Nutritional Content and Microbial Load of Fresh Liang, Gnetum gnemon var. tenerum Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Leaf Preparation and Sampling
2.3. Physiochemical and Chemical Characteristics
2.3.1. Proximate Compositions
Moisture Content [8]
Carbohydrate Content [9]
Crude Protein Content
- 1.4007 = milliequivalent weight N × 100 (%)
- M = molarity of HCl
- 6.25 = protein factor for meat products (16% N).
Fat Content
Ash Content
- W1 = weight of sample (g)
- W2 = weight of crucible (g)
- W3 = weight of crucible + ash (g).
Total Dietary Fibre
- P and A = weights (mg) of protein and ash, respectively.
- Weight test portion = average of 2 duplicate weights (mg) and
- B = blank.
Insoluble and Soluble Dietary Fibre
- BR1 and BR2 = residue weights (mg) for blank
- PB and AB = weights (mg) of protein and ash of blank.
- R1 and R2 = residue weights (mg) for duplicate samples
- P and A = weights (mg) of protein and ash, respectively
- B = blank weight (mg)
- M1 and M2 = weights (mg) of sample.
2.3.2. Amino Acid Profiles
2.3.3. Vitamin and Mineral Contents
Vitamin A
Vitamin B9 [12]
Vitamin C
Mineral Determination
- A = concentration (µg/mL) of element as determined using ICP
- B = volume or weight of sample as ml or g
- C = elemental concentration in the sample solution (µg/mL or µg/g).
2.4. Weight Gain and Loss
- W1 = initial weight of the sample
- W2 = weight of the sample after storage.
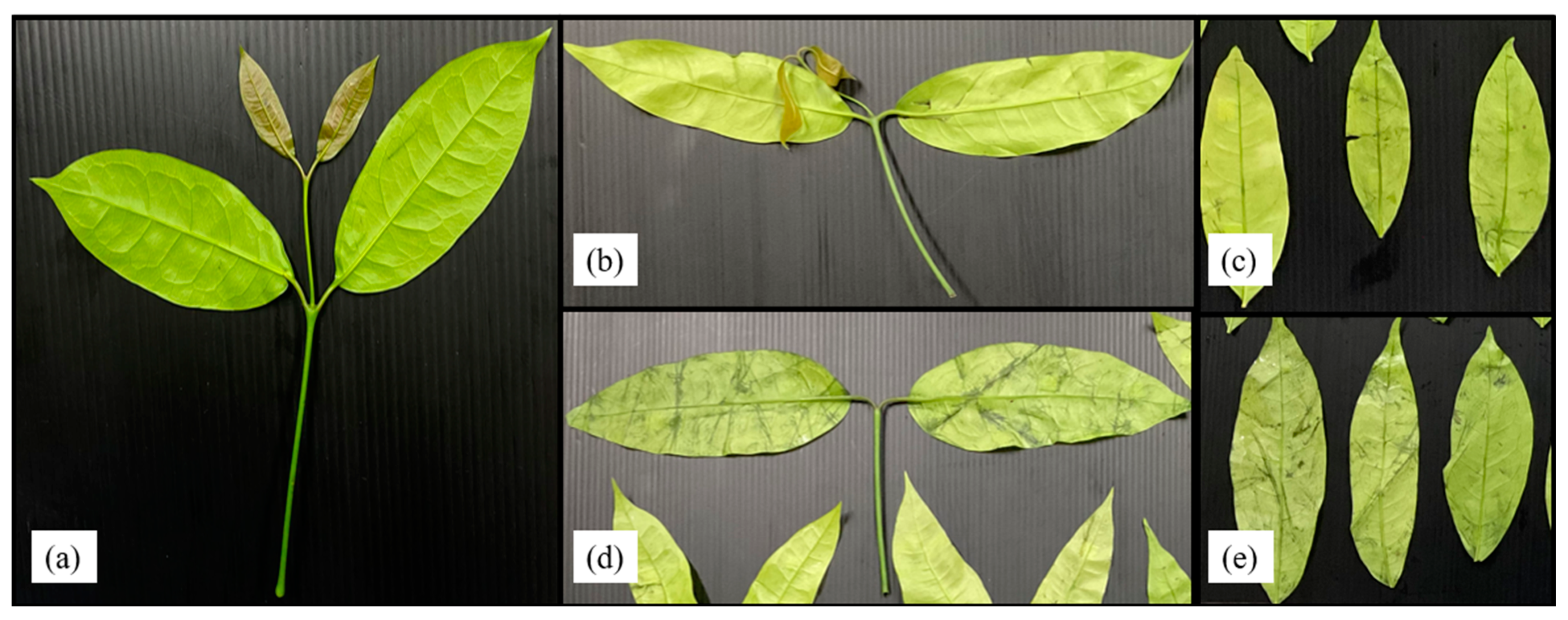
2.5. Damage Detection and Classification
2.6. Microbial Load
2.6.1. TVC Determination
2.6.2. Coliform and E. coli Determination
2.6.3. Yeast and Mould Determination
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate Compositions
