Raman Multi-Omic Snapshots of Koshihikari Rice Kernels Reveal Important Molecular Diversities with Potential Benefits in Healthcare
Abstract
1. Introduction
2. Experimental Procedures
2.1. Rice Samples
2.2. Raman Equipment
2.3. Data Treatments, Machine Learning Approach, and Statistics
3. Experimental Results
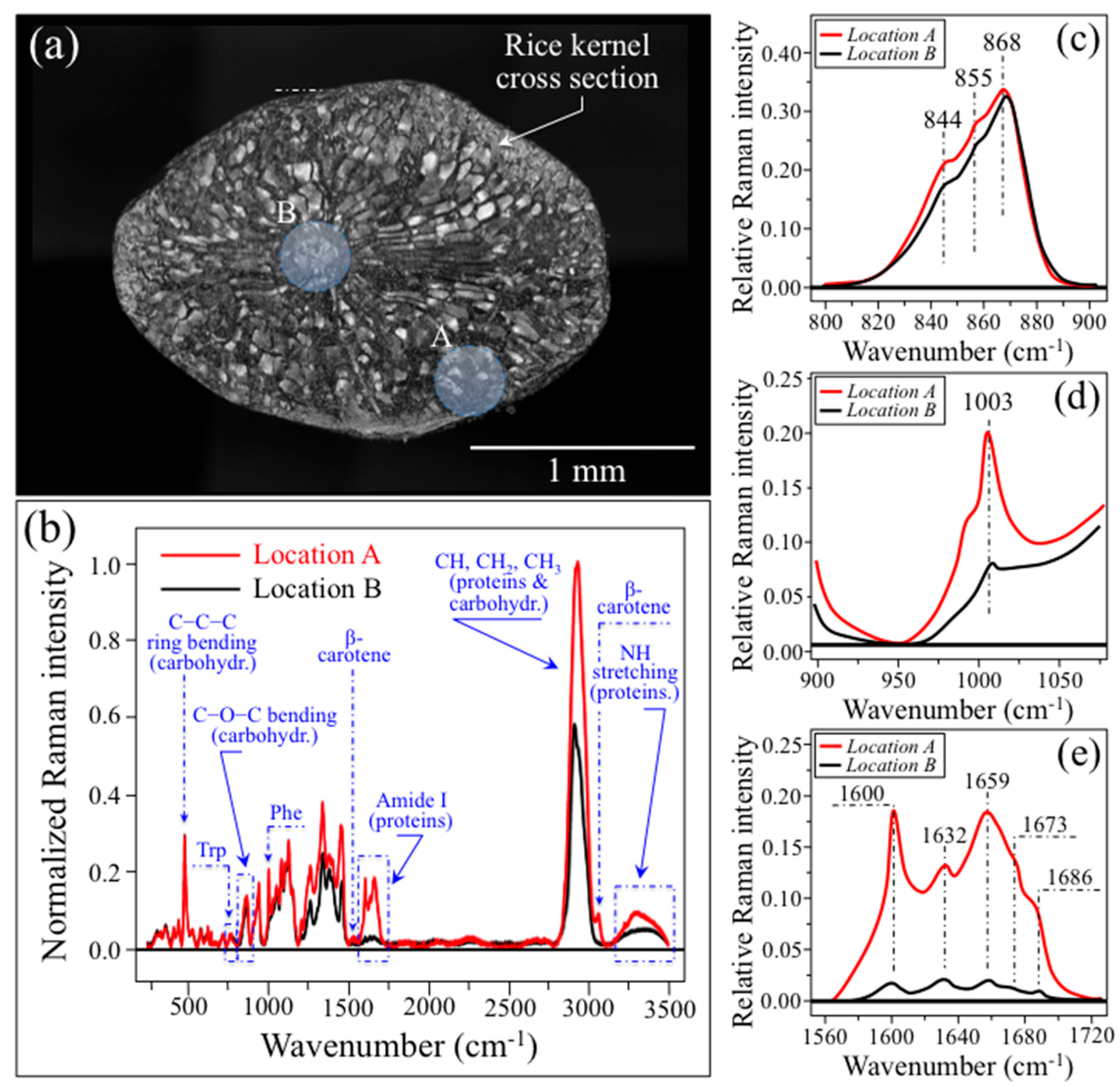
3.1. The Raman Spectrum of Rice and Related Quantitative Algorithms
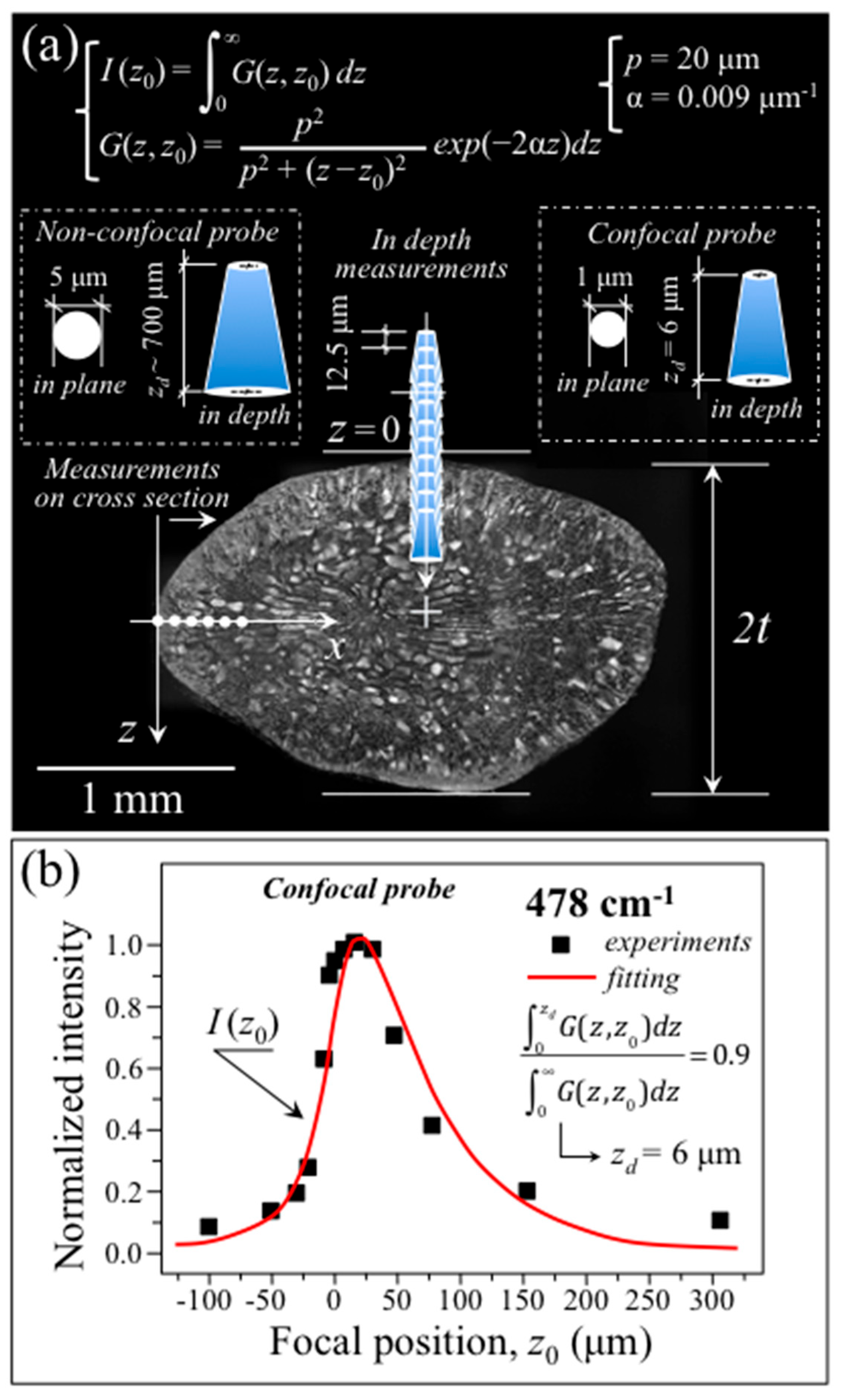
3.2. Raman Probe Size and Probe Calibrations for Single-Shot Analyses
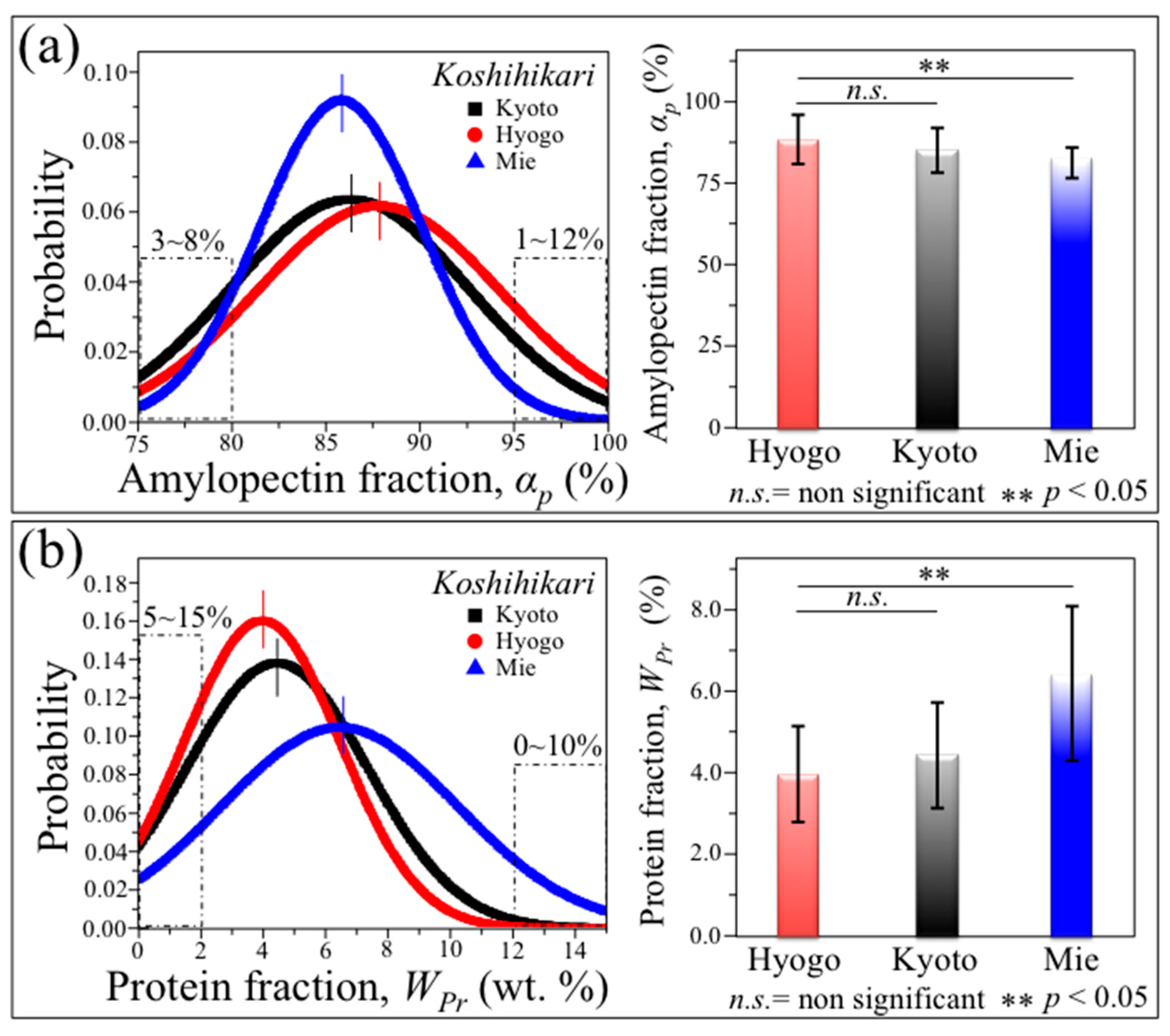
3.3. Statistical Distributions among Genetically Homogeneous Rice Kernels
4. Discussion
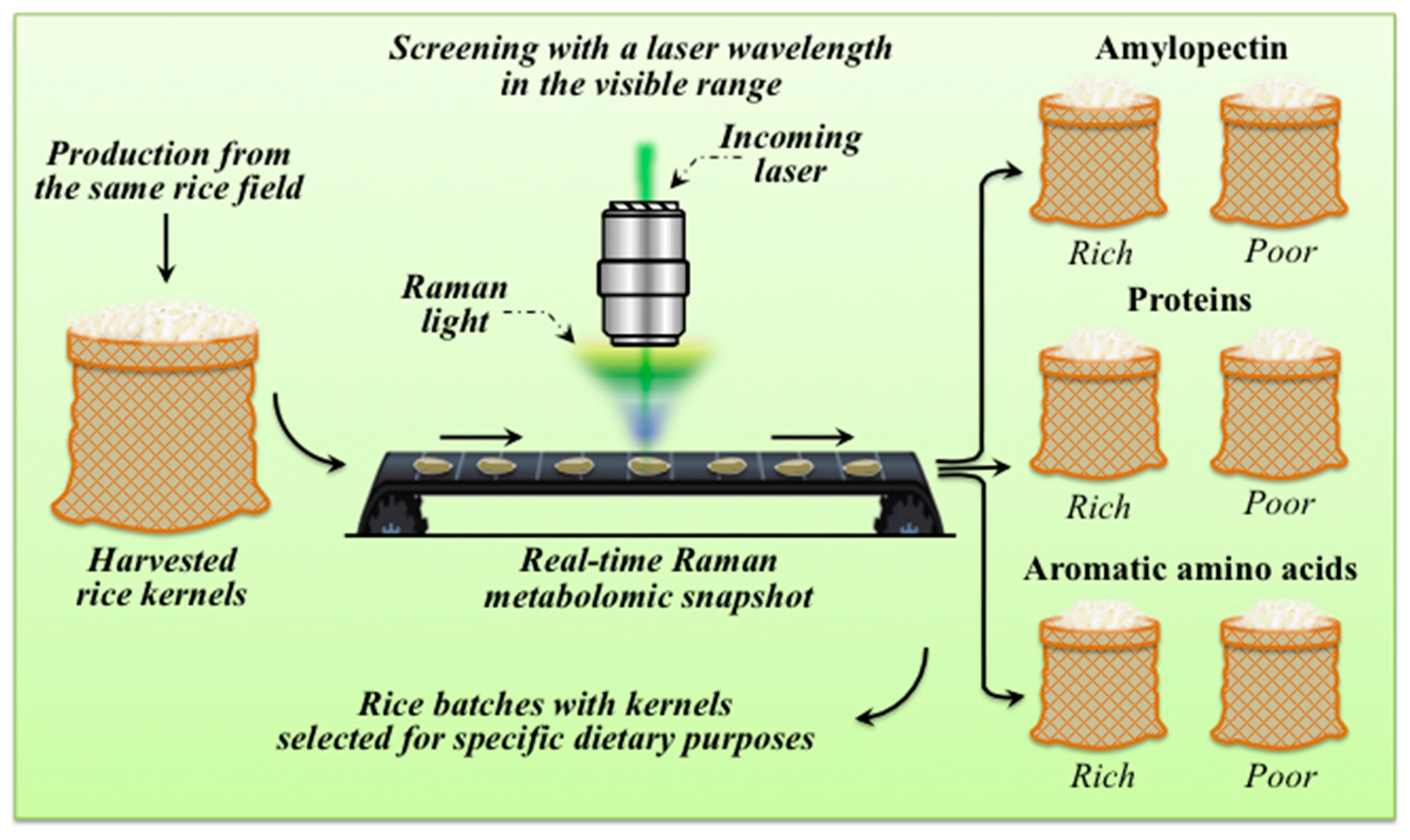
4.1. Feasibility of Real-Time Raman Multi-Omic Snapshot of Rice Kernels
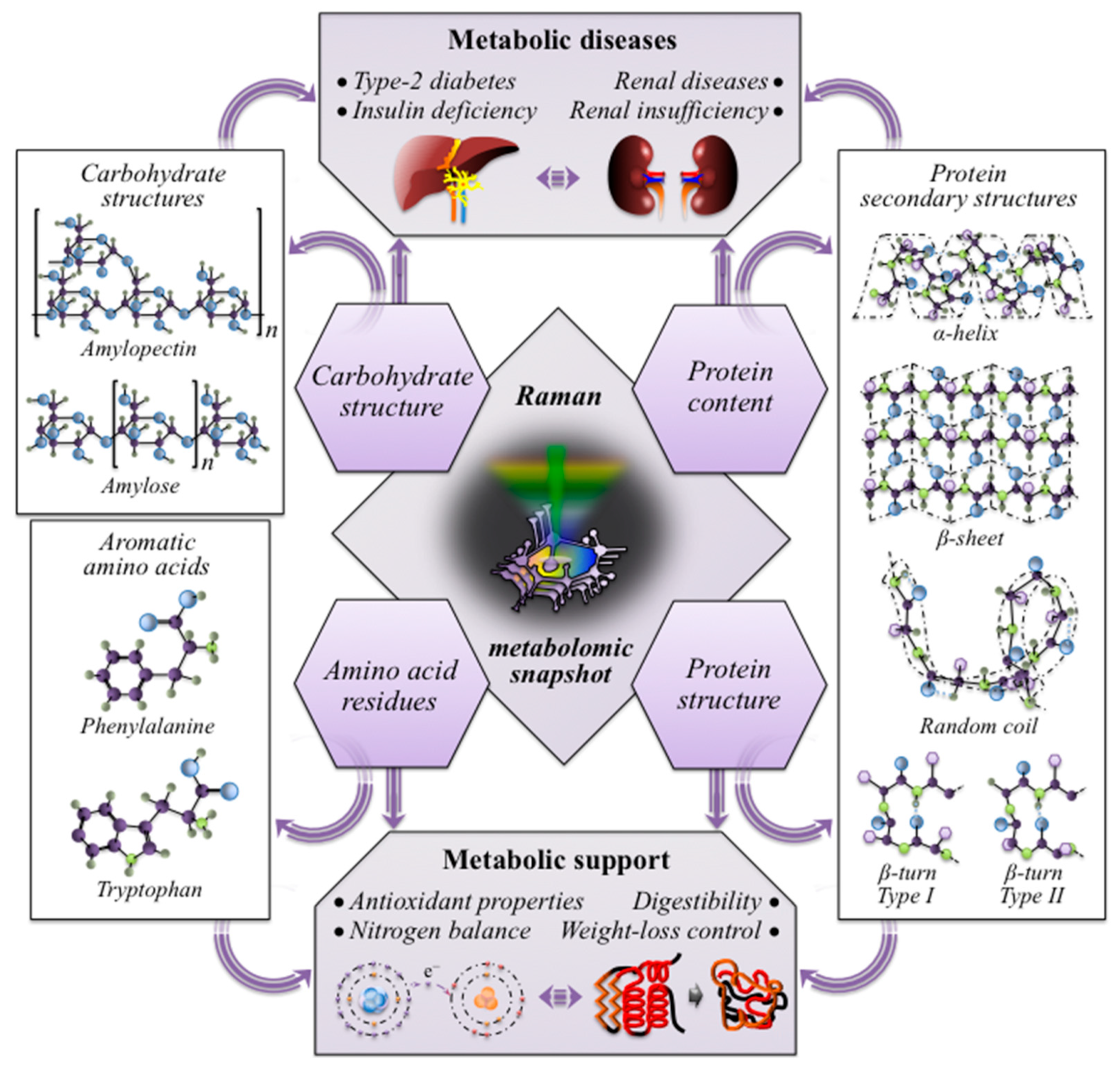
4.2. The Importance of Rice Kernel Multi-Omics in Modern Healthcare
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, W.; Chen, Z.; Hong, J.; Shi, J. Promoting human nutrition and health through plant metabolomics: Current status and challenges. Biology 2021, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Jukanti, A.K.; Pautong, P.A.; Liu, Q.; Sreenivasulu, N. Low glycemic index rice—A desired trait in starchy staples. Trends Food Sci. Technol. 2020, 106, 132–149. [Google Scholar] [CrossRef]
