Applying Different Vinification Techniques in Teran Red Wine Production: Impact on Bioactive Compounds and Sensory Attributes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Minivinification
2.4. Standard Physico-Chemical Analysis
2.5. Analysis of Phenolic Compounds
2.6. Analysis of Macro- and Microelements
2.7. Analysis of Vitamins
2.8. Sensory Analysis
2.9. Statistical Data Analysis
3. Results and Discussion
3.1. Standard Physico-Chemical Parameters
3.2. Phenolic Compounds
3.3. Macroelements and Microelements
3.4. Vitamins
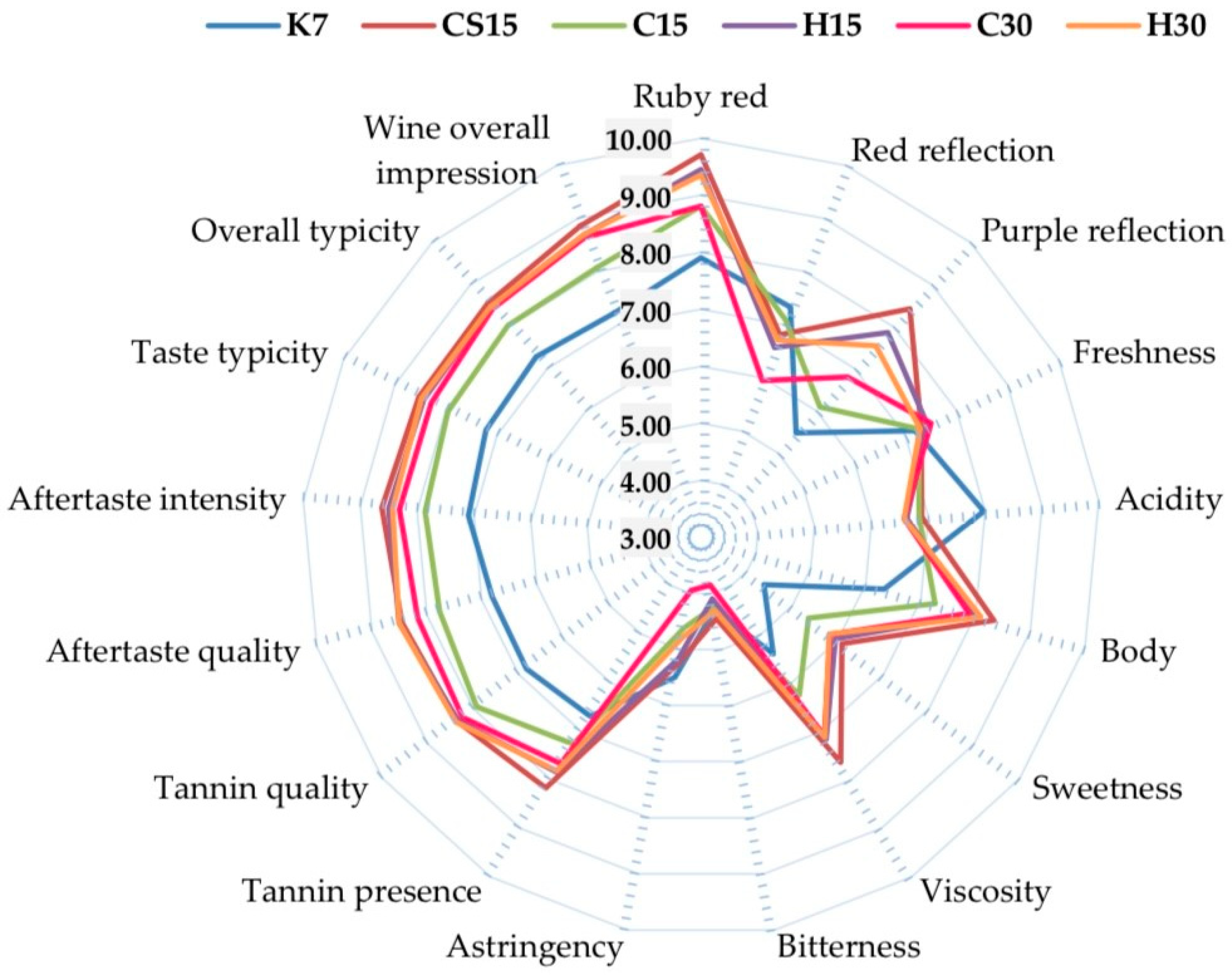
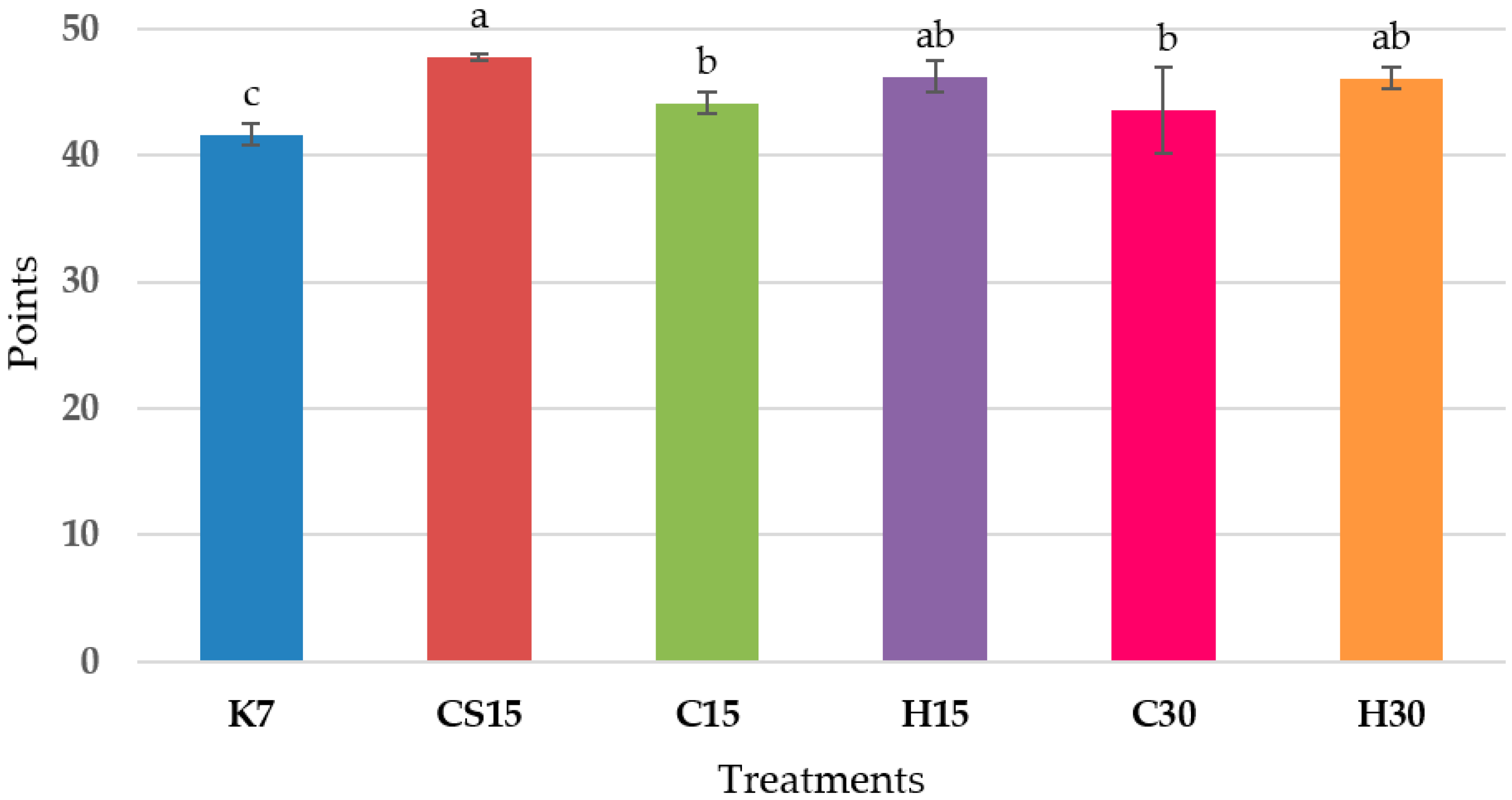
3.5. Sensory Analysis
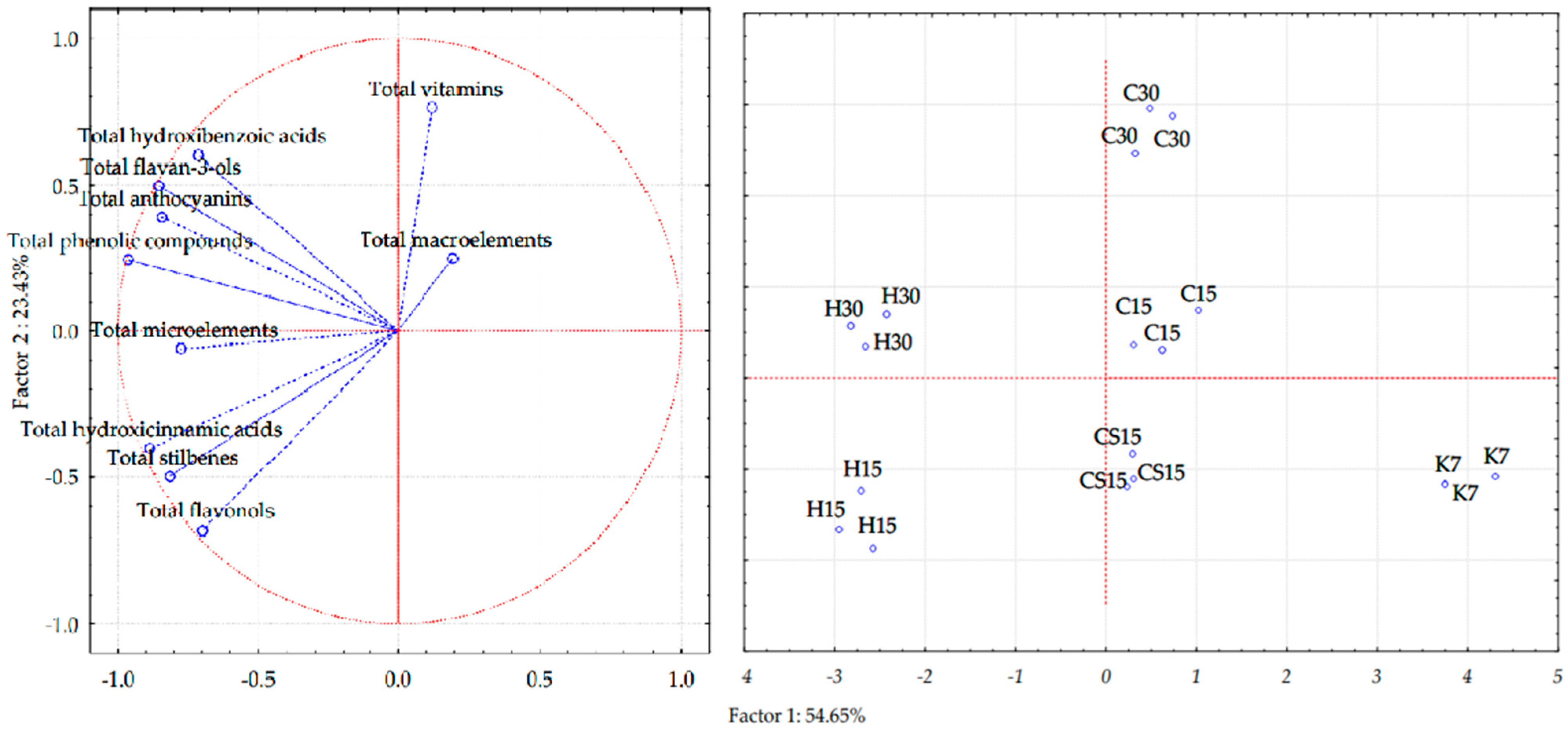
3.6. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vejarano, R.; Luján-Corro, M. Red Wine and Health: Approaches to Improve the Phenolic Content During Winemaking. Front. Nutr. 2022, 9, 890066. [Google Scholar] [CrossRef] [PubMed]
- Ruskovska, T.; Budić-Leto, I.; Corral-Jara, K.F.; Ajdžanović, V.; Arola-Arnal, A.; Bravo, F.I.; Deligiannidou, G.-E.; Havlik, J.; Janeva, M.; Kistanova, E.; et al. Systematic Bioinformatic Analyses of Nutrigenomic Modifications by Polyphenols Associated with Cardiometabolic Health in Humans—Evidence from Targeted Nutrigenomic Studies. Nutrients 2021, 13, 2326. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Radeka, S.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Lukić, I.; Orbanić, F.; Zaninović Jurjević, T.; Dvornik, Š. Bioactive Compounds and Antioxidant Activity of Red and White Wines Produced from Autochthonous Croatian Varieties: Effect of Moderate Consumption on Human Health. Foods 2022, 11, 1804. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; García-Estévez, I.; Ferreras-Charro, R.; Rivas-Gonzalo, J.C.; Ferrer-Gallego, R.; Escribano-Bailón, M.T. Adding Oenological Tannin vs. Overripe Grapes: Effect on the Phenolic Composition of Red Wines. J. Food Compos. Anal. 2014, 34, 99–113. [Google Scholar] [CrossRef]
- Avizcuri, J.-M.; Sáenz-Navajas, M.-P.; Echávarri, J.-F.; Ferreira, V.; Fernández-Zurbano, P. Evaluation of the Impact of Initial Red Wine Composition on Changes in Color and Anthocyanin Content during Bottle Storage. Food Chem. 2016, 213, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Flavonoids in Wine. In Flavonoids; Andersen, Ø., Markham, K., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 263–318. ISBN 978-0-8493-2021-7. [Google Scholar]
- Rentzsch, M.; Wilkens, A.; Winterhalter, P. Non-Flavonoid Phenolic Compounds. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 509–527. ISBN 978-0-387-74118-5. [Google Scholar]