3.2. Amino Acid Profile
3.3. Vitamins and Minerals
3.4. Weight Changes and Damage Levels from Field to Bagging and Storage
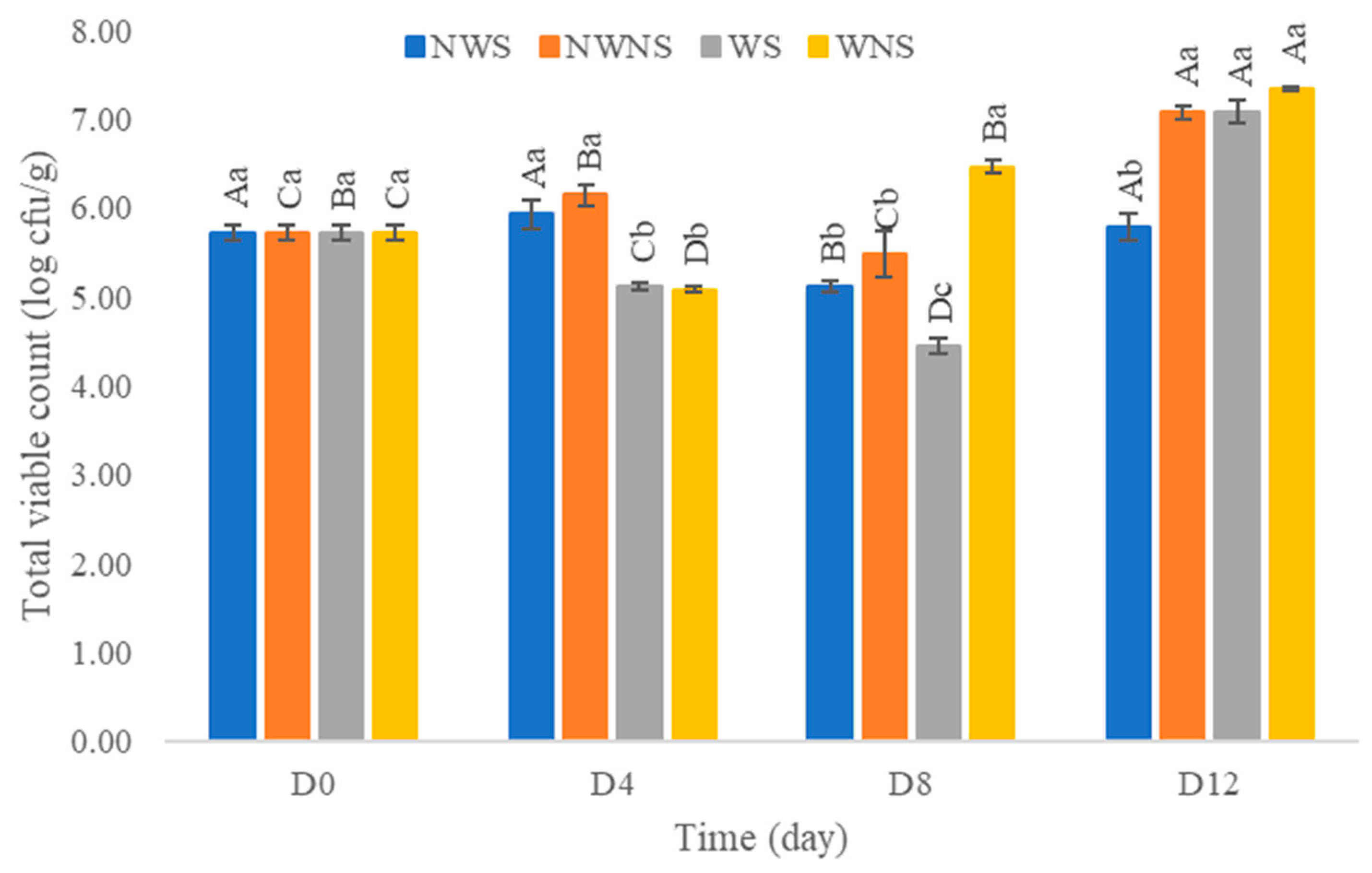
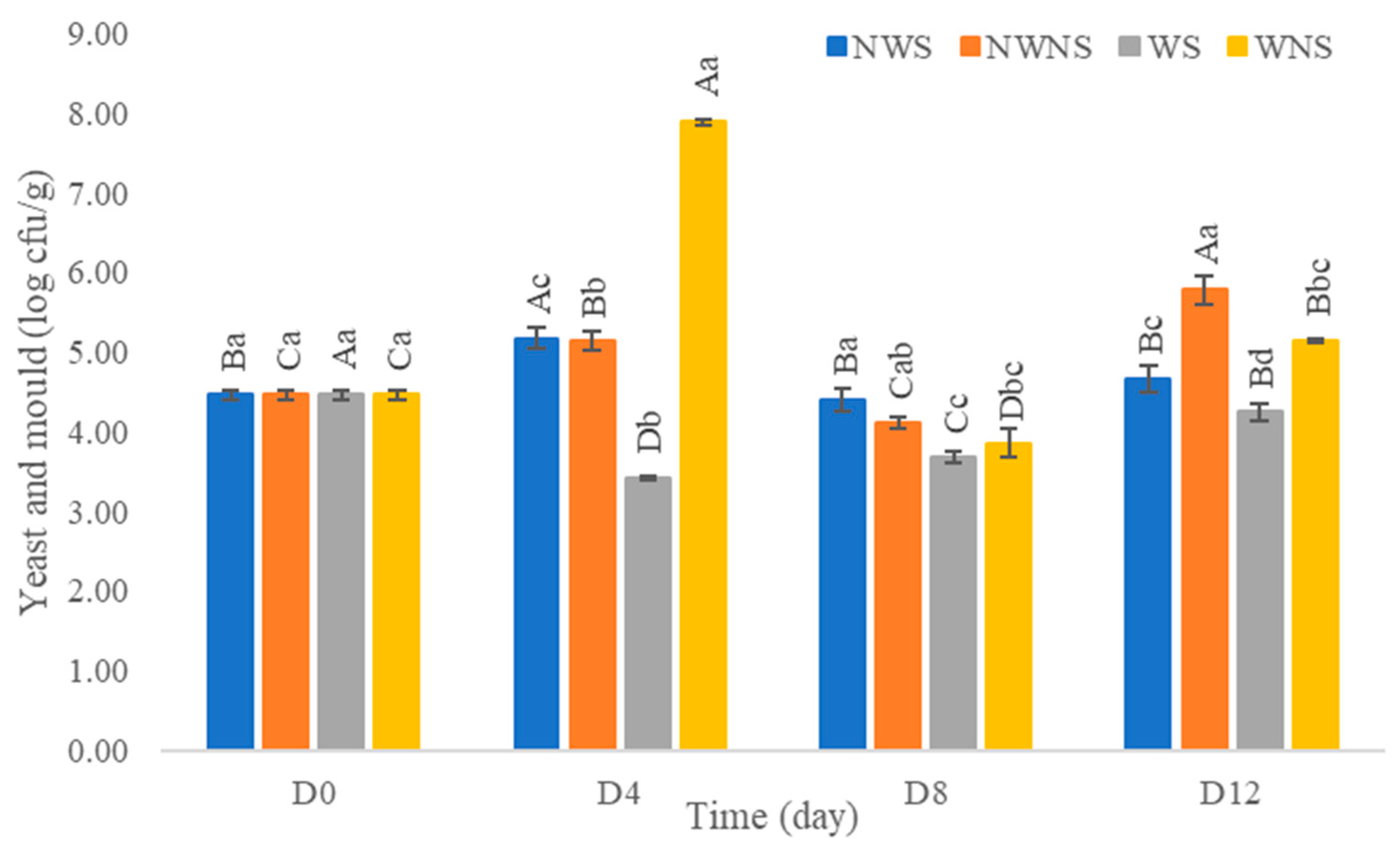
3.5. Microbial Load
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anisong, N.; Siripongvutikorn, S.; Wichienchot, S.; Puttarak, P. A comprehensive review on nutritional contents and functional properties of Gnetum gnemon Linn. Food Sci. Technol. 2022, 42, e100121. [Google Scholar] [CrossRef]
- Suksanga, A.; Siripongvutikorn, S.; Yupanqui, T.C.; Leelawattana, R. The potential antidiabetic properties of Liang (Gnetum gnemon var. tenerum) leaves. Food Sci. Technol. 2022, 42, e64522. [Google Scholar] [CrossRef]
- Rosberg, A.K.; Darlison, J.; Mogren, L.; Alsanius, B.W. Commercial wash of leafy vegetables do not significantly decrease bacterial load but leads to shifts in bacterial species composition. Food Microbiol. 2021, 94, 103667. [Google Scholar] [CrossRef] [PubMed]
- Mogren, L.; Windstam, S.; Boqvist, S.; Vagsholm, I.; Soderqvist, K.; Rosberg, A.K.; Linden, J.; Mulaosmanovic, E.; Karlsson, M.; Uhlig, E.; et al. The hurdle approach—A holistic concept for controlling food safety risks associated with pathogenic bacterial contamination of leafy green vegetables: A review. Front. Microbiol. 2018, 9, 1965. [Google Scholar] [CrossRef] [PubMed]
- López-Gálvez, F.; Allende, A.; Gil, M.I. Recent progress on the management of the industrial washing of fresh produce with a focus on microbiological risks. Curr. Opin. Food Sci. 2021, 38, 46–51. [Google Scholar] [CrossRef]
