP6 vs. TP6_H | 3.252050367 | up | |
| Hsp70Bb | C vs. C_H | 4.044325616 | up |
| TP vs. TP_H | 3.265791372 | up | |
| TP6 vs. TP6_H | 2.048701979 | up |
| Gene.ID | log2FC | Regulated | NR_Annotation |
|---|---|---|---|
| Hsp23 | 10.39543125 | up | heat shock protein 23 |
| Hsp26 | 6.710522365 | up | heat shock protein 26 |
| Hsp60C | 4.111382603 | up | heat shock protein 60C |
| Hsp68 | 6.263508306 | up | heat shock protein 68 |
| Hsp70Bb | 5.252050367 | up | heat shock protein 70Bb |
| Hsp70Ab | 5.359082364 | up | heat shock protein 70Ab |
| Sod1 | 2.367690793 | up | Superoxide dismutase 1 |
| Sod2 | 2.643104457 | up | Superoxide dismutase 2 |
| Dop1R1 | 2.403899212 | up | dopamine receptor gene R1 |
| Dop1R2 | 2.739719490 | up | dopamine receptor gene R2 |
| GABA-B-R1 | 3.714866365 | up | g-aminobutyric acid (GABA) type B receptor subunit 1 |
| GABA-B-R3 | 2.085191171 | up | g-aminobutyric acid (GABA) type B receptor subunit 3 |
| 5-HT1A | 2.33974916 | up | 5-hydroxytryptamine-1A |
| 5-HT2B | 2.51624293 | up | 5-hydroxytryptamine-2B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Su, X.; Jia, Q.; Chen, H.; Zeng, S.; Xu, H. Influence of Heat Treatment on Tea Polyphenols and Their Impact on Improving Heat Tolerance in Drosophila melanogaster. Foods 2023, 12, 3874. https://doi.org/10.3390/foods12203874
Huang J, Su X, Jia Q, Chen H, Zeng S, Xu H. Influence of Heat Treatment on Tea Polyphenols and Their Impact on Improving Heat Tolerance in Drosophila melanogaster. Foods. 2023; 12(20):3874. https://doi.org/10.3390/foods12203874
Chicago/Turabian StyleHuang, Jianfeng, Xinxin Su, Qiyan Jia, Haoran Chen, Shaoxiao Zeng, and Hui Xu. 2023. "Influence of Heat Treatment on Tea Polyphenols and Their Impact on Improving Heat Tolerance in Drosophila melanogaster" Foods 12, no. 20: 3874. https://doi.org/10.3390/foods12203874
APA StyleHuang, J., Su, X., Jia, Q., Chen, H., Zeng, S., & Xu, H. (2023). Influence of Heat Treatment on Tea Polyphenols and Their Impact on Improving Heat Tolerance in Drosophila melanogaster. Foods, 12(20), 3874. https://doi.org/10.3390/foods12203874