- Galgano, F.; Favati, F.; Caruso, M.; Scarpa, T.; Palma, A. Analysis of Trace Elements in Southern Italian Wines and Their Classification According to Provenance. LWT Food Sci. Technol. 2008, 41, 1808–1815. [Google Scholar] [CrossRef]
- Vrček, I.V.; Bojić, M.; Žuntar, I.; Mendaš, G.; Medić-Šarić, M. Phenol Content, Antioxidant Activity and Metal Composition of Croatian Wines Deriving from Organically and Conventionally Grown Grapes. Food Chem. 2011, 124, 354–361. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The Importance of Minerals in Human Nutrition: Bioavailability, Food Fortification, Processing Effects and Nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Núñez, M.; Peña, R.M.; Herrero, C.; García-Martín, S. Analysis of Some Metals in Wine by Means of Capillary Electrophoresis. Application to the Differentiation of Ribeira Sacra Spanish Red Wines. Analusis 2000, 28, 432–437. [Google Scholar] [CrossRef]
- Ball, G.F.M. Vitamins: Their Role in the Human Body; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-1-4051-4810-8. [Google Scholar]
- Jackson, R.S. Wine, Food, and Health. In Wine Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 947–978. ISBN 978-0-12-816118-0. [Google Scholar]
- Evers, M.S.; Roullier-Gall, C.; Morge, C.; Sparrow, C.; Gobert, A.; Alexandre, H. Vitamins in Wine: Which, What for, and How Much? Compr. Rev. Food Sci. Food Saf. 2021, 20, 2991–3035. [Google Scholar] [CrossRef] [PubMed]
- Heredia, F.J.; Escudero-Gilete, M.L.; Hernanz, D.; Gordillo, B.; Meléndez-Martínez, A.J.; Vicario, I.M.; González-Miret, M.L. Influence of the Refrigeration Technique on the Colour and Phenolic Composition of Syrah Red Wines Obtained by Pre-Fermentative Cold Maceration. Food Chem. 2010, 118, 377–383. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Piccardo, D.; González-Neves, G. Extracción de polifenoles y composición de vinos tintos Tannat elaborados por técnicas de maceración prefermentativa. Agrociencia 2013, 17, 36–44. [Google Scholar] [CrossRef]
- de Beer, D.; Joubert, E.; Marais, J.; Manley, M. Maceration Before and During Fermentation: Effect on Pinotage Wine Phenolic Composition, Total Antioxidant Capacity and Objective Colour Parameters. SAJEV 2017, 27, 137–150. [Google Scholar] [CrossRef][Green Version]
- Girard, B.; Yuksel, D.; Cliff, M.A.; Delaquis, P.; Reynolds, A.G. Vinification Effects on the Sensory, Colour and GC Profiles of Pinot Noir Wines from British Columbia. Food Res. Int. 2001, 34, 483–499. [Google Scholar] [CrossRef]
- Maza, M.; Álvarez, I.; Raso, J. Thermal and Non-Thermal Physical Methods for Improving Polyphenol Extraction in Red Winemaking. Beverages 2019, 5, 47. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. (Eds.) Handbook of Enology, Volume 2: The Chemistry of Wine—Stabilization and Treatments, 2nd ed.; Wiley: Chichester, UK; Hoboken, NJ, USA, 2006; ISBN 978-0-470-01037-2. [Google Scholar]