- Allende, A.; Selma, M.V.; Lopez-Galvez, F.; Villaescusa, R.; Gil, M.I. Impact of wash water quality on sensory and microbial quality, including Escherichia coli cross-contamination, of fresh-cut escarole. J. Food Prot. 2008, 71, 2514–2518. [Google Scholar] [CrossRef]
- Holvoet, K.; Jacxsens, L.; Sampers, I.; Uyttendaele, M. Insight into the prevalence and distribution of microbial contamination to evaluate water management in the fresh produce processing industry. J. Food Prot. 2012, 75, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemist. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemist: Washington, DC, USA, 2019. [Google Scholar]
- Ellefson, W. Provisions of nutrition labeling and education act. In Methods of Analysis for Nutrition Labeling; Sullivan, D.M., Carpenter, D.E., Eds.; AOAC International: Arlington, VA, USA, 1993; p. 8. [Google Scholar]
- Sarwar, G.; Botting, H.G.; Peace, R.W. Complete amino acid analysis in hydrolysates of food and feces by liquid chromatography of precolumn phenylisothiocyanate derivatives. J. Assoc. Off. Anal. Chem. 1988, 71, 1172–1175. [Google Scholar] [CrossRef]
- Visuthi, V. Development of Dry Pellets for Broodstock Yellowtail. Master’s Thesis, Department of Aquatic Biosciences, Tokyo University of Fisheries, Tokyo, Japan, 1994. [Google Scholar]
- Chen, Z.; Chen, B.; Yao, S. High-performance liquid chromatography/electrospray ionization-mass spectrometry for simultaneous determination of taurine and 10 water-soluble vitamins in multivitamin tablets. Anal. Chim. Acta 2006, 569, 169–175. [Google Scholar] [CrossRef]
- National Bureau of Agricultural Commodity and Food Standards. Compendium of Methods for Food Analysis; Department of Medical Sciences and National Bureau of Agriculture Commodity and Food Standards: Bangkok, Thailand, 2003.