- Boers, H.M.; Hoorn, J.S.T.; Mela, D.J. A systematic review of the influence of rice characteristics and processing methods on postprandial glycaemic and insulinaemic responses. Br. J. Nutr. 2015, 114, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Mahender, A.; Anandan, A.; Pradhan, S.K.; Pandit, E. Rice grain nutritional traits and their enhancement using relevant genes and QTLs through advanced approaches. SpringerPlus 2016, 5, 2086. [Google Scholar] [CrossRef] [PubMed]
- Wakasa, K.; Ishihara, A. Metabolic engineering of the tryptophan and phenylalanine biosynthetic pathways in rice. Plant Technol. 2009, 26, 523–533. [Google Scholar] [CrossRef]
- Dixit, S.; Singh, U.M.; Abbai, R.; Ram, T.; Singh, V.K.; Paul, A.; Virk, P.S.; Kumar, A. Identification of genomic region(s) responsible for high iron and zinc content in rice. Sci. Rep. 2019, 9, 8136. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.A.; Rahman, S.; Resurreccion, A.P.; Concepcion, J.; Daygon, V.D.; Dipti, S.S.; Kabir, K.A.; Klingner, B.; Morell, M.K.; Bird, A.R. Identification of a major genetic determinant of glycaemic index in rice. Rice 2011, 5, 66–74. [Google Scholar] [CrossRef]
- Shao, Y.; Bao, J. Polyphenols in whole rice grain: Genetic diversity and health benefits. Food Chem. 2015, 180, 86–97. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Chikaguchi, H.; Marin, E.; Masumura, T.; Sato, Y.-I.; Nakazaki, T. Raman spectroscopic analysis of polysaccharides in popular Japanese rice cultivars. Food Chem. 2021, 354, 129434. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Chikaguchi, H.; Marin, E.; Boschetto, F.; Masumura, T.; Sato, Y.-I.; Nakazaki, T. Raman molecular fingerprints of rice nutritional quality and the concept of Raman barcode. Front. Nutr. 2021, 8, 663569. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Zhu, W.; Hashimoto, Y.; Marin, E.; Masumura, T.; Sato, Y.-I.; Nakazaki, T. Raman fingerprints of rice nutritional quality: A comparison between Japanese Koshihikari and internationally renowned cultivars. Foods 2021, 10, 2936. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.; Cordeiro, C.; Roessner, U.; Figueiredo, A. Editorial: Metabolomics in crop research—Current and emerging methodologies. Front. Plant Sci. 2019, 10, 1013. [Google Scholar] [CrossRef] [PubMed]
- Garris, J.; Tai, T.H.; Coburn, J.; Kresovich, S.; McCouch, S. Genetic structure and diversity in Oriza sativa L. Genetics 2005, 169, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.W.; Morishima, H. QTL clusters reflect character associations in wild and cultivated rice. Theor. Appl. Genet. 2002, 104, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, Q.; Zhou, X.; Zheng, S.; Wang, Y.; Li, P.; Wang, Y. Genetic diversity and population structure of 93 rice cultivars (lines) (Oryza sativa Xian group) in Qinba in China by 3 types of genetic markers. BMC Genom. 2022, 23, 550. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wu, L.; Wang, Y.; Xu, Q.; Xu, Z.; Chen, W. Research progress on the divergence and genetic basis of agronomic traits in Xian and Geng rice. Crop J. 2022, 10, 924–931. [Google Scholar] [CrossRef]
