Biofortification of Broccoli Microgreens (Brassica oleracea var. italica) with Glucosinolates, Zinc, and Iron through the Combined Application of Bio- and Nanofertilizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
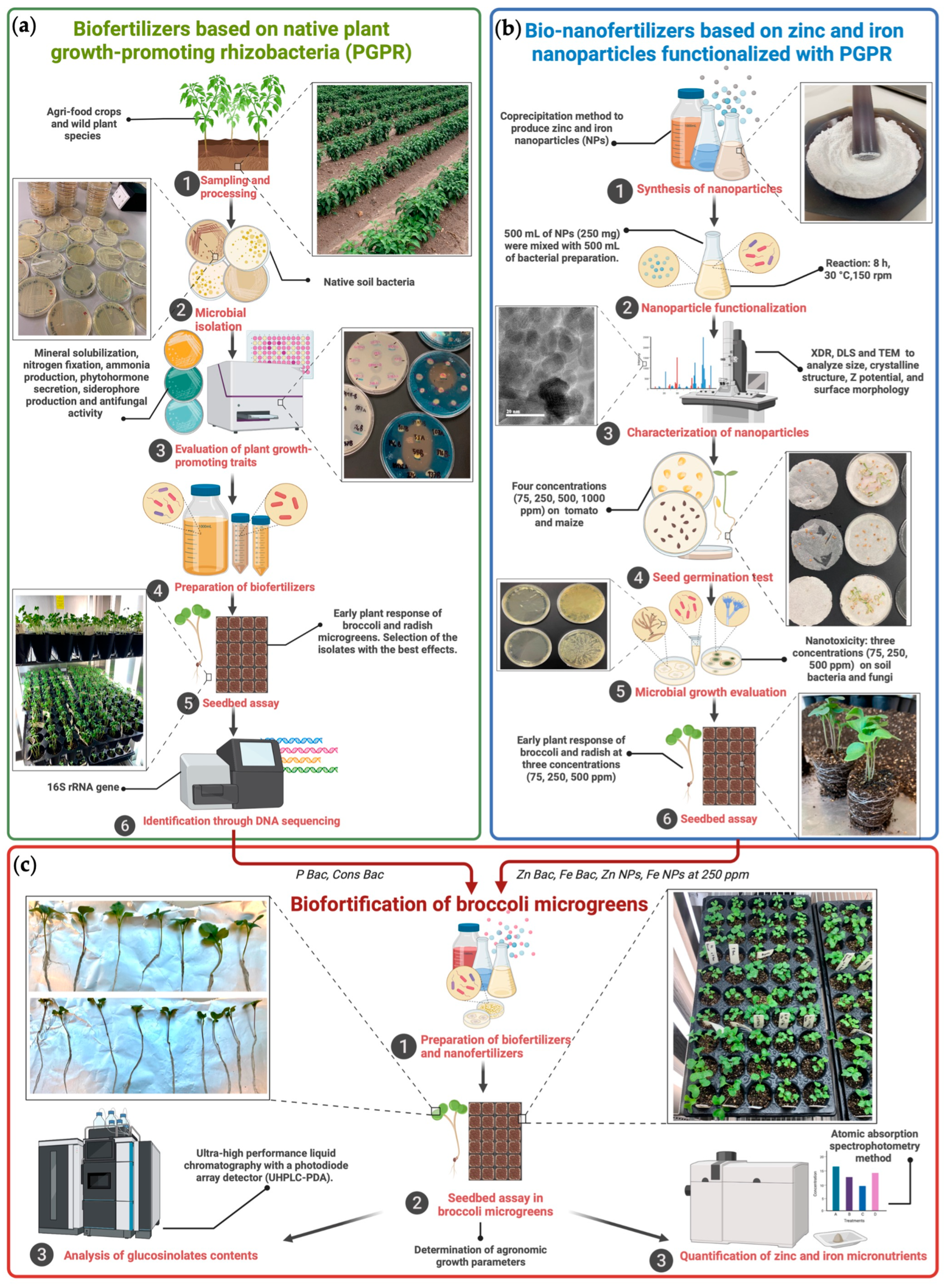
2.2. Formulation of Biofertilizers Based on Native Plant Growth-Promoting Microorganisms
2.3. Preparation of Zinc and Iron Nanofertilizer
2.4. Evaluation of Bio- and Nanoformulations in Broccoli Microgreens
2.4.1. Determination of Plant Growth Parameters
2.4.2. Analysis of Glucosinolates Contents
2.4.3. Quantification of Zinc and Iron Micronutrients
2.4.4. Statistical Analysis
3. Results
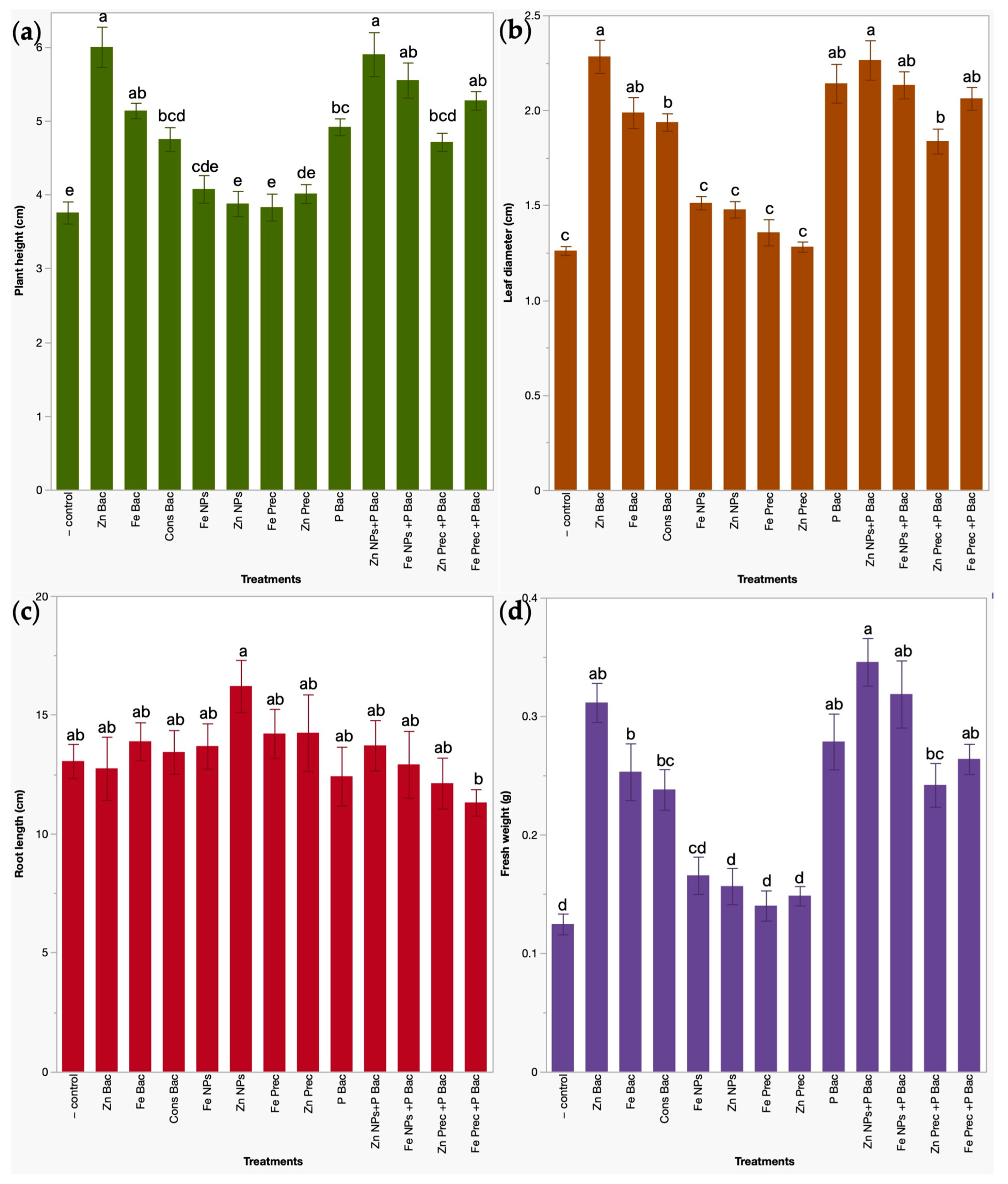
3.1. Effects of Bio- and Nanoformulations on the Growth of Broccoli Microgreens
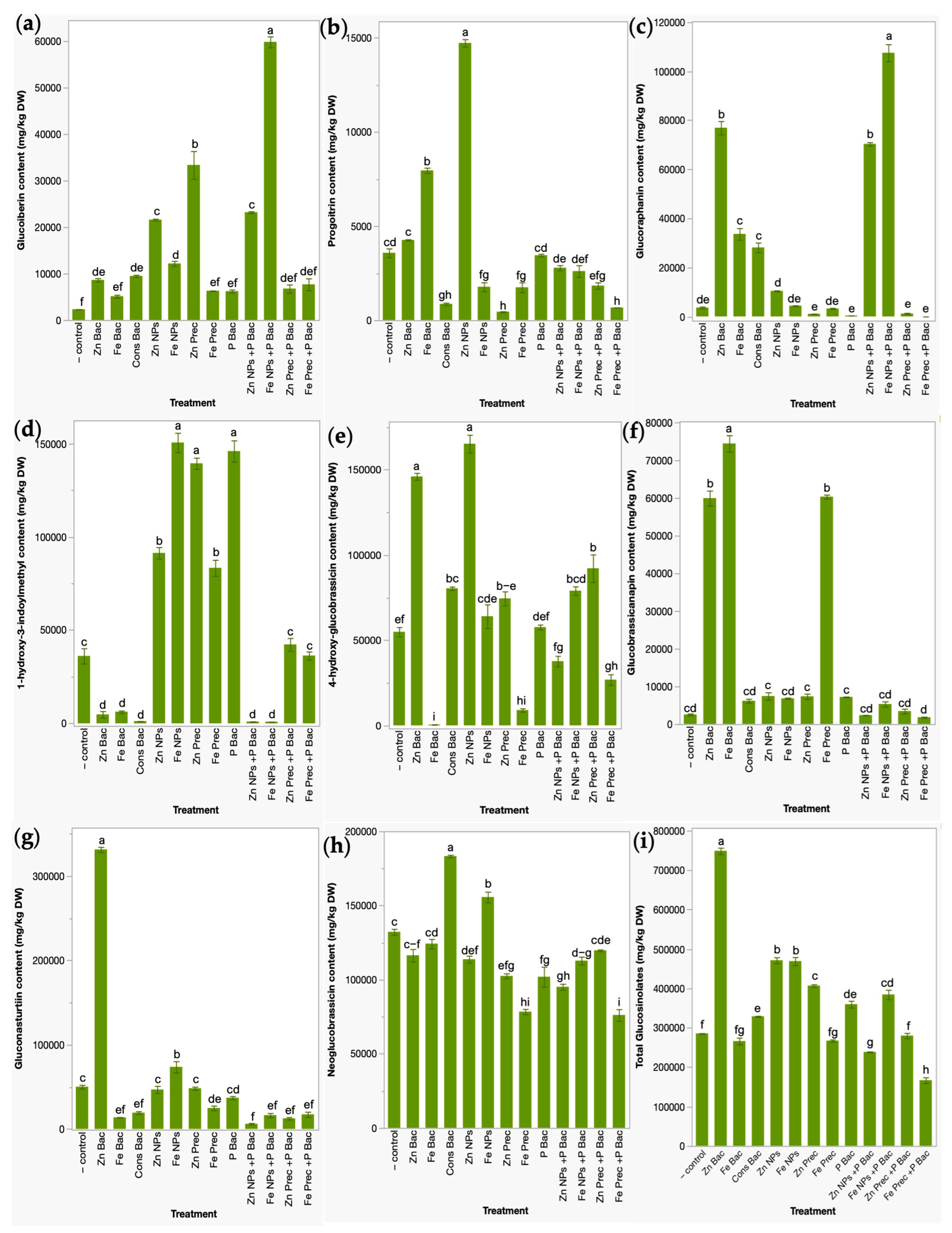
3.2. Glucosinolates Profile in Broccoli Microgreens after the Application of Bio- and Nanofertilizers
3.3. Effects on the Concentration of Zinc and Iron Micronutrients in Broccoli Microgreens
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guardiola-Márquez, C.E.; Santos-Ramírez, M.T.; Segura-Jiménez, M.E.; Figueroa-Montes, M.L.; Jacobo-Velázquez, D.A. Fighting obesity-related micronutrient deficiencies through biofortification of agri-food crops with sustainable fertilization practices. Plants 2022, 11, 3477. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Márquez, C.E.; Figueroa-Montes, M.L.; Pacheco Moscoa, A.; Senés-Guerrero, C. Native microbial consortia improve maize shoot and root systems at early developmental stages in a seedbed assay. Sci. Fungorum 2021, 51, e1329. [Google Scholar] [CrossRef]
- Hesham, A.E.-L.; Kaur, T.; Devi, R.; Kour, D.; Prasad, S.; Yadav, N.; Singh, C.; Singh, J.; Yadav, A.N. Current trends in microbial biotechnology for agricultural sustainability: Conclusion and future challenges. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Environmental and Microbial Biotechnology; Yadav, A.N., Singh, J., Singh, C., Yadav, N., Eds.; Springer: Singapore, 2021; pp. 555–572. [Google Scholar] [CrossRef]
- Acharya, A.; Pal, P.K. Agriculture nanotechnology: Translating research outcome to field applications by influencing environmental sustainability. NanoImpact 2020, 19, 100232. [Google Scholar] [CrossRef]
