Food-Dependent Exercise-Induced Anaphylaxis: A Distinct Form of Food Allergy—An Updated Review of Diagnostic Approaches and Treatments
Abstract
1. Introduction
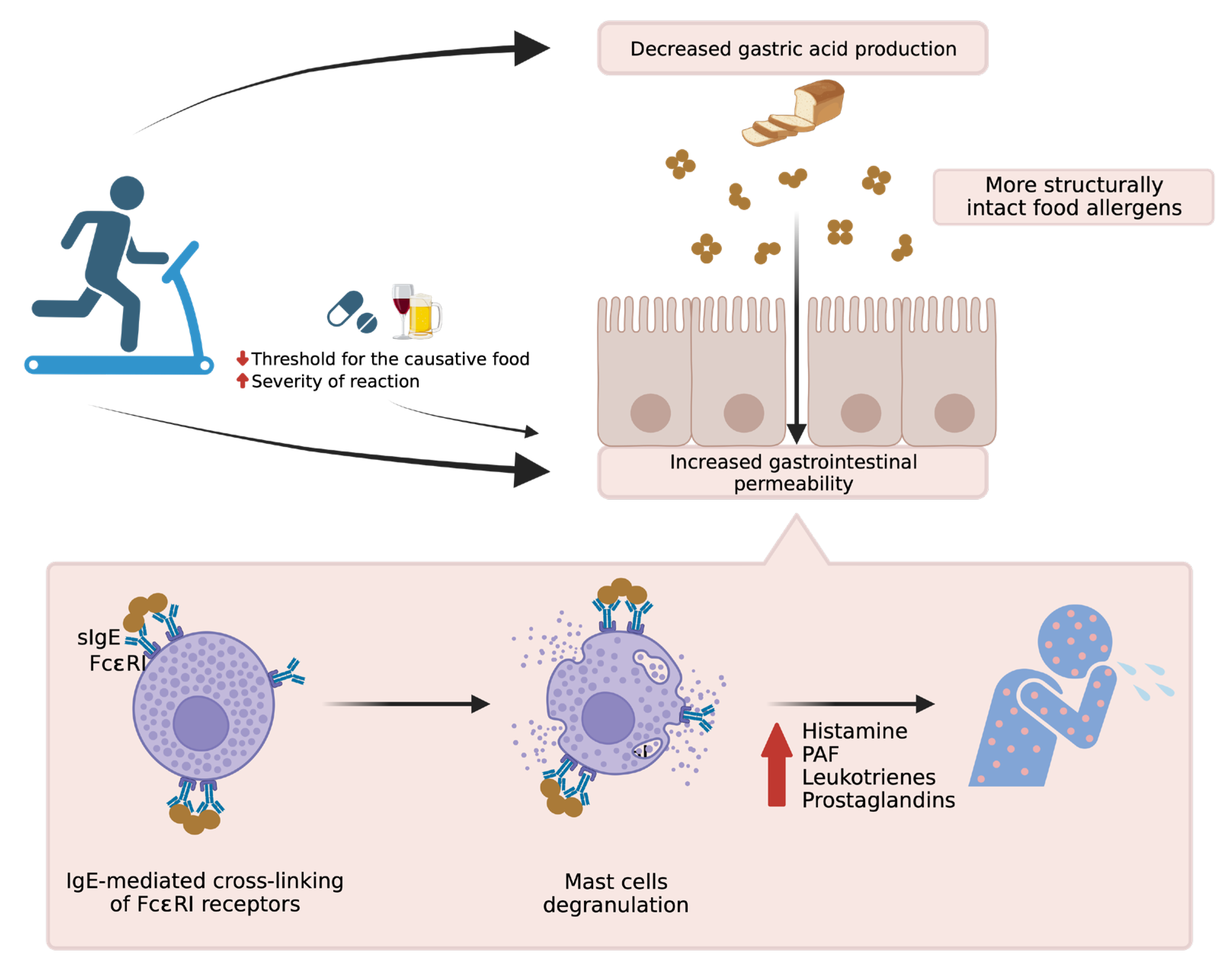
2. The Importance of Cofactors
3. Atopic Diseases and Related Comorbidities of FDEIA