- Mulaosmanovic, E.; Lindblom, T.U.T.; Windstam, S.T.; Bengtsson, M.; Rosberg, A.K.; Mogren, L.; Alsanius, B. Processing of leafy vegetables matters: Damage and microbial community structure from field to bag. Food Control 2021, 125, 107894. [Google Scholar] [CrossRef]
- Maturin, L.; Peeler, J.T. BAM Chapter 3: Aerobic plate count. In Bacteriological Analytical Manual; The United States Food and Drug Administration: Silver Spring, MD, USA, 2001. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-3-aerobic-plate-count (accessed on 17 August 2022).
- Feng, P.; Weagant, S.D.; Grant, M.A.; Burkhardt, W. BAM Chapter 4: Enumeration of Escherichia coli and the Coliform Bacteria. In Bacteriological Analytical Manual; The United States Food and Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-4-enumeration-escherichia-coli-and-coliform-bacteria (accessed on 17 August 2022).
- Tournas, V.; Stack, M.E.; Mislivec, P.B.; Koch, H.A.; Bandler, R. BAM Chapter 18: Yeasts, molds and mycotoxins. In Bacteriological Analytical Manual; The United States Food and Drug Administration: Silver Spring, MD, USA, 2001. Available online: www.fda.gov/food/laboratory-methods-food/bam-chapter-18-yeasts-molds-and-mycotoxins (accessed on 17 August 2022).
- Salim, A.; Hasyim, M.; Adam, A. Nutrient contents of Moringa leaves based on leaf age. Indian J. Public Health Res. Dev. 2018, 9, 397. [Google Scholar] [CrossRef]
- Ncube, S.; Saidi, P.T.; Halimani, T.E. Effect of stage of growth and processing methods on the nutritional content of Acacia angustissima leaf meal harvested for broiler feeding. Livest. Res. Rural. Dev. 2015, 27, 89. [Google Scholar]
- Kitajima, K.; Wright, S.J.; Westbrook, J.W. Leaf cellulose density as the key determinant of inter- and intra-specific variation in leaf fracture toughness in a species-rich tropical forest. Interface Focus 2016, 6, 20150100. [Google Scholar] [CrossRef] [PubMed]
- Patricia, O.; Zoue, L.; Megnanou, R.M.; Doue, R.; Niamke, S. Proximate composition and nutritive value of leafy vegetables consumed in northern Cote d’Ivoire. Eur. Sci. J. 2014, 10, 212–227. [Google Scholar]
- Opazo-Navarrete, M.; Burgos-Díaz, C.; Soto-Cerda, B.; Barahona, T.; Anguita-Barrales, F.; Mosi-Roa, Y. Assessment of the nutritional value of traditional vegetables from Southern Chile as potential sources of natural ingredients. Plant Foods Hum. Nutr. 2021, 76, 523–532. [Google Scholar] [CrossRef]
- Ministry of Public Health. Announcement of Ministry of Public Health; Nutrition Claim. Thai Government. 1998. Available online: http://www.rbpho.moph.go.th/upload-file/doc/files/27072022-121229-9336.pdf (accessed on 19 March 2023). (In Thai)
- Dabbou, S.; Maatallah, S.; Antonelli, A.; Montevecchi, G. Variation of amino acids in Prunus persica cultivars leaves with regard to leaf age. Sci. Hortic. 2021, 281, 110001. [Google Scholar] [CrossRef]
- Gorelova, V.; Ambach, L.; Rébeillé, F.; Stove, C.; Straeten, D.V.D. Folates in plants: Research advances and progress in crop biofortification. Front. Chem. 2017, 5, 21. [Google Scholar] [CrossRef]
- Ayala-Rodríguez, J.A.; Barrera-Oztiz, S.; Ruiz-Herrera, L.F.; López-Bucio, J. Folic acid orchestrates root development linking cell elongation with auxin response and acts independently of the target of Rapamycin signaling in Arabidopsis thaliana. Plant Sci. 2017, 264, 168–178. [Google Scholar] [CrossRef]
- Johansson, M.; Jägerstad, M.; Frølich, W. Folates in lettuce: A pilot study. Scand. J. Food Nutr. 2007, 51, 22–30. [Google Scholar] [CrossRef]
- Rubóczki, T.; Hájos, M. Folic acid content of beetroot leaf and root by different growing stages and genotypes. Acta Agrar. Debreceniensis 2019, 2, 115–119. [Google Scholar] [CrossRef]
- Xu, P.; Chukhutsina, V.H.; Nawrocki, W.J.; Schansker, G.; Bielczynski, L.W.; Lu, Y.; Karcher, D.; Bock, R.; Croce, R. Photosynthesis without β-carotene. eLife 2020, 9, e58984. [Google Scholar] [CrossRef] [PubMed]