- Rabadán-Chávez, G.; de la Garza, R.I.D.; Jacobo-Velázquez, D.A. White adipose tissue: Distribution, molecular insights of impaired expandability, and its implication in fatty liver disease. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166853. [Google Scholar] [CrossRef] [PubMed]
- Assunção, A.G.; Cakmak, I.; Clemens, S.; González-Guerrero, M.; Nawrocki, A.; Thomine, S. Micronutrient homeostasis in plants for more sustainable agriculture and healthier human nutrition. J. Exp. Bot. 2022, 73, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Kathi, S.; Laza, H.; Singh, S.; Thompson, L.; Li, W.; Simpson, C. Vitamin C biofortification of broccoli microgreens and resulting effects on nutrient composition. Front. Plant Sci. 2023, 14, 1145992. [Google Scholar] [CrossRef]
- Praharaj, S.; Skalicky, M.; Maitra, S.; Bhadra, P.; Shankar, T.; Brestic, M.; Hejnak, V.; Vachova, P.; Hossain, A. Zinc biofortification in food crops could alleviate the zinc malnutrition in human health. Molecules 2021, 26, 3509. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Ma, S.; Tian, S.; Sun, J.; Pang, X.; Hu, Q.; Li, X.; Lu, Y. Broccoli microgreens have hypoglycemic effect by improving blood lipid and inflammatory factors while modulating gut microbiota in mice with type 2 diabetes. J. Food Biochem. 2022, 46, e14145. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential effects of low light intensity on broccoli microgreens growth and phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
- Abdalla, Z.F.; El-Bassiony, A.E.M.; El-Ramady, H.; El-Sawy, S.; Shedeed, S. Broccoli biofortification using biological nano-and mineral fertilizers of selenium: A comparative study under soil nutrient deficiency stress. Egypt. J. Soil Sci. 2023, 63, 57–66. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Li, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Combination of selenium and UVA radiation affects growth and phytochemicals of broccoli microgreens. Molecules 2021, 26, 4646. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of Brassicaceae: Genetic diversity of phytochemical concentrations and antioxidant capacity. LWT 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of glucosinolate profiles in different tissues of nine Brassica crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef]