4. Diagnosis of FDEIA
4.1. Clinical Diagnosis
4.2. In Vivo and In Vitro Tests
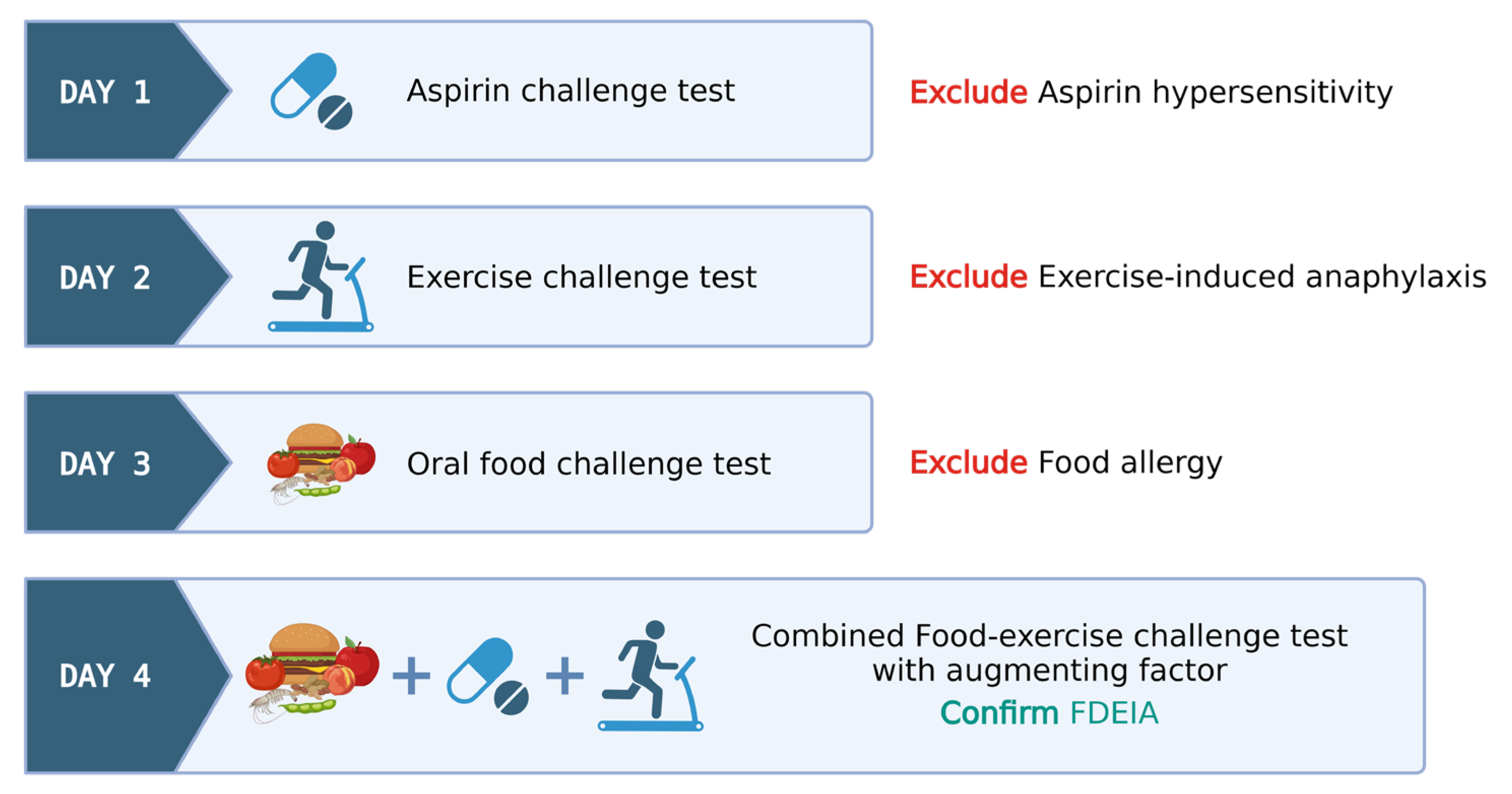
4.3. Food–Exercise Cofactor Challenge Test
- Day 1: ASA provocation test, cumulative 421.5 mg, observed for 6 h to rule out ASA hypersensitivity.