- Cheptoo, G.; Willis, O.O.; Kenji, G.M. Nutritional quality, bioactive compounds and antioxidant activity of selected African indigenous leafy vegetables as influenced by maturity and minimal processing. Afr. J. Food Agric. Nutr. Dev. 2019, 19, 14769–14789. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; de Pascle, S.; Rouphael, Y. Variation in macronutrient content, phytochemical constitution and In vitro antioxidant capacity of green and red butter head lettuce dictated by different developmental stage of harvest maturity. Antioxidants 2020, 9, 300. [Google Scholar] [CrossRef]
- Rudani, K.; Prajapati, K.; Patel, V. The importance of zinc in plant growth—A review. Int. Res. J. Nat. Sci. 2018, 5, 38–48. [Google Scholar]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Schmidt, W.; Thomine, S.T.; Buckhout, T.J. Editorial: Iron nutrition and interactions in plants. Front. Plant Sci. 2020, 10, 1670. [Google Scholar] [CrossRef]
- Chen, Z.C.; Peng, W.T.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Lu, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- Pongrac, P.; Tolrà, R.; Hajiboland, R.; Vogel-Mikuš, K.; Kelemen, M.; Vavpetič, P.; Pelicon, P.; Barceló, J.; Regvar, M.; Poschenrieder, C. Contrasting allocation of magnesium, calcium and manganese in leaves of tea (Camellia sinensis (L.) Kuntze) plants may explain their different extraction efficiency into tea. Food Chem. Toxicol. 2020, 135, 110974. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jákli, M.; Tränkner, M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: A systematic review and meta-analysis from 70 years of research. Front. Plant Sci. 2019, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium-nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- US; FDA. Water Activity (aw) in Foods. United States of America. 2014. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/water-activity-aw-foods (accessed on 4 June 2023).
- Zander, A.; Bunning, M. Guide to Washing Fresh Produce. Fact Sheet No 9.380. Food and Nutrition Series, Food Safety. Colorado State University, U.S. Department of Agriculture and Colorado Counties Cooperating. 2010. Available online: https://www.nifa.usda.gov/sites/default/files/resource/Guide%20to%20Washing%20Fresh%20Produce508.pdf (accessed on 8 May 2023).
- Ali, H.; Bacha, K.; Ketema, T. Bacteriological quality and antimicrobial susceptibility of some isolates of well waters used for drinking in Jimma Town, Southwest Ethiopia. Ethiop. J. Educ. Sci. 2012, 6, 95–108. [Google Scholar]
- Kambire, O.; Yao, K.M.; Aka, N.; Bamba, K.E.; Nevry, R.K. Microbiological and physicochemical characterization of Irrigation water in market gardening in Korhogo. Sci. World J. 2020, 2020, 3167581. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, C.; Peng, Z.; Tan, B.; Wang, K.; Liu, Z. Distribution pattern of endophytic bacteria and fungi in tea plants. Front. Microbiol. 2020, 13, 872034. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, A.; Shukla, A.; Sharma, A.; Kumar, R.; Choudhary, D.K. Endophytic bacteria in plant salt stress tolerance: Current and future prospects. J. Plant Growth Regul. 2019, 38, 650–668. [Google Scholar] [CrossRef]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.A.; Mohi-Ud-Din, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef]
- Islam, M.T.; Rahman, M.M.; Pandey, P.; Jha, C.K.; Aeron, A. Bacilli and Agrobiotechnology; Springer International Publishing: New York, NY, USA, 2017; pp. 143–162. [Google Scholar]
- Bashan, Y.; Holguin, G. Proposal for the division of plant growth-promoting rhizobacteria into two classifications biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol. Biochem. 1998, 30, 1225–1230. [Google Scholar] [CrossRef]
- Sawana, A.; Adeolu, M.; Gupta, R.S. Molecular signatures and phylogenomic analysis of the genus Burkholderia: Proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harbouring environmental species. Front. Genet 2014, 5, 429. [Google Scholar] [CrossRef] [PubMed]
- McHlynn, W. Guidelines for the Use of Chlorine Bleach as a Sanitizer in Food Processing Operations. Oklahoma State University. 2016. Available online: https://extension.okstate.edu/fact-sheets/guidelines-for-the-use-of-chlorine-bleach-as-a-sanitizer-in-food-processing-operations.html (accessed on 3 July 2023).