- Li, X.; Tian, S.; Wang, Y.; Liu, J.; Wang, J.; Lu, Y. Broccoli microgreens juice reduces body weight by enhancing insulin sensitivity and modulating gut microbiota in high-fat diet-induced C57BL/6J obese mice. Eur. J. Nutr. 2021, 60, 3829–3839. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Xiao, Z.; Yu, L.; Pham, Q.; Sun, J.; Wang, T.T. Red cabbage microgreens lower circulating low-density lipoprotein (LDL), liver cholesterol, and inflammatory cytokines in mice fed a high-fat diet. J. Agric. Food Chem. 2016, 64, 9161–9171. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Rhee, J.; Choi, C.S.; Jo, J.S.; Shin, Y.K.; Lee, J.G. Profiling of individual desulfo-glucosinolate content in cabbage head (Brassica oleracea var. capitata) germplasm. Molecules 2020, 25, 1860. [Google Scholar] [CrossRef]
- Yang, R.; Guo, L.; Jin, X.; Shen, C.; Zhou, Y.; Gu, Z. Enhancement of glucosinolate and sulforaphane formation of broccoli sprouts by zinc sulphate via its stress effect. J. Funct. Foods. 2015, 13, 345–349. [Google Scholar] [CrossRef]
- Bojorquez-Rodríguez, E.M.; Guajardo-Flores, D.; Jacobo-Velázquez, D.A.; Serna-Saldívar, S.O. Evaluation of the effects of process conditions on the extraction of glucosinolates from broccoli sprouts. Horticulturae 2022, 8, 1090. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A. Definition of biofortification revisited. ACS Food Sci. Technol. 2022, 2, 782–783. [Google Scholar] [CrossRef]
- Jaiswal, D.K.; Krishna, R.; Chouhan, G.K.; de Araujo Pereira, A.P.; Ade, A.B.; Prakash, S.; Verma, J.P. Bio-fortification of minerals in crops: Current scenario and future prospects for sustainable agriculture and human health. J. Plant Growth Regul. 2022, 98, 5–22. [Google Scholar] [CrossRef]
- Koç, E.; Karayiğit, B. Assessment of biofortification approaches used to improve micronutrient-dense plants that are a sustainable solution to combat hidden hunger. J. Soil Sci. Plant Nutr. 2022, 22, 475–500. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Guardiola-Márquez, C.E.; Santos-Ramírez, M.T.; Figueroa-Montes, M.L.; Valencia-de los Cobos, E.O.; Stamatis-Félix, I.J.; Navarro-López, D.E.; Jacobo-Velázquez, D.A. Identification and characterization of beneficial soil microbial strains for the formulation of biofertilizers based on native plant growth-promoting microorganisms isolated from northern Mexico. Plants 2023, 12, 3262. [Google Scholar] [CrossRef]