- Kobayashi, A.; Hori, K.; Yamamoto, T.; Yano, M. Koshihikari: A premium short-grain rice cultivar—Its expansion and breeding in Japan. Rice 2018, 11, 15. [Google Scholar] [CrossRef]
- Pezzotti, G. Raman spectroscopy in cell biology and microbiology. J. Raman Spectrosc. 2021, 52, 2348–2443. [Google Scholar] [CrossRef]
- Pezzotti, G.; Kobara, M.; Asai, T.; Nakaya, T.; Miyamoto, N.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Ohgitani, E.; Marin, E.; et al. Raman imaging of pathogenic Candida auris: Visualization of structural characteristics and machine-learning identification. Front. Microbiol. 2021, 12, 769597. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Hilton, A.C. One-way analysis of variance (Anova). In Statistical Analysis in Microbiology: StatNotes; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 33–37. [Google Scholar]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.; Pflueger, F.; Kruglik, S.G.; Ghomi, M. Characteristic Raman lines of phenylalanine analyzed by a multiconformational approach. J. Raman Spectrosc. 2013, 44, 827–833. [Google Scholar] [CrossRef]
- Miura, T.; Takeuchi, H.; Harada, I. Tryptophan Raman bands sensitive to hydrogen bonding and side-chain conformation. J. Raman Spectrosc. 1989, 20, 667–671. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak fitting applied to Fourier transform infrared and Raman spectroscopic analysis of proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Ren, B.; Young, B.; Variola, F.; Delatolla, R. Protein to polysaccharide ratio in EPS as an indicator of non-optimized operation of tertiary nitrifying MBBR. Water Qual. Res. J. Can. 2016, 51, 197–306. [Google Scholar] [CrossRef]
- Kupenko, I.; Dubrovinsky, L.; Dmitriev, V.; Dubrovinskaia, N. In situ Raman spectroscopic study of the pressure induced structural changes in ammonia borane. J. Chem. Phys. 2012, 137, 074506. [Google Scholar] [CrossRef]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman spectra of carotenoids in natural products. Spectrochim. Acta Part A 2003, 59, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.F.; Spielmann, A.A.; de Oliveira, L.F.C. Raman spectroscopy as a tool to the in situ study of three lichens species from Antarctica and Brazil. J. Raman Spectrosc. 2015, 46, 70–75. [Google Scholar] [CrossRef]
- Burkhardt, P.K.; Beyer, P.; Wunn, J.; Kloti, A.; Armstrong, G.A.; Schledz, M.; von Lintig, J.; Potrykus, I. Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J. 1997, 11, 1071–1078. [Google Scholar] [CrossRef]
- Pezzotti, G. Spatially resolved Raman and cathodoluminescence probes in electronic materials: Basics and applications. Phys. Stat. Sol. A Appl. Mater. 2011, 208, 976–999. [Google Scholar] [CrossRef]