- Day 2: Exercise challenge at 27–30 °C ambient temperature. Treadmill exercise adjusted for target heart rate (>80% max HR) for 15 min. Test ceased at any positive reaction or gradually tapered if negative.
- Day 3: Wheat challenge using common wheat source (Farmhouse® bread, Thailand)—incremental dosing up to five slices observed for 6 h.
- Day 4: Combined wheat–exercise–ASA challenge. ASA (300 mg) followed by four slices of bread, exercise as per Day 1.
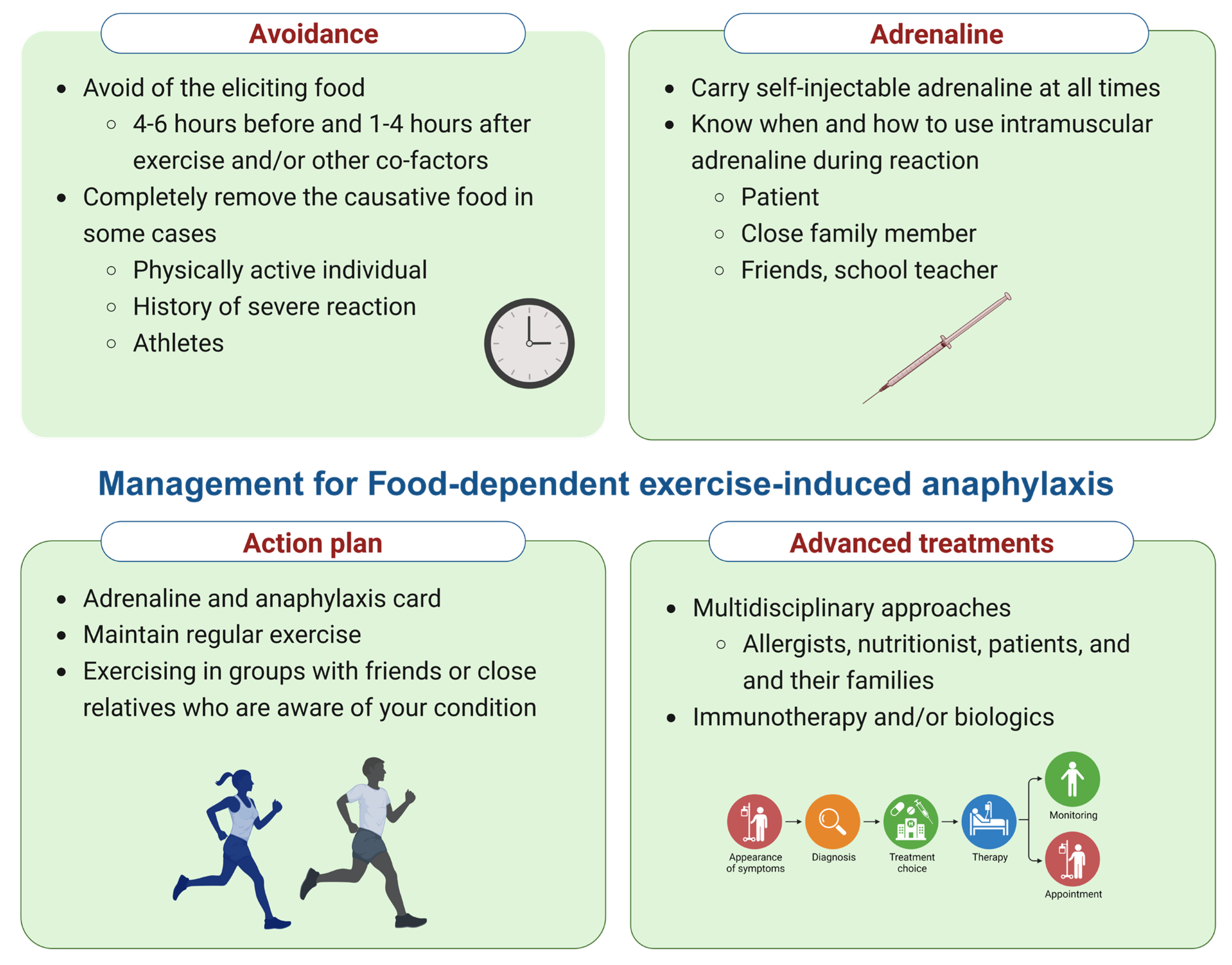
5. Management of FDEIA
6. Gaps in Knowledge and Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feldweg, A.M. Food-Dependent, Exercise-Induced Anaphylaxis: Diagnosis and Management in the Outpatient Setting. J. Allergy Clin. Immunol. Pract. 2017, 5, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Kulthanan, K.; Ungprasert, P.; Jirapongsananuruk, O.; Rujitharanawong, C.; Munprom, K.; Trakanwittayarak, S.; Pochanapan, O.; Panjapakkul, W.; Maurer, M. Food-Dependent Exercise-Induced Wheals/Angioedema, Anaphylaxis, or Both: A Systematic Review of Phenotypes. J. Allergy Clin. Immunol. Pract. 2023, 11, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Borges, M.S.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020, 13, 100472. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Food allergy: A practice parameter update-2014. J. Allergy Clin. Immunol. 2014, 134, 1016–1025.e43. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Miyazawa, M.; Osuna, H.; Tsubaki, K.; Ikebe, T.; Aihara, Y.; Ikezawa, Z. Food-dependent exercise-induced anaphylaxis: Influence of concurrent aspirin administration on skin testing and provocation. Br. J. Dermatol. 2002, 146, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.J.; Eller, E.; Mortz, C.G.; Brockow, K.; Bindslev-Jensen, C. Exercise Lowers Threshold and Increases Severity, but Wheat-Dependent, Exercise-Induced Anaphylaxis Can Be Elicited at Rest. J. Allergy Clin. Immunol. Pract. 2018, 6, 514–520. [Google Scholar] [CrossRef]
- Motomura, C.; Matsuzaki, H.; Ono, R.; Iwata, M.; Okabe, K.; Akamine, Y.; Wakatsuki, M.; Murakami, Y.; Taba, N.; Odajima, H. Aspirin is an enhancing factor for food-dependent exercise-induced anaphylaxis in children. Clin. Exp. Allergy 2017, 47, 1497–1500. [Google Scholar] [CrossRef]
- Scherf, K.A.; Lindenau, A.-C.; Valentini, L.; Collado, M.C.; García-Mantrana, I.; Christensen, M.; Tomsitz, D.; Kugler, C.; Biedermann, T.; Brockow, K. Cofactors of wheat-dependent exercise-induced anaphylaxis do not increase highly individual gliadin absorption in healthy volunteers. Clin. Transl. Allergy 2019, 9, 19. [Google Scholar] [CrossRef]