- Guardiola-Márquez, C.E.; Jacobo-Velázquez, D.A. Potential of enhancing anti-obesogenic agriceuticals by applying sustainable fertilizers during plant cultivation. Front. Sustain. Food Syst. 2022, 6, 1034521. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; El-Banna, A.A.A.; Salama, E.A.A.; Ali, M.M.; Al-Huqail, A.A.; Ali, H.M.; Paszt, L.S.; El-Sorady, G.A.; Lamlom, S.F. The individual and combined effect of nanoparticles and biofertilizers on growth, yield, and biochemical attributes of peanuts (Arachis hypogea L.). Agronomy 2022, 12, 398. [Google Scholar] [CrossRef]
- El-Ramady, H.; Prokisch, J.; El-Baily, S.; Elasawi, T.H.; Elmahrouk, M.; Omara, A.E.D.; Elsakhawy, T.A.; Amer, M.M.; Brevik, E. Biological nanofertilizer for horticultural crops: A diagrammatic mini-review. Env. Biodivers. Soil Secur. 2022, 6, 339–348. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Cantu, J.; Tamez, C.; Zuverza-Mena, N.; Hamdi, H.; Adisa, I.O.; Elmer, W.; Gardea-Torresdey, J.; White, J.C. Seed biofortification by engineered nanomaterials: A pathway to alleviate malnutrition? J. Agric. Food Chem. 2020, 68, 12189–12202. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Joshi, A.; Rajput, V.D.; Singh, M.; Sharma, A.; Singh, R.K.; Li, D.M.; Arora, J.; Minkina, T.; et al. Nanofertilizer possibilities for healthy soil, water, and food in future: An overview. Front. Plant Sci. 2022, 13, 865048. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Singh, K.T.T.; Raliya, R. Nanofertilizers and their role in sustainable agriculture. Ann. Plant Soil Res. 2021, 23, 238–255. [Google Scholar] [CrossRef]
- Umar, W.; Hameed, M.K.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Rasheed, N. Synthesis, characterization and application of ZnO nanoparticles for improved growth and Zn biofortification in maize. Arch. Agron. Soil Sci. 2021, 67, 1164–1176. [Google Scholar] [CrossRef]
- Eissa, N.H.; Zayed, M.S.; Hassanein, M.K.; Abdallah, M.M.F. Green pea sprout response to microbial inoculation and increasing atmospheric CO2 concentration. Arab. Univ. J. Agric. Sci. 2018, 26, 2513–2523. [Google Scholar] [CrossRef]
- Briatia, X.; Jomduang, S.; Park, C.H.; Lumyong, S.; Kanpiengjai, A.; Khanongnuch, C. Enhancing growth of buckwheat sprouts and microgreens by endophytic bacterium inoculation. Int. J. Agric. Biol. 2017, 19, 374–380. [Google Scholar] [CrossRef]