- Nakayama, Y. Relationship between food properties and sensory evaluation of cooked low-protein rice. Kiryu Daigaku Kiyo 2012, 23, 31–36. [Google Scholar]
- Alseekh, S.; Bermudez, L.; de Haro, L.A.; Fernie, A.R.; Carrari, F. Crop metabolomics: From diagnostics to assisted breeding. Metabolomics 2018, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Scossa, F.; Alseekh, S.; Fernie, A.R. Integrating multi-omics data for crop improvement. J. Plant Physiol. 2021, 257, 153352. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Huhman, D.V.; Sumner, L.W. Mass spectrometry strategies in metabolomics. J. Biol. Chem. 2011, 286, 25435–25442. [Google Scholar] [CrossRef] [PubMed]
- Scigelova, M.; Hornshaw, M.; Giannakopulos, A.; Makarov, A. Fourier transform mass spectrometry. Mol. Cell. Proteom. 2011, 10, M111.009431. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.A.; Jabaji, S. FT-ICR/MS and GC-EI/MS metabolomics networking unravels global potato sprout’s responses to Rhizoctonia solani infection. PLoS ONE 2012, 7, e42576. [Google Scholar] [CrossRef]
- Adrian, M.; Lucio, M.; Roullier-Gall, C.; Heloir, M.C.; Trouvelot, S.; Daire, X.; Kanawati, B.; Lemaitre-Guillier, C.; Poinssot, B.; Gougeon, R.; et al. Metabolic fingerprint of PS3-induced resistance of grapevine leaves against Plasmopara viticola revealed differences in elicitor-triggered defenses. Front. Plant Sci. 2017, 8, 101. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Silva, A.B.