- Unhapipatpong, C.; Julanon, N.; Krikeerati, T.; Vichara-Anont, I.; Sompornrattanaphan, M. Adult IgE-mediated food allergy is on the rise: A review of phenotypes, pathophysiologic mechanisms, diagnosis, and advances in management. Asian Pac. J. Allergy Immunol. 2022, 40, 308–320. [Google Scholar]
- Srisuwatchari, W.; Sompornrattanaphan, M.; Jirapongsananuruk, O.; Visitsunthorn, N.; Pacharn, P. Exercise-food challenge test in patients with wheat-dependent exercise-induced anaphylaxis. Asian Pac. J. Allergy Immunol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Kulthanan, K.; Ungprasert, P.; Jirapongsananuruk, O.; Rujitharanawong, C.; Munprom, K.; Trakanwittayarak, S.; Pochanapan, O.; Panjapakkul, W.; Maurer, M. Food-Dependent Exercise-Induced Wheals, Angioedema, and Anaphylaxis: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2022, 10, 2280–2296. [Google Scholar] [CrossRef] [PubMed]
- Thongngarm, T.; Wongsa, C.; Pacharn, P.; Piboonpocanun, S.; Sompornrattanaphan, M. Clinical Characteristics and Proposed Wheat-Cofactor Challenge Protocol with a High Diagnostic Yield in Adult-Onset IgE-Mediated Wheat Allergy. J. Asthma Allergy 2020, 13, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Palosuo, K. Update on wheat hypersensitivity. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.J.; Eller, E.; Mortz, C.G.; Brockow, K.; Bindslev-Jensen, C. Wheat-Dependent Cofactor-Augmented Anaphylaxis: A Prospective Study of Exercise, Aspirin, and Alcohol Efficacy as Cofactors. J. Allergy Clin. Immunol. Pract. 2019, 7, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Kunie, K.; Matsuo, H. Food-dependent exercise-induced anaphylaxis. J. Dermatol. Sci. 2007, 47, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Pravettoni, V.; Incorvaia, C. Diagnosis of exercise-induced anaphylaxis: Current insights. J. Asthma Allergy 2016, 9, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Kneissl, D.; Valentini, L.; Zelger, O.; Grosber, M.; Kugler, C.; Werich, M.; Darsow, U.; Matsuo, H.; Morita, E.; et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 2015, 135, 977–984.e4. [Google Scholar] [CrossRef]
- Scherf, K.A.; Brockow, K.; Biedermann, T.; Koehler, P.; Wieser, H. Wheat-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2016, 46, 10–20. [Google Scholar] [CrossRef]
- Sompornrattanaphan, M.; Ajalasaereewong, S.; Wongsa, C.; Thongngarm, T.; Chokevittaya, P.; Vichara-Anont, I.; Sirimaskasem, K.; Surapinij, J.; Laomoleethron, J.; Wihakhaphirom, S.; et al. Prevalence and characteristics of adult patients with adult-onset and childhood-onset food allergy. Asian Pac. J. Allergy Immunol. 2023. online ahead of print. [Google Scholar]