- Casassa, L.F.; Huff, R.; Steele, N.B. Chemical Consequences of Extended Maceration and Post-Fermentation Additions of Grape Pomace in Pinot Noir and Zinfandel Wines from the Central Coast of California (USA). Food Chem. 2019, 300, 125147. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.-M.; Kozina, B.; Preiner, D.; Tomaz, I.; Volarević, J.; Jeromel, A. The Effect of Seed Removal and Extraction Time on the Phenolic Profile of Plavac Mali Wine. Appl. Sci. 2023, 13, 5411. [Google Scholar] [CrossRef]
- Scudamore-Smith, P.D.; Hooper, R.L.; McLaran, E.D. Color and Phenolic Changes of Cabernet Sauvignon Wine Made by Simultaneous Yeast/Bacterial Fermentation and Extended Pomace Contact. Am. J. Enol. Vitic. 1990, 41, 57–67. [Google Scholar] [CrossRef]
- Ivanova, V.; Dörnyei, Á.; Márk, L.; Vojnoski, B.; Stafilov, T.; Stefova, M.; Kilár, F. Polyphenolic Content of Vranec Wines Produced by Different Vinification Conditions. Food Chem. 2011, 124, 316–325. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Fernández-Fernández, J.I. Maintenance of Colour Composition of a Red Wine During Storage. Influence of Prefermentative Practices, Maceration Time and Storage. LWT Food Sci. Technol. 2002, 35, 46–53. [Google Scholar] [CrossRef]
- Rossi, S.; Bestulić, E.; Horvat, I.; Plavša, T.; Lukić, I.; Bubola, M.; Ganić, K.K.; Ćurko, N.; Jagatić Korenika, A.-M.; Radeka, S. Comparison of Different Winemaking Processes for Improvement of Phenolic Composition, Macro- and Microelemental Content, and Taste Sensory Attributes of Teran (Vitis vinifera L.) Red Wines. LWT 2022, 154, 112619. [Google Scholar] [CrossRef]
- Rusjan, D.; Bubola, M.; Janjanin, D.; Užila, Z.; Radeka, S.; Poljuha, D.; Pelengić, R.; Javornik, D.; Štajner, N. Ampelographic Characterisation of Grapevine Accessions Denominated “Refošk”, “Refosco”, “Teran” and “Terrano” (Vitis vinifera L.) from Slovenia, Croatia and Italy. VITIS J. Grapevine Res. 2015, 54, 77–80. [Google Scholar] [CrossRef]
- Vrhovšek, U. Extraction of Hydroxycinnamoyltartaric Acids from Berries of Different Grape Varieties. J. Agric. Food Chem. 1998, 46, 4203–4208. [Google Scholar] [CrossRef]
- Bubola, M.; Sivilotti, P.; Janjanin, D.; Poni, S. Early Leaf Removal Has a Larger Effect than Cluster Thinning on Grape Phenolic Composition in Cv. Teran. Am. J. Enol. Vitic. 2017, 68, 234–242. [Google Scholar] [CrossRef]
- Croatian Wine Law (NN 32/2019). Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_03_32_641.html (accessed on 16 August 2023).
- Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 Establishing a Common Organisation of the Markets in Agricultural Products and Repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32013R1308 (accessed on 16 July 2023).
- Ordinance on Wine Production (NN 2/2005). Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2005_01_2_17.html (accessed on 8 October 2023).
- International Organisation of Vine and Wine. OIV Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine (OIV): Paris, France, 2018; Volume 1. [Google Scholar]
- Pati, S.; Crupi, P.; Benucci, I.; Antonacci, D.; Di Luccia, A.; Esti, M. HPLC-DAD–MS/MS Characterization of Phenolic Compounds in White Wine Stored without Added Sulfite. Food Res. Int. 2014, 66, 207–215. [Google Scholar] [CrossRef]
- Mark, L.; Nikfardjam, M.S.P.; Avar, P.; Ohmacht, R. A Validated HPLC Method for the Quantitative Analysis of Trans-Resveratrol and Trans-Piceid in Hungarian Wines. J. Chromatogr. Sci. 2005, 43, 445–449. [Google Scholar] [CrossRef]
- Ćurko, N.; Kovačević Ganić, K.; Gracin, L.; Đapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of Seed and Skin Polyphenolic Extracts of Two Red Grape Cultivars Grown in Croatia and Their Sensory Perception in a Wine Model Medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef]
- Larcher, R.; Nicolini, G. Survey of 22 Mineral Elements in Wines from Trentino (Italy) Using ICP-OES. Ital. J. Food Sci. 2001, 13, 233–241. [Google Scholar]
- Leder, R.; Kubanović, V.; Petric, I.V.; Vahčić, N.; Banović, M. Chemometric Prediction of the Geographical Origin of Croatian Wines through Their Elemental Profiles. J. Food Nutr. Res. 2015, 54, 229–238. [Google Scholar]
- Trang, H. Development of HPLC Methods for the Determination of Water-Soluble Vitamins in Pharmaceuticals and Fortified Food Products. Master’s Thesis, Clemson University, Clemson, SC, USA, 2013. [Google Scholar]
- ISO 3591:1977; Sensory Analysis. Apparatus Wine-Tasting Glass. Group B; International Organization for Standardization: Geneva, Switzerland, 1997. Available online: https://www.iso.org/standard/9002.html (accessed on 18 August 2023).
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/63/36385.html (accessed on 18 August 2023).
- Radeka, S.; Bestulić, E.; Rossi, S.; Orbanić, F.; Bubola, M.; Plavša, T.; Lukić, I.; Jeromel, A. Effect of Different Vinification Techniques on the Concentration of Volatile Aroma Compounds and Sensory Profile of Malvazija Istarska Wines. Fermentation 2023, 9, 676. [Google Scholar] [CrossRef]
- OIV Resolution 332a/2009; OIV Standard for International Wine and Spirituous Beverages of Vitivinicultural Origin Competitions. International Organisation of Vine and Wine: Paris, France, 2009.
- ISO 17025:2017; ISO/IEC 17025:2017—General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/66912.html (accessed on 18 August 2023).
- Ordinance on Wine and Fruit Wine Sensory Testing (NN 106/04). Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2004_07_106_2061.html (accessed on 17 July 2023).
- Commission Regulation (EC) No 606/2009 of 10 July 2009 Laying down Certain Detailed Rules for Implementing Council Regulation (EC) No 479/2008 as Regards the Categories of Grapevine Products, Oenological Practices and the Applicable Restrictions. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32009R0606 (accessed on 17 July 2023).
- Parenti, A.; Spugnoli, P.; Calamai, L.; Ferrari, S.; Gori, C. Effects of Cold Maceration on Red Wine Quality from Tuscan Sangiovese Grape. Eur. Food Res. Technol. 2004, 218, 360–366. [Google Scholar] [CrossRef]
- Giriboni, P.P.; Xavier, A.; Roque, V.; Vargas, G.; Souza, F.; Costa, V.B. Physical and Chemical Analyzis of Chardonnay Wine with Different Periods of Skin Contact. BIO Web Conf. 2016, 7, 02021. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Gonzalez-Rodriguez, J.; Perez Juan, P. Analytical Methods in Wineries: Is It Time to Change? Food Rev. Int. 2005, 21, 231–265. [Google Scholar] [CrossRef]
- Hidalgo Togores, J. Tratado de Enología. Volumen I y II; Mundi-Prensa: Madrid, Spain, 2003; ISBN 978-84-8476-752-7. [Google Scholar]
- Commission Delegated Regulation (EU) 2019/934 of 12 March 2019 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Wine-Growing Areas Where the Alcoholic Strength May Be Increased, Authorised Oenological Practices and Restrictions Applicable to the Production and Conservation of Grapevine Products, the Minimum Percentage of Alcohol for By-Products and Their Disposal, and Publication of OIV Files. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019R0934 (accessed on 8 October 2023).