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Vitis vinifera ‘Pinot Noir’ leaves as a source of bioactive nutraceutical compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Caoa, H. Raman spectroscopy of graphene and related materials. In New Developments in Photon and Materials Research; Jang, J.I., Ed.; Nova Science Publ. Inc.: London, UK, 2013; pp. 403–418. [Google Scholar]
- Pezzotti, G. Raman spectroscopy of biomedical polyethylenes. Acta Biomater. 2017, 55, 28–99. [Google Scholar] [CrossRef]
- Pezzotti, G. Raman spectroscopy of piezoelectrics. J. Appl. Phys. 2013, 113, 211–301. [Google Scholar] [CrossRef]
- Vankeirsbilck, T.; Vercauteren, A.; Baeyens, W.; Van der Weken, G.; Verpoort, F.; Vergote, G.; Remon, J.P. Application of Raman spectroscopy in pharmaceutical analysis. TrAC Trends Anal. Chem. 2002, 21, 869–877. [Google Scholar] [CrossRef]
- Roggo, Y.; Degardin, K.; Margot, P. Identification of pharmaceutical tablets by Raman spectroscopy and chemometrics. Talanta 2010, 81, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Matousek, P.; Parker, A.W. Non-invasive probing of pharmaceutical capsules using transmission Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 563–567. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Gong, C.Y.; Wang, T. Use of proteomics to understand seed development in rice. Proteomics 2013, 13, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Butardo, V.M., Jr.; Misra, G.; Cuevas, R.P.; Anacleto, R.; Kishor, P.B.K. Designing