- Lertvipapath, P.; Jameekornrak Taweechue, A.; Wongsa, C.; Thongngarm, T.; Uawattanasakul, W.; Sompornrattanaphan, M. Concomitant chronic spontaneous urticaria treatment might hinder the diagnosis of occupational latex-induced anaphylaxis: A case report. Asian Pac. J. Allergy Immunol. 2021. online ahead of print. [Google Scholar]
- Kraft, M.; Dölle-Bierke, S.; Renaudin, J.-M.; Ruëff, F.; Hofmeier, K.S.; Treudler, R.; Pföhler, C.; Hawranek, T.; Poziomkowska-Gęsicka, I.; Jappe, U.; et al. Wheat Anaphylaxis in Adults Differs from Reactions to Other Types of Food. J. Allergy Clin. Immunol. Pract. 2021, 9, 2844–2852.e5. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.J.; Eller, E.; Kjaer, H.F.; Broesby-Olsen, S.; Mortz, C.G.; Bindslev-Jensen, C. Exercise-induced anaphylaxis: Causes, consequences, and management recommendations. Expert. Rev. Clin. Immunol. 2019, 15, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Barg, W.; Medrala, W.; Wolanczyk-Medrala, A. Exercise-induced anaphylaxis: An update on diagnosis and treatment. Curr. Allergy Asthma Rep. 2011, 11, 45–51. [Google Scholar] [CrossRef]
- Foong, R.X.; Giovannini, M.; du Toit, G. Food-dependent exercise-induced anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 224–228. [Google Scholar] [CrossRef]
- Asaumi, T.; Yanagida, N.; Sato, S.; Shukuya, A.; Nishino, M.; Ebisawa, M. Provocation tests for the diagnosis of food-dependent exercise-induced anaphylaxis. Pediatr. Allergy Immunol. 2016, 27, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.; Judge, C.; Redenbaugh, V.; Awad, H.; Conlon, N. Food-dependent exercise-induced reactions: Lessons from a 15-year retrospective study. Ir. J. Med. Sci. 2019, 188, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Palosuo, K.; Alenius, H.; Varjonen, E.; Koivuluhta, M.; Mikkola, J.; Keskinen, H.; Kalkkinen, N.; Reunala, T. A novel wheat gliadin as a cause of exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 1999, 103, 912–917. [Google Scholar] [CrossRef]
- Matsuo, H.; Dahlström, J.; Tanaka, A.; Kohno, K.; Takahashi, H.; Furumura, M.; Morita, E. Sensitivity and specificity of recombinant omega-5 gliadin-specific IgE measurement for the diagnosis of wheat-dependent exercise-induced anaphylaxis. Allergy 2008, 63, 233–236. [Google Scholar] [CrossRef]
- Pacharn, P.; Jirapongsananuruk, O.; Daengsuwan, T.; Vichyanond, P.; Visitsunthorn, N. Wheat-dependent, exercise-induced anaphylaxis in Thai children: A report of 5 cases. Asian Pac. J. Allergy Immunol. 2009, 27, 115–120. [Google Scholar] [CrossRef]