- Neto, F.S.; de Castilhos, M.B.; Telis, V.R.; Telis-Romero, J. Effect of Ethanol, Dry Extract and Reducing Sugars on Density and Viscosity of Brazilian Red Wines. J. Sci. Food Agric. 2015, 95, 1421–1427. [Google Scholar] [CrossRef]
- Budić-Leto, I.; Lovrić, T.; Kljusurić, J.G.; Pezo, I.; Vrhovšek, U. Anthocyanin Composition of the Red Wine Babić Affected by Maceration Treatment. Eur. Food Res. Technol. 2006, 222, 397–402. [Google Scholar] [CrossRef]
- Lukić, I.; Budić-Leto, I.; Bubola, M.; Damijanić, K.; Staver, M. Pre-Fermentative Cold Maceration, Saignée, and Various Thermal Treatments as Options for Modulating Volatile Aroma and Phenol Profiles of Red Wine. Food Chem. 2017, 224, 251–261. [Google Scholar] [CrossRef]
- González-Neves, G.; Gil, G.; Barreiro, L.; Favre, G. Pigment Profile of Red Wines Cv. Tannat Made with Alternative Winemaking Techniques. J. Food Compos. Anal. 2010, 23, 447–454. [Google Scholar] [CrossRef]
- Gonzalez-Manzano, S.; Duenas, M.; Rivasgonzalo, J.; Escribanobailon, M.; Santosbuelga, C. Studies on the Copigmentation between Anthocyanins and Flavan-3-Ols and Their Influence in the Colour Expression of Red Wine. Food Chem. 2009, 114, 649–656. [Google Scholar] [CrossRef]
- Sims, C.A.; Bates, R.P. Effects of Skin Fermentation Time on the Phenols, Anthocyanins, Ellagic Acid Sediment, and Sensory Characteristics of a Red Vitis Rotundifolia Wine. Am. J. Enol. Vitic. 1994, 45, 56–62. [Google Scholar] [CrossRef]
- Şener, H. Effect of Temperature and Duration of Maceration on Colour and Sensory Properties of Red Wine: A Review. S. Afr. J. Enol. Vitic. 2018, 39, 1–8. [Google Scholar] [CrossRef]
- Koyama, K.; Goto-Yamamoto, N.; Hashizume, K. Influence of Maceration Temperature in Red Wine Vinification on Extraction of Phenolics from Berry Skins and Seeds of Grape (Vitis vinifera). Biosci. Biotechnol. Biochem. 2007, 71, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Vanzo, A.; Nemanic, J. Effect of Red Wine Maceration Techniques on Oligomeric and Polymeric Proanthocyanidins in Wine, Cv. Blaufränkisch. Vitis 2002, 41, 47–51. [Google Scholar]
- Garrido, J.; Borges, F. Wine and Grape Polyphenols—A Chemical Perspective. Food Res. Int. 2011, 44, 3134–3148. [Google Scholar] [CrossRef]
- Bloomfield, D.G.; Heatherbell, D.A.; Nikfardjam, M.S.P. Effect of P-Coumaric Acid on the Color in Red Wine. Mitteilungen Klosterneubg. 2003, 53, 195–198. [Google Scholar]
- Bimpilas, A.; Panagopoulou, M.; Tsimogiannis, D.; Oreopoulou, V. Anthocyanin Copigmentation and Color of Wine: The Effect of Naturally Obtained Hydroxycinnamic Acids as Cofactors. Food Chem. 2016, 197, 39–46. [Google Scholar] [CrossRef]
- Darias-Martín, J.; Rodr’iguez, O.; D’Iaz, E.; Lamuela-Raventós, R.M. Effect of Skin Contact on the Antioxidant Phenolics in White Wine. Food Chem. 2000, 71, 483–487. [Google Scholar] [CrossRef]
- Lisov, N.; Čakar, U.; Milenković, D.; Čebela, M.; Vuković, G.; Despotović, S.; Petrović, A. The Influence of Cabernet Sauvignon Ripeness, Healthy State and Maceration Time on Wine and Fermented Pomace Phenolic Profile. Fermentation 2023, 9, 695. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Hornedo-Ortega, R.; Garcia, F.; El Khawand, T.; Saucier, C.; Richard, T. Stilbenes in Grape Berries and Wine and Their Potential Role as Anti-Obesity Agents: A Review. Trends Food Sci. Technol. 2021, 112, 362–381. [Google Scholar] [CrossRef]
- Sun, X.; Chen, X.; Li, L.; Ma, T.; Zhao, F.; Huang, W.; Zhan, J. Effect of Ultra-High Pressure Treatment on the Chemical Properties, Colour and Sensory Quality of Young Red Wine. S. Afr. J. Enol. Vitic. 2015, 36, 393–401. [Google Scholar] [CrossRef][Green Version]
- Carrizzo, A.; Izzo, C.; Vecchione, C. Protective Activity of Resveratrol in Cardio- and Cerebrovascular Diseases. In Resveratrol—Adding Life to Years, Not Adding Years to Life; IntechOpen: London, UK, 2018; ISBN 978-1-78984-995-0. [Google Scholar]