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef]
- Bacilio, M.; Moreno, M.; Lopez-Aguilar, D.R.; Bashan, Y. Scaling from the growth chamber to the greenhouse to the field: Demonstration of diminishing effects of mitigation of salinity in peppers inoculated with plant growth-promoting bacterium and humic acids. Appl. Soil Ecol. 2017, 119, 327–338. [Google Scholar] [CrossRef]
- Guardiola-Márquez, C.E.; López-Mena, E.R.; Segura-Jiménez, M.E.; Gutierrez-Marmolejo, I.; Flores-Matzumiya, M.A.; Mora-Godínez, S.; Hernández-Brenes, C.; Jacobo-Velázquez, D.A. Development and evaluation of zinc and iron nanoparticles functionalized with plant growth-promoting rhizobacteria (PGPR) and microalgae for their application as bio-nanofertilizers. Plants 2023. Under Review. [Google Scholar]
- Jiménez-Gómez, A.; Flores-Félix, J.D.; García-Fraile, P.; Mateos, P.F.; Menéndez, E.; Velázquez, E.; Rivas, R. Probiotic activities of Rhizobium laguerreae on growth and quality of spinach. Sci. Rep. 2018, 8, 295. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Zepeda-Hernández, A.; Uribe-Velázquez, T.; Rosales-De la Cruz, M.F.; Raygoza-Murguía, L.V.; Garcia-Amezquita, L.E.; García-Cayuela, T. Utilization of blueberry-based ingredients for formulating a synbiotic Petit Suisse cheese: Physicochemical, microbiological, sensory, and functional characterization during cold storage. LWT 2023, 183, 114955. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10, 2858. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Hernández, E.; Acevedo-Pacheco, L.; Jacobo-Velázquez, D.A.; Antunes-Ricardo, M. Bioaccessibility and potential biological activities of lutein, glucosinolates, and phenolic compounds accumulated in kale sprouts treated with selenium, sulfur, and methyl jasmonate. ACS Food Sci. Technol. 2023, 3, 404–413. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef]
- Chiriac, E.R.; Chiţescu, C.L.; Sandru, C.; Geană, E.-I.; Lupoae, M.; Dobre, M.; Borda, D.; Gird, C.E.; Boscencu, R. Comparative study of the bioactive properties and elemental composition of red clover (Trifolium pratense) and alfalfa (Medicago sativa) sprouts during germination. Appl. Sci. 2020, 10, 7249. [Google Scholar] [CrossRef]
- Awan, S.; Shahzadi, K.; Javad, S.; Tariq, A.; Ahmad, A.; Ilyas, S. A preliminary study of influence of zinc oxide nanoparticles on growth parameters of Brassica oleracea var italic. J. Saudi Soc. Agric. Sci. 2021, 20, 18–24. [Google Scholar] [CrossRef]