- Parsons, J.P.; Hallstrand, T.S.; Mastronarde, J.G.; Kaminsky, D.A.; Rundell, K.W.; Hull, J.H.; Storms, W.W.; Weiler, J.M.; Cheek, F.M.; Wilson, K.C.; et al. An official American Thoracic Society clinical practice guideline: Exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 2013, 187, 1016–1027. [Google Scholar] [CrossRef]
- Jo, E.-J.; Yang, M.-S.; Kim, Y.-J.; Kim, H.-S.; Kim, M.-Y.; Kim, S.-H.; Cho, S.-H.; Min, K.-U.; Chang, Y.-S. Food-dependent exercise-induced anaphylaxis occurred only in a warm but not in a cold environment. Asia Pac. Allergy 2012, 2, 161–164. [Google Scholar] [CrossRef][Green Version]
- Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGalvin, A.; Garvey, L.H.; Riggioni, C.; de Silva, D.; Angier, E.; Arasi, S.; et al. EAACI guidelines: Anaphylaxis (2021 update). Allergy 2022, 77, 357–377. [Google Scholar] [CrossRef]
- Shaker, M.S.; Wallace, D.V.; Golden, D.B.K.; Oppenheimer, J.; Bernstein, J.A.; Campbell, R.L.; Dinakar, C.; Ellis, A.; Greenhawt, M.; Khan, D.A.; et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020, 145, 1082–1123. [Google Scholar] [CrossRef]
- Sompornrattanaphan, M.; Jitvanitchakul, Y.; Malainual, N.; Wongsa, C.; Jameekornrak, A.; Theankeaw, O.; Thongngarm, T. Dust mite ingestion-associated, exercise-induced anaphylaxis: A case report and literature review. Allergy Asthma Clin. Immunol. 2020, 16, 2. [Google Scholar] [CrossRef]
| Study, Country | Population | Protocol | Challenge Food | Exercise | Cofactors | Positive Challenge Rate |
|---|---|---|---|---|---|---|
| Studies in children and adolescents | ||||||
| Motomura et al. [8] 2015, Japan | 51 children | 3 days | Noodles (70 g of wheat), boiled shrimp (50 g) | 30 min of aerobic exercise followed by 6 min of anaerobic exercise | ASA 10 mg/kg/dose (Max 500 mg) | 51% |
| Asauimi et al. [26] 2016, Japan | 41 children and adolescents | 1 day | Udon noodles (200–400 g of wheat, 5.2–10.4 g of wheat protein), shrimp (40–100 g), or one whole apple | Ergometer ≥ 6 min | ASA 10 mg/kg/dose (Max 500 mg) | 49% |
| Studies in children, adolescents, and adults | ||||||
| Aihara et al. [6] 2002, Japan | 10 children, adolescents, and adults | 2–5 days | Wheat (100 g of wheat) | No detail | ASA 500 mg | 80% |
| Srisuwatchari et al., [11] 2021, Thailand | 14 children, adolescents, and adults | 3 days | Bread (60–75 g of wheat, 7.6–9.5 g of wheat protein) | Motor-driven treadmill Children and adolescents ≥ 4 min and adults ≥ 15 min | ASA Children 10 mg/kg/dose (Max 300 mg) Adults 300–381 mg | 71.4% |
| Studies in adults | ||||||
| Brockow et al. [18] 2015, Germany | 16 adults | 6 days | Baked bread (10–80 g of pure gluten flour) | 45 min of aerobic exercise followed by 8 min of anaerobic exercise | ASA 500–1000 mg and 10–30 mL of 95% ethanol | 100% |