- Kostadinović, S.; Wilkens, A.; Stefova, M.; Ivanova, V.; Vojnoski, B.; Mirhosseini, H.; Winterhalter, P. Stilbene Levels and Antioxidant Activity of Vranec and Merlot Wines from Macedonia: Effect of Variety and Enological Practices. Food Chem. 2012, 135, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Petrović, A.; Lisov, N.; Čakar, U.; Marković, N.; Matijašević, S.; Cvejić, J.; Atanacković, M.; Gojković-Bukarica, L. The Effects of Prokupac Variety Clones and Vinification Method on the Quantity of Resveratrol in Wine. Food Feed. Res. 2019, 46, 189–198. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds During Red Wine Maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Kocabey, N.; Yilmaztekin, M.; Hayaloglu, A.A. Effect of Maceration Duration on Physicochemical Characteristics, Organic Acid, Phenolic Compounds and Antioxidant Activity of Red Wine from Vitis vinifera L. Karaoglan. J. Food Sci. Technol. 2016, 53, 3557–3565. [Google Scholar] [CrossRef]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The Roles of Iron in Health and Disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M.; Stefanou, S. Preliminary Study of Microelements, Phenolics as Well as Antioxidant Activity in Local, Homemade Wines from North-East Greece. Foods 2020, 9, 1607. [Google Scholar] [CrossRef]
- Bora, F.-D.; Bunea, C.-I.; Rusu, T.; Pop, N. Vertical Distribution and Analysis of Micro-, Macroelements and Heavy Metals in the System Soil-Grapevine-Wine in Vineyard from North-West Romania. Chem. Cent. J. 2015, 9, 19. [Google Scholar] [CrossRef][Green Version]
- Ournac, A.; Flanzy, M. The Increase in Vitamin B1 in Wine Stored with Its Lees. Ann. Technol. Agric. 1967, 16, 41–54. [Google Scholar]
- Lafourcade, S.; Peynaud, E.; Ribereau-Gayon, J. Different forms of nicotinamide in wine. Bull. Soc. Chim. Biol. 1956, 38, 923–930. [Google Scholar]
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial Properties of Red Wine Polyphenols: Astringency and Bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Enhancement of Chardonnay Antioxidant Activity and Sensory Perception through Maceration Technique. LWT Food Sci. Technol. 2016, 65, 152–157. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Sensory Evaluation of Bitterness and Astringency Sub-Qualities of Wine Phenolic Compounds: Synergistic Effect and Modulation by Aromas. Food Res. Int. 2014, 62, 1100–1107. [Google Scholar] [CrossRef]
- Casassa, F.; Sari, S.; Avagnina, S.; Díaz, M.; Jofré, V.; Fanzone, M. Influencia de dos técnicas de maceración sobre la composición polifenólica, aromática y las características organolépticas de vinos cv. Merlot. Vitic. Enol. Prof. 2007, 5–20. [Google Scholar]
- González-Neves, G.; Ferrer, M. Efectos del sistema de conducción y del raleo de racimos en la composición de uvas Merlot. Agrociencia 2008, 12, 10–18. [Google Scholar] [CrossRef]
- Budić-Leto, I.; Gracin, L.; Lovric, T.; Vrhovsek, U. Effects of Maceration Conditions on the Polyphenolic Composition of Red Wine “Plavac Mali”. Vitis J. Grapevine Res. 2008, 47, 245–250. [Google Scholar]
- Ofoedu, C.E.; Ofoedu, E.O.; Chacha, J.S.; Owuamanam, C.I.; Efekalam, I.S.; Awuchi, C.G. Comparative Evaluation of Physicochemical, Antioxidant, and Sensory Properties of Red Wine as Markers of Its Quality and Authenticity. Int. J. Food Sci. 2022, 2022, e8368992. [Google Scholar] [CrossRef]

| Treatment | Pre-Fermentative Procedure | Fermentation and Maceration | Pre-Fermentative Procedure + Maceration Duration | |||
|---|---|---|---|---|---|---|
| Vinification Technique—Maceration | Fermentation/Maceration Temperature | Maceration Duration | ||||
| K7 | / | Standard maceration | 24 °C | 7 days | / | |
| CS15 | Cooling at 8 °C, 48 h | Saignée | Fermentation/maceration + prolonged post-fermentative maceration | 13 days | 15 days | |
| C15 | 13 days | 15 days | ||||
| C30 | 28 days | 30 days | ||||
| H15 | Heating at 50 °C, 48 h | 13 days | 15 days | |||
| H30 | 28 days | 30 days | ||||
| Parameters | Treatments | |||||
|---|---|---|---|---|---|---|
| K7 | CS15 | C15 | H15 | C30 | H30 | |
| Alcohol (vol. %) | 12.08 ± 0.03 c | 11.62 ± 0.01 e | 12.33 ± 0.03 a | 12.03 ± 0.02 c | 12.22 ± 0.04 b | 11.94 ± 0.07 d |
| Total dry extract (g/L) | 23.0 ± 0.15 d | 24.6 ± 0.35 c | 25.3 ± 0.4 a | 25.3 ± 0.12 ab | 25.6 ± 0.10 a | 24.8 ± 0.30 bc |
| Reducing sugars (g/L) | 1.3 ± 0.10 b | 1.5 ± 0.06 b | 1.5 ± 0.01 b | 1.2 ± 0.15 b | 2.6 ± 0.78 a | 1.4 ± 0.10 b |