- Shakir, W.M.; AL-Mahdawe, M.M.I.; Hammadi, M. Effect of ZnO nanoparticles on the content of sulforaphane in broccoli plant. NeuroQuantology 2022, 20, 361–367. [Google Scholar] [CrossRef]
- Al-Tameemi, A.J.H.; Al-Aloosy, Y.A.M.; Al-Saedi, N.J. Effect of spraying chelated and nano of both iron and zinc on the growth and yield of broccoli (Brassica oleracea var. italica). Plant Arch. 2019, 19, 1783–1790. [Google Scholar]
- Farhan, K.J.; Mahdi, L.E.; Al-Falahi, M.N.A.; Sallume, M.O.; Alkhateb, B.A. The role of iron nanoparticles and humic acid in iron concentration, growth and yield of broccoli (Brassica oleracea var Italic). IOP Conf. Ser. Earth Environ. Sci. 2023, 1158, 022033. [Google Scholar] [CrossRef]
- Elabd, H.S. Effect of nano-fertilization and some bio-fertilizer on growth, yield and fiber quality of Egyptian cotton. Ann. Agric. Sci. Moshtohor. 2020, 58, 35–44. [Google Scholar] [CrossRef]
- Seyed Sharifi, R.; Khalilzadeh, R.; Pirzad, A.; Anwar, S. Effects of biofertilizers and nano zinc-iron oxide on yield and physicochemical properties of wheat under water deficit conditions. Commun. Soil Sci. Plant Anal. 2020, 51, 2511–2524. [Google Scholar] [CrossRef]
- Singhal, U.; Khanuja, M.; Prasad, R.; Varma, A. Impact of synergistic association of ZnO-nanorods and symbiotic fungus Piriformospora indica DSM 11827 on Brassica oleracea var. botrytis (Broccoli). Front Microbiol. 2017, 8, 1909. [Google Scholar] [CrossRef] [PubMed]
- Kitainda, V.; Jez, J.M. Structural studies of aliphatic glucosinolate chain-elongation enzymes. Antioxidants 2021, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Jiráček, V.; Kutáček, M.; Salkade, S.; Koštíř, J. Effect of zinc on the biosynthesis of indole glucosinolates glucobrassicin and neoglucobrassicin in etiolated seedlings of rape (Brassica napus var. arvensis (Lam.) Thell). Biol. Plant. 1974, 16, 454–461. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Bączek-Kwinta, R.; Bartoszek, A.; Piekarska, A.; Huk, A.; Manikowska, A.; Konieczka, P. The dose-dependent influence of zinc and cadmium contamination of soil on their uptake and glucosinolate content in white cabbage (Brassica oleracea var. capitata f. alba). Environ. Toxicol. Chem. 2012, 31, 2482–2489. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef]