- US; FDA. Selecting and Serving Produce Safely. United States of America. 2022. Available online: https://www.fda.gov/food/buy-store-serve-safe-food/selecting-and-serving-produce-safely (accessed on 21 July 2023).
- Suntornsuk, L. Dissemination Article of Knowledge to the Public “Method for Decrease Chemical Residue in Fruits and Vegetables”. 2019. Available online: https://pharmacy.mahidol.ac.th/th/knowledge/article/452/%E0%B8%AA%E0%B8%B2%E0%B8%A3%E0%B8%95%E0%B8%81%E0%B8%84%E0%B9%89%E0%B8%B2%E0%B8%87-%E0%B8%81%E0%B8%B2%E0%B8%A3%E0%B8%81%E0%B8%B3%E0%B8%88%E0%B8%B1%E0%B8%94/ (accessed on 25 September 2023).
- Ministry of Public Health. Notifications of Ministry of Public Health: Washing Agent and Microbial Decontaminated Substance for Food. 2019. Available online: https://www.ratchakitcha.soc.go.th/DATA/PDF/2562/E/278/T_0034.PDF (accessed on 27 September 2023).




| Compound | Treatments | Nutrition Claim Criteria (Wet) * | ||||
|---|---|---|---|---|---|---|
| Tip | Young | Intermediate | Stem | Good Source | High | |
| Moisture | 82.08 ± 0.29 a | 82.93 ± 1.40 a | 83.04 ± 1.06 a | 82.95 ± 2.08 a | - | - |
| Carbohydrate | 55.13 ± 1.36 c (9.88 ± 0.24 c) | 63.74 ± 2.36 b (10.88 ± 0.40 b) | 65.63 ± 2.63 b (11.13 ± 0.45 b) | 78.42 ± 1.02 a (13.37 ± 0.17 a) | - | - |
| Protein | 33.43 ± 1.21 a (5.99 ± 0.22 a) | 26.77 ± 0.82 b (4.57 ± 0.14 b) | 25.41 ± 0.68 b (4.31 ± 0.12 b) | 14.25 ± 0.37 c (2.43 ± 0.06 c) | ≥2.50 | ≥5.00 |
| Fat | 4.13 ± 0.15 a (0.74 ± 0.03 a) | 2.69 ± 0.13 b (0.46 ± 0.02 b) | 2.30 ± 0.10 c (0.39 ± 0.00 c) | 1.47 ± 0.02 d (0.25 ± 0.01 d) | - | - |
| Ash | 7.31 ± 0.06 a (1.31 ± 0.01 a) | 6.80 ± 0.06 b (1.16 ± 0.01 b) | 6.66 ± 0.06 b (1.13 ± 0.01 b) | 5.87 ± 0.16 c (1.00 ± 0.03 c) | - | - |
| Total dietary fibre | 30.92 ± 0.37 d (5.54 ± 0.07 d) | 37.67 ± 0.21 c (6.43 ± 0.04 c) | 41.27 ± 0.16 b (7.00 ± 0.03 b) | 48.97 ± 0.94 a (8.35 ± 0.16 a) | ≥3.00 | ≥6.00 |
| Insoluble dietary fibre | 27.51 ± 0.20 d (4.93 ± 0.04 d) | 32.22 ± 1.01 c (5.50 ± 0.17 c) | 34.43 ± 0.46 b (5.84 ± 0.08 b) | 41.88 ± 0.42 a (7.14 ± 0.07 a) | - | - |
| Soluble dietary fibre | 3.40 ± 0.17 c (0.61 ± 0.03 c) | 5.45 ± 0.23 b (0.93 ± 0.04 b) | 6.84 ± 0.29 a (1.16 ± 0.05 a) | 7.10 ± 0.06 a (1.21 ± 0.01 a) | - | - |
| Amino Acids (g/100 g DW) | Treatments | |||
|---|---|---|---|---|
| Tip | Young | Intermediate | Stem | |
| Essential | ||||
| Histidine | 0.73 ± 0.028 a (0.13 ± 0.005 a) | 0.70 ± 0.027 a (0.12 ± 0.005 a) | 0.59 ± 0.016 b (0.10 ± 0.003 b) | 0.47 ± 0.016 c (0.08 ± 0.003 c) |
| Isoleucine | 0.39 ± 0.015 a (0.07 ± 0.003 a) | 0.35 ± 0.010 b (0.06 ± 0.002 b) | 0.12 ± 0.04 c (0.02 ± 0.001 c) | ND d (ND d) |
| Leucine | 1.40 ± 0.010 a (0.25 ± 0.002 a) | 1.23 ± 0.015 b (0.21 ± 0.003 b) | 0.88 ± 0.006 c (0.15 ± 0.001 c) | 0.29 ± 0.012 d (0.05 ± 0.002 d) |