| Christensen et al. [7] 2018 Denmark | 71 adults | 2 days | Baked bread (80 g of pure gluten flour) | 4 treadmill aerobic phases of 15 min | None | 62% |
| Christensen et al. [15] 2019, Denmark | 25 adults | 5 days | Baked gluten rolls (80 g) | Treadmill aerobic exercise for 15 min | ASA 1000 mg, 37.5% alcohol | 100% |
| Thongngarm et al., [13] 2020, Thailand | 18 adults | 3 days | Bread (60–75 g of wheat, 7.6–9.5 g of wheat protein) | Motor-driven treadmill for 15 min | ASA 300 mg | 94.4% |
| Treatment of the acute episode of FDEIA | |
| Treatment for anaphylaxis |
|
| Patient education | |
| Anaphylaxis action plan |
|
| Food avoidance |
|
| Physical activity |
|
| Underlying patient knowledge | Convey the need to take β-blockers and angiotensin-converting enzyme inhibitors with caution since they increase the severity of anaphylaxis and reduce the response of epinephrine |
| Prophylaxis and treatment | |
| Pharmacological prophylaxis | Not recommended to take any premedication to prevent the allergic reaction, e.g., antihistamine, oral corticosteroid, or leukotriene antagonist inhibitor |
| Biological agents | Absence of current recommendations |
| Desensitization of the causative food | Absence of current recommendations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisuwatchari, W.; Kanchanaphoomi, K.; Nawiboonwong, J.; Thongngarm, T.; Sompornrattanaphan, M. Food-Dependent Exercise-Induced Anaphylaxis: A Distinct Form of Food Allergy—An Updated Review of Diagnostic Approaches and Treatments. Foods 2023, 12, 3768. https://doi.org/10.3390/foods12203768
Srisuwatchari W, Kanchanaphoomi K, Nawiboonwong J, Thongngarm T, Sompornrattanaphan M. Food-Dependent Exercise-Induced Anaphylaxis: A Distinct Form of Food Allergy—An Updated Review of Diagnostic Approaches and Treatments. Foods. 2023; 12(20):3768. https://doi.org/10.3390/foods12203768
Chicago/Turabian StyleSrisuwatchari, Witchaya, Kantima Kanchanaphoomi, Jutamard Nawiboonwong, Torpong Thongngarm, and Mongkhon Sompornrattanaphan. 2023. "Food-Dependent Exercise-Induced Anaphylaxis: A Distinct Form of Food Allergy—An Updated Review of Diagnostic Approaches and Treatments" Foods 12, no. 20: 3768. https://doi.org/10.3390/foods12203768
APA StyleSrisuwatchari, W., Kanchanaphoomi, K., Nawiboonwong, J., Thongngarm, T., & Sompornrattanaphan, M. (2023). Food-Dependent Exercise-Induced Anaphylaxis: A Distinct Form of Food Allergy—An Updated Review of Diagnostic Approaches and Treatments. Foods, 12(20), 3768. https://doi.org/10.3390/foods12203768