| Extract without reducing sugars (g/L) | 20.7 ± 0.25 d | 22.1 ± 0.36 bc | 22.8 ± 0.40 ab | 23.1 ± 0.10 ab | 22.0 ± 0.78 c | 22.4 ± 0.40 abc |
| Ash (g/L) | 2.53 ± 0.03 d | 2.71 ± 0.02 c | 2.77 ± 0.05 c | 2.94 ± 0.07 a | 2.76 ± 0.01 c | 2.85 ± 0.01 b |
| pH | 3.17 ± 0.03 e | 3.33 ± 0.01 c | 3.31 ± 0.01 d | 3.41 ± 0.01 b | 3.34 ± 0.01 c | 3.44 ± 0.01 a |
| Total acidity * (g/L) | 8.6 ± 0.25 a | 6.4 ± 0.06 c | 6.2 ± 0.01 c | 5.6 ± 0.01 d | 6.6 ± 0.01 b | 5.6 ± 0.01 d |
| Volatile acidity ** (g/L) | 0.26 ± 0.02 c | 0.38 ± 0.04 b | 0.37 ± 0.05 b | 0.30 ± 0.03 c | 0.46 ± 0.01 a | 0.40 ± 0.01 b |
| Free SO2 (mg/L) | 11 ±1 a | 10 ± 1 a | 10 ± 2 a | 11 ± 2 a | 9 ± 1 b | 10 ± 2 a |
| Total SO2 (mg/L) | 80 ± 2 a | 79 ± 3 a | 78 ± 3 a | 77 ± 3 a | 78 ± 4 a | 79 ± 3 a |
| Phenolic Compounds | Treatments | |||||
|---|---|---|---|---|---|---|
| K7 | CS15 | C15 | H15 | C30 | H30 | |
| Anthocyanins | ||||||
| Delphinidin-3-O-glucoside | 1.47 ± 0.06 d | 1.65 ± 0.12 c | 1.47 ± 0.10 d | 1.95 ± 0.04 b | 1.61 ± 0.06 c | 2.08 ± 0.02 a |
| Cyanidin-3-O-glucoside | 0.20 ± 0.01 d | 0.26 ± 0.04 bc | 0.30 ± 0.02 a | 0.28 ± 0.01 ab | 0.23 ± 0.01 cd | 0.26 ± 0.01 ab |
| Petunidin-3-O-glucoside | 1.62 ± 0.09 d | 1.78 ± 0.05 c | 1.76 ± 0.03 c | 2.3 ± 0.05 a | 1.77 ± 0.04 c | 2.04 ± 0.01 b |
| Peonidin-3-O-glucoside | 1.51 ± 0.07 a | 1.92 ± 0.07 a | 1.58 ± 0.05 a | 1.64 ± 0.01 a | 1.35 ± 0.03 a | 1.57 ± 0.08 a |
| Malvidin-3-O-glucoside | 23.34 ± 0.95 d | 27.54 ± 0.67 c | 27.09 ± 0.93 c | 27.77 ± 0.74 bc | 29.13 ± 0.54 a | 29.04 ± 0.26 ab |
| Peonidin-3-O-acetylglucoside | 1.64 ± 0.12 c | 2.58 ± 0.10 b | 2.65 ± 0.03 b | 2.52 ± 0.05 b | 2.88 ± 0.17 a | 2.97 ± 0.03 a |
| Malvidin-3-O-acetylglucoside | 0.50 ± 0.01 b | 0.58 ± 0.02 a | 0.47 ± 0.01 cd | 0.49 ± 0.01 bc | 0.46 ± 0.01 d | 0.48 ± 0.01 bcd |
| Peonidin-3-O-cumarylglucoside | 0.29 ± 0.03 b | 0.35 ± 0.03 a | 0.22 ± 0.01 c | 0.26 ± 0.01 b | 0.32 ± 0.01 a | 0.26 ± 0.01 b |
| Malvidin-3-O-cumarylglucoside | 3.18 ± 0.12 a | 2.92 ± 0.06 b | 2.86 ± 0.16 b | 2.93 ± 0.23 b | 2.84 ± 0.10 b | 2.79 ± 0.02 b |
| Total detected anthocyanins | 33.76 ± 1.34 d | 39.58 ± 0.73 bc | 38.4 ± 1.17 c | 40.14 ± 1.07 ab | 40.6 ± 0.60 ab | 41.50 ± 0.32 a |
| Phenolic acids | ||||||
| Gallic acid | 12.41 ± 0.3 e | 35.98 ± 0.5 c | 35.54 ± 0.84 c | 33.56 ± 1.25 d | 44.96 ± 0.69 a | 43.47 ± 0.66 b |
| Protocatechuic acid | 3.10 ± 0.09 d | 4.37 ± 0.32 ab | 4.22 ± 0.20 ab | 4.11 ± 0.33 b | 4.57 ± 0.14 a | 4.60 ± 0.08 a |
| p-Hydroxybenzoic acid | 0.43 ± 0.01 cd | 0.68 ± 0.07 b | 0.89 ± 0.03 a | 0.38 ± 0.04 d | 0.63 ± 0.03 b | 0.46 ± 0.02 c |
| Syringic acid | 2.46 ± 0.04 d | 3.75 ± 0.05 c | 3.74 ± 0.19 c | 4.30 ± 0.06 ab | 3.96 ± 0.16 bc | 4.32 ± 0.42 a |
| Total detected hydroxybenzoic acids | 18.39 ± 0.31 d | 44.78 ± 0.69 b | 44.39 ± 0.98 b | 42.35 ± 1.66 c | 54.12 ± 0.94 a | 52.85 ± 1.07 a |
| cis-Caftaric acid | 0.46 ± 0.01 ab | 0.47 ± 0.02 a | 0.48 ± 0.04 a | 0.43 ± 0.01 bc | 0.40 ± 0.02 c | 0.45 ± 0.02 ab |
| trans-Caftaric acid | 39.94 ± 1.66 d | 46.95 ± 0.22 c | 46.86 ± 0.62 c | 67.18 ± 1.78 a | 39.33 ± 0.39 d | 60.55 ± 0.53 b |
| Caffeic acid | 1.70 ± 0.04 c | 2.39 ± 0.04 a | 2.18 ± 0.05 b | 1.63 ± 0.10 c | 2.42 ± 0.08 a | 1.46 ± 0.08 d |
| p-Coumaric acid | 1.11 ± 0.01 c | 0.88 ± 0.03 c | 1.49 ± 0.06 b | 0.48 ± 0.08 d | 2.13 ± 0.07 a | 0.51 ± 0.03 d |
| Ferulic acid | 1.40 ± 0.02 e | 2.06 ± 0.02 c | 1.80 ± 0.03 d | 4.15 ± 0.18 a | 1.14 ± 0.11 f | 2.68 ± 0.08 b |
| Total detected hydroxycinnamic acids | 44.60 ± 1.69 d | 52.75 ± 0.22 c | 52.80 ± 0.71 c | 73.88 ± 1.98 a | 45.41 ± 0.49 d | 65.65 ± 0.60 b |
| Flavonols | ||||||
| Quercetin 3-glucoside + Quercetin 3-glucuronide | 3.78 ± 0.29 e | 6.46 ± 0.21 c | 4.99 ± 0.17 d | 13.21 ± 0.64 a | 2.65 ± 0.10 f | 8.74 ± 0.15 b |
| Myricetin | 1.54 ± 0.34 ab | 2.14 ± 0.59 a | 1.52 ± 0.19 ab | 1.32 ± 0.30 b | 1.20 ± 0.26 b | 1.08 ± 0.31 b |
| Quercetin | 9.19 ± 0.48 cd | 11.48 ± 1.47 a | 10.06 ± 0.47 bc | 11.01 ± 0.61 ab | 8.33 ± 0.84 d | 9.60 ± 0.37 bcd |
| Total detected flavonols | 14.52 ± 0.67 d | 20.08 ± 1.97 b | 16.57 ± 0.81 c | 25.54 ± 1.13 a | 12.18 ± 1.12 e | 19.41 ± 0.52 b |
| Flavan-3-ols | ||||||
| Procyanidin B1 | 8.97 ± 0.35 f | 22.1 ± 0.64 d | 20.95 ± 0.60 e | 27.46 ± 0.72 b | 25.45 ± 0.24 c | 30.36 ± 0.45 a |