- Schreiner, M.; Krumbein, A.; Ruppel, S. Interaction between plants and bacteria: Glucosinolates and phyllospheric colonization of cruciferous vegetables by Enterobacter radicincitans DSM 16656. Microb. Physiol. 2009, 17, 124–135. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, J.; Lu, X.; Zhou, C. Development of plant systemic resistance by beneficial rhizobacteria: Recognition, initiation, elicitation and regulation. Front. Plant Sci. 2022, 13, 952397. [Google Scholar] [CrossRef]
- Messa, V.R. Biocontrol by induced systemic resistance using plant growth promoting rhizobacteria. Rhizosphere 2021, 17, 100323. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Behera, S.K.; Singh, P.; Alotaibi, S.S.; Gaber, A.; Hossain, A. Comparative efficiency of mineral, chelated and nano forms of zinc and iron for improvement of zinc and iron in chickpea (Cicer arietinum L.) through biofortification. Agronomy 2021, 11, 2436. [Google Scholar] [CrossRef]
- Yang, G.; Yuan, H.; Ji, H.; Liu, H.; Zhang, Y.; Wang, G.; Guo, Z. Effect of ZnO nanoparticles on the productivity, Zn biofortification, and nutritional quality of rice in a life cycle study. Plant Physiol. Biochem. 2021, 163, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sundariae, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed priming with iron oxide nanoparticles triggers iron acquisition and biofortification in wheat (Triticum aestivum L.) grains. J. Plant Growth Regul. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, S.; Luo, L.; Zhang, J.; Yang, K.; Christie, P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ. Sci. Nano 2015, 2, 68–77. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Manag. 2011, 13, 822–828. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano. 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Seshadri, B.; Bolan, N.S.; Naidu, R. Rhizosphere-induced heavy metal(loid) transformation in relation to bioavailability and remediation. J. Soil Sci. Plant Nutr. 2015, 15, 524–548. [Google Scholar] [CrossRef]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, translocation, and transformation of silver nanoparticles in plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Boutchuen, A.; Zimmerman, D.; Aich, N.; Masud, A.M.; Arabshahi, A.; Palchoudhury, S. Increased plant growth with hematite nanoparticle fertilizer drop and determining nanoparticle uptake in plants using multimodal approach. J. Nanomater. 