| Lysine | 2.34 ± 0.028 a (0.42 ± 0.005 a) | 1.76 ± 0.059 b (0.3 ± 0.010 b) | 1.30 ± 0.059 c (0.22 ± 0.010 c) | 0.70 ± 0.029 d (0.12 ± 0.005 d) |
| Methionine | 0.73 ± 0.024 a (0.13 ± 0.004 a) | 0.70 ± 0.006 a (0.12 ± 0.001 b) | 0.59 ± 0.021 b (0.10 ± 0.004 c) | 0.59 ± 0.010 b (0.10 ± 0.002 c) |
| Phenylalanine | 0.78 ± 0.015 a (0.14 ± 0.003 a) | 0.76 ± 0.010 a (0.13 ± 0.002 b) | 0.59 ± 0.020 b (0.1 ± 0.003 c) | 0.47 ± 0.005 c (0.08 ± 0.001 d) |
| Threonine | 0.67 ± 0.024 a (0.12 ± 0.004 a) | 0.53 ± 0.015 b (0.09 ± 0.003 b) | 0.35 ± 0.008 c (0.06 ± 0.001 c) | 0.23 ± 0.010 d (0.04 ± 0.002 d) |
| Tryptophan | 0.39 ± 0.010 b (0.07 ± 0.002 a) | 0.41 ± 0.007 a (0.07 ± 0.001 a) | 0.41 ± 0.003 a (0.07 ± 0.001 a) | ND c (ND b) |
| Valine | 1.45 ± 0.010 a (0.26 ± 0.002 a) | 1.35 ± 0.015 b (0.23 ± 0.003 b) | 0.94 ± 0.006 c (0.16 ± 0.001 c) | 0.59 ± 0.026 d (0.10 ± 0.004 d) |
| Non-essential | ||||
| Alanine | 1.90 ± 0.056 a (0.34 ± 0.010 a) | 1.46 ± 0.006 b (0.25 ± 0.001 b) | 0.83 ± 0.016 c (0.14 ± 0.003 c) | 0.41 ± 0.016 d (0.07 ± 0.003 d) |
| Arginine | 5.58 ± 0.097 b (1.00 ± 0.017 a) | 5.68 ± 0.020 b (0.97 ± 0.003 b) | 5.96 ± 0.010 a (1.01 ± 0.002 a) | 5.92 ± 0.006 a (1.01 ± 0.001 a) |
| Cystine | ND | ND | ND | ND |
| Glutamic acid | 2.06 ± 0.015 b (0.37 ± 0.003 b) | 1.29 ± 0.021 a (0.39 ± 0.004 a) | 0.77 ± 0.010 c (0.13 ± 0.002 c) | 0.70 ± 0.026 d (0.12 ± 0.004 d) |
| Glutamine | ND | ND | ND | ND |
| Aspartic acid | 3.74 ± 0.148 a (0.67 ± 0.026 a) | 2.28 ± 0.015 d (0.13 ± 0.003 d) | 1.30 ± 0.010 b (0.22 ± 0.002 b) | 1.06 ± 0.027 c (0.18 ± 0.005 c) |
| Asparagine | ND | ND | ND | ND |
| Glycine | 0.95 ± 0.015 a (0.17 ± 0.003 a) | 0.76 ± 0.015 b (0.13 ± 0.003 b) | 0.41 ± 0.010 c (0.07 ± 0.002 c) | 0.23 ± 0.006 d (0.04 ± 0.001 d) |
| Hydroxylysine | ND | ND | ND | ND |
| Hydroxyproline | ND | ND | ND | ND |
| Proline | 1.23 ± 0.010 a (0.22 ± 0.002 a) | 1.11 ± 0.041 b (0.19 ± 0.007 b) | 0.65 ± 0.016 c (0.11 ± 0.003 c) | 0.29 ± 0.012 d (0.05 ± 0.002 d) |
| Serine | 0.95 ± 0.015 a (0.17 ± 0.003 a) | 0.70 ± 0.015 b (0.12 ± 0.003 b) | 0.53 ± 0.016 c (0.09 ± 0.003 c) | 0.47 ± 0.006 d (0.08 ± 0.001 d) |
| Tyrosine | 0.39 ± 0.015 a (0.07 ± 0.003 a) | 0.41 ± 0.010 a (0.07 ± 0.002 a) | 0.35 ± 0.010 b (0.06 ± 0.002 b) | 0.23 ± 0.011 c (0.04 ± 0.002 c) |
| Cysteine | ND | ND | ND | ND |
| Total essential | 8.87 (1.59) | 7.79 (1.33) | 5.78 (0.98) | 3.34 (0.57) |
| Total non-essential | 16.80 (3.01) | 13.71 (2.34) | 10.79 (1.83) | 9.33 (1.59) |
| Total | 25.67 (4.60) | 21.50 (3.67) | 16.57 (2.81) | 12.67 (2.16) |
| Compound | Treatments | Nutrition Claim Criteria (Wet) * | ||||
|---|---|---|---|---|---|---|
| Tip | Young | Intermediate Leaves | Stem | Good Source | High | |
| Vitamins | ||||||