| Procyanidin B3 | 2.21 ± 0.03 e | 7.26 ± 0.17 b | 5.58 ± 0.28 d | 8.99 ± 0.22 c | 8.83 ± 0.43 c | 10.3 ± 0.61 a |
| (+)-Catechin | 11.84 ± 0.45 e | 27.06 ± 0.41 d | 27.98 ± 0.51 d | 33.56 ± 1.12 c | 37.98 ± 0.70 b | 41.07 ± 0.91 a |
| Procyanidin B2 | 5.11 ± 0.16 f | 15.91 ± 0.59 e | 16.77 ± 0.31 d | 18.06 ± 0.43 c | 21.45 ± 0.26 b | 25.27 ± 0.29 a |
| (−)-Epicatechin | 3.71 ± 0.09 f | 11.47 ± 0.28 e | 12.79 ± 0.38 d | 14.33 ± 0.45 c | 20.34 ± 0.27 b | 21.04 ± 0.39 a |
| Procyanidin C1 | 0.90 ± 0.03 f | 3.45 ± 0.04 d | 3.14 ± 0.02 e | 3.92 ± 0.01 c | 4.56 ± 0.13 b | 5.47 ± 0.03 a |
| Total detected flavan-3-ols | 32.74 ± 1.01 e | 87.24 ± 1.89 d | 87.20 ± 2.03 d | 106.32 ± 2.85 c | 118.61 ± 1.62 b | 133.51 ± 2.42 a |
| Stilbenes | ||||||
| trans-Piceid | 10.48 ± 0.17 e | 12.94 ± 0.55 c | 12.65 ± 0.46 d | 19.51 ± 0.18 a | 9.31 ± 0.47 f | 16.68 ± 0.06 b |
| Piceatannol | 0.60 ± 0.08 bc | 0.58 ± 0.07 c | 0.81 ± 0.05 a | 0.68 ± 0.04 b | 0.69 ± 0.02 b | 0.47 ± 0.02 d |
| trans-Resveratrol | 1.12 ± 0.03 c | 1.83 ± 0.36 ab | 1.46 ± 0.18 bc | 2.20 ± 0.16 a | 1.54 ± 0.35 b | 2.00 ± 0.1 a |
| cis-Piceid | 5.74 ± 0.12 bc | 5.74 ± 2.52 bc | 6.38 ± 0.16 b | 9.10 ± 0.06 a | 4.27 ± 0.01 c | 8.22 ± 0.13 a |
| Total detected stilbenes | 17.95 ± 0.25 d | 21.09 ± 2.19 c | 21.30 ± 0.81 c | 31.49 ± 0.33 a | 15.82 ± 0.50 e | 27.38 ± 0.09 b |
| Total detected phenolic compounds | 163.23 ± 4.13 e | 266.54 ± 0.76 d | 261.48 ± 5.56 d | 321.17 ± 6.32 b | 287.87 ± 3.45 c | 341.20 ± 4.70 a |
| Macroelements | Treatments | |||||
|---|---|---|---|---|---|---|
| K7 | CS15 | C15 | H15 | C30 | H30 | |
| K | 822.50 ± 54.89 a | 736.50 ± 43.02 b | 785.33 ± 51.75 a | 770.83 ± 50.51 a | 794.00 ± 28.25 a | 786 ± 28.51 a |
| Ca | 129.50 ± 7.05 b | 115.83 ± 6.53 c | 127.33 ± 4.86 b | 132.50 ± 6.56 ab | 129.50 ± 4.00 b | 140.33 ± 4.37 a |
| Mg | 78.30 ± 1.00 c | 78.75 ± 1.13 c | 83.07 ± 1.35 b | 83.42 ± 1.21 ab | 85.22 ± 1.08 a | 84.65 ± 1.08 ab |
| Na | 8.27 ± 0.77 a | 7.95 ± 0.71 a | 7.51 ± 0.69 a | 7.79 ± 0.74 a | 7.62 ± 0.61 a | 7.89 ± 0.53 a |
| Total macroelements | 1038.6 ± 63.59 a | 939.03 ± 50.21 b | 1003.3 ± 58.53 ab | 994.54 ± 58.94 ab | 1016.3 ± 32.17 ab | 1018.9 ± 34.35 ab |
| Microelements | ||||||
| Al | 0.38 ± 0.06 b | 0.26 ± 0.03 c | 0.32 ± 0.04 bc | 0.65 ± 0.10 a | 0.30 ± 0.02 bc | 0.62 ± 0.06 a |
| Cu | 0.03 ± 0.01 a | 0.02 ± 0.01 b | 0.01 ± 0.01 e | 0.02 ± 0.01 c | 0.02 ± 0.01 d | 0.02 ± 0.01 b |
| Fe | 2.49 ± 0.03 e | 2.01 ± 0.03 f | 2.88 ± 0.03 d | 4.69 ± 0.02 b | 2.98 ± 0.01 c | 4.75 ± 0.02 a |
| Mn | 0.97 ± 0.06 a | 0.80 ± 0.07 b | 0.90 ± 0.06 ab | 0.98 ± 0.08 a | 0.90 ± 0.07 ab | 0.98 ± 0.08 a |
| Total microelements | 3.86 ± 0.12 c | 3.09 ± 0.10 d | 4.12 ± 0.08 b | 6.33 ± 0.15 a | 4.19 ± 0.08 b | 6.37 ± 0.16 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orbanić, F.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Plavša, T.; Bubola, M.; Lukić, I.; Jeromel, A.; et al. Applying Different Vinification Techniques in Teran Red Wine Production: Impact on Bioactive Compounds and Sensory Attributes. Foods 2023, 12, 3838. https://doi.org/10.3390/foods12203838
Orbanić F, Rossi S, Bestulić E, Budić-Leto I, Kovačević Ganić K, Horvat I, Plavša T, Bubola M, Lukić I, Jeromel A, et al. Applying Different Vinification Techniques in Teran Red Wine Production: Impact on Bioactive Compounds and Sensory Attributes. Foods. 2023; 12(20):3838. https://doi.org/10.3390/foods12203838
Chicago/Turabian StyleOrbanić, Fumica, Sara Rossi, Ena Bestulić, Irena Budić-Leto, Karin Kovačević Ganić, Ivana Horvat, Tomislav Plavša, Marijan Bubola, Igor Lukić, Ana Jeromel, and et al. 2023. "Applying Different Vinification Techniques in Teran Red Wine Production: Impact on Bioactive Compounds and Sensory Attributes" Foods 12, no. 20: 3838. https://doi.org/10.3390/foods12203838
APA StyleOrbanić, F., Rossi, S., Bestulić, E., Budić-Leto, I., Kovačević Ganić, K., Horvat, I., Plavša, T., Bubola, M., Lukić, I., Jeromel, A., & Radeka, S. (2023). Applying Different Vinification Techniques in Teran Red Wine Production: Impact on Bioactive Compounds and Sensory Attributes. Foods, 12(20), 3838. https://doi.org/10.3390/foods12203838