2019, 2019, e6890572. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The future of food security and food safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Srivastava, R.; Pandiyan, K.; Singh, A.; Chakdar, H.; Kashyap, P.L.; Bhardwaj, A.K.; Murugan, K.; Karthikeyan, N.; Bagul, S.Y.; et al. Enhancement in plant growth and zinc biofortification of chickpea (Cicer arietinum L.) by Bacillus altitudinis. J. Soil Sci. Plant Nutr. 2021, 21, 922–935. [Google Scholar] [CrossRef]
- Harmanescu, M.; Alda, L.M.; Bordean, D.M.; Gogoasa, I.; Gergen, I. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem. Cent. J. 2011, 5, 64. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guardiola-Márquez, C.E.; García-Sánchez, C.V.; Sánchez-Arellano, Ó.A.; Bojorquez-Rodríguez, E.M.; Jacobo-Velázquez, D.A. Biofortification of Broccoli Microgreens (Brassica oleracea var. italica) with Glucosinolates, Zinc, and Iron through the Combined Application of Bio- and Nanofertilizers. Foods 2023, 12, 3826. https://doi.org/10.3390/foods12203826
Guardiola-Márquez CE, García-Sánchez CV, Sánchez-Arellano ÓA, Bojorquez-Rodríguez EM, Jacobo-Velázquez DA. Biofortification of Broccoli Microgreens (Brassica oleracea var. italica) with Glucosinolates, Zinc, and Iron through the Combined Application of Bio- and Nanofertilizers. Foods. 2023; 12(20):3826. https://doi.org/10.3390/foods12203826
Chicago/Turabian StyleGuardiola-Márquez, Carlos Esteban, C. Valentina García-Sánchez, Óscar Armando Sánchez-Arellano, Erika Melissa Bojorquez-Rodríguez, and Daniel A. Jacobo-Velázquez. 2023. "Biofortification of Broccoli Microgreens (Brassica oleracea var. italica) with Glucosinolates, Zinc, and Iron through the Combined Application of Bio- and Nanofertilizers" Foods 12, no. 20: 3826. https://doi.org/10.3390/foods12203826
APA StyleGuardiola-Márquez, C. E., García-Sánchez, C. V., Sánchez-Arellano, Ó. A., Bojorquez-Rodríguez, E. M., & Jacobo-Velázquez, D. A. (2023). Biofortification of Broccoli Microgreens (Brassica oleracea var. italica) with Glucosinolates, Zinc, and Iron through the Combined Application of Bio- and Nanofertilizers. Foods, 12(20), 3826. https://doi.org/10.3390/foods12203826