| A (Beta-Carotene) (mg/100 g) | 1.35 ± 0.06 c (0.24 ± 0.01 c) | 2.35 ± 0.09 b (0.40 ± 0.02 b) | 3.03 ± 0.07 a (0.51 ± 0.01 a) | 2.23 ± 0.08 b (0.38 ± 0.01 b) | ≥0.72 | ≥1.44 |
| B9 (Folic acid) (mg/100 g) | 0.0290 ± 0.0013 b (0.0052 ± 0.0002 b) | 0.0375 ± 0.0002 a (0.0064 ± 0.0000 a) | ND c (ND c) | ND c (ND c) | ≥0.03 | ≥0.06 |
| C (Ascorbic acid) (mg/100 g) | 5.25 ± 0.05 a (0.94 ± 0.01 a) | 2.93 ± 0.05 b (0.50 ± 0.01 b) | 2.71 ± 0.04 c (0.46 ± 0.01 c) | ND d (ND d) | ≥9 | ≥18 |
| Minerals | ||||||
| Calcium (mg/100 g) | 374.11 ± 6.42 d (67.04 ± 1.15 b) | 425.89 ± 16.26 b (72.7 ± 2.78 a) | 450.71 ± 5.37 a (76.44 ± 0.91 a) | 400.18 ± 3.08 c (68.23 ± 0.53 b) | ≥120 | ≥240 |
| Copper (mg/100 g) | 0.56 ± 0.02 a (0.10 ± 0.00 a) | 0.47 ± 0.02 b (0.08 ± 0.00 b) | 0.47 ± 0.01 b (0.08 ± 0.00 b) | 0.47 ± 0.00 b (0.08 ± 0.00 b) | ≥0.3 | ≥0.6 |
| Iron (mg/100 g) | 4.46 ± 0.20 a (0.80 ± 0.04 a) | 3.63 ± 0.05 b (0.62 ± 0.01 b) | 3.63 ± 0.04 b (0.61 ± 0.01 b) | 1.94 ± 0.05 c (0.3 ± 0.013 c) | ≥2.25 | ≥4.5 |
| Magnesium (mg/100 g) | 196.76 ± 0.60 a (35.26 ± 0.11 a) | 197.01 ± 8.23 a (33.63 ± 1.41 ab) | 193.1 ± 0.27 a (32.75 ± 0.05 b) | 124.52 ± 6.21 b (21.23 ± 1.06 c) | ≥52.5 | ≥105 |
| Zinc (mg/100 g) | 4.58 ± 0.02 a (0.82 ± 0.00 a) | 3.51 ± 0.16 b (0.60 ± 0.03 b) | 3.18 ± 0.07 c (0.54 ± 0.01 c) | 2.23 ± 0.07 d (0.38 ± 0.01 d) | ≥2.25 | ≥4.5 |
| Time/Condition | NWS | NWNS | WS | WNS |
|---|---|---|---|---|
| D0 | >1100 | >1100 | >1100 | >1100 |
| D4 | >1100 | >1100 | 240 | 240 |
| D8 | 1100 | >1100 | <3 | 150 |
| D12 | >1100 | >1100 | 7.4 | >1100 |
| Time/Condition | NWS | NWNS | WS | WNS |
|---|---|---|---|---|
| D0 | 93 | 93 | 93 | 93 |
| D4 | 150 | 150 | <3 | 3.6 |
| D8 | 43 | >1100 | <3 | 150 |
| D12 | 43 | 460 | <3 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siripongvutikorn, S.; Usawakesmanee, W.; Pisuchpen, S.; Khatcharin, N.; Rujirapong, C. Nutritional Content and Microbial Load of Fresh Liang, Gnetum gnemon var. tenerum Leaves. Foods 2023, 12, 3848. https://doi.org/10.3390/foods12203848
Siripongvutikorn S, Usawakesmanee W, Pisuchpen S, Khatcharin N, Rujirapong C. Nutritional Content and Microbial Load of Fresh Liang, Gnetum gnemon var. tenerum Leaves. Foods. 2023; 12(20):3848. https://doi.org/10.3390/foods12203848
Chicago/Turabian StyleSiripongvutikorn, Sunisa, Worapong Usawakesmanee, Supachai Pisuchpen, Nicha Khatcharin, and Chanonkarn Rujirapong. 2023. "Nutritional Content and Microbial Load of Fresh Liang, Gnetum gnemon var. tenerum Leaves" Foods 12, no. 20: 3848. https://doi.org/10.3390/foods12203848
APA StyleSiripongvutikorn, S., Usawakesmanee, W., Pisuchpen, S., Khatcharin, N., & Rujirapong, C. (2023). Nutritional Content and Microbial Load of Fresh Liang, Gnetum gnemon var. tenerum Leaves. Foods, 12(20), 3848. https://doi.org/10.3390/foods12203848