climate-resilient rice with ideal grain quality suited for high-temperature stress. J. Exp. Bot. 2015, 66, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.S.; Li, Q.F.; Zhang, C.Q.; Zhang, C.; Yang, Q.Q.; Pan, L.X.; Ren, X.Y.; Lu, J.; Gu, M.H.; Liu, Q.Q. GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat. Comm. 2018, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Tohge, T.; Chan, S.N.; Song, Y.; Rao, J.; Cui, B.; Lin, H.; Wang, L.; Fernie, A.R.; Zhang, D.B.; et al. Identification of conserved and diverse metabolic shifts during rice grain development. Sci. Rep. 2016, 6, 20942. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, J.; Su, L.; Sun, K.; Li, D.X.; Liu, Y.Z.; Wang, H.; Chen, Z.Q.; Guo, T. Metabolic profile analysis and identification of key metabolites during rice seed germination under low-temperature stress. Plant Sci. 2019, 289, 110282. [Google Scholar] [CrossRef]
- Yang, Y.H.; Guo, M.; Sun, S.Y.; Zou, Y.L.; Yin, S.Y.; Liu, Y.N.; Tang, S.Z.; Gu, M.H.; Yang, Z.F.; Yan, C.J. Natural variation of OsGluA2 is involved in grain protein content regulation in rice. Nat. Comm. 2019, 10, 1949. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.S.; Peng, M.; Gong, L.; Gao, Y.Q.; Wan, J.; Wang, S.C.; Shi, L.; Zhou, B.; Li, Z.M.; et al. Comparative and parallel genome-wide association studies for metabolic and agronomic traits in cereals. Nat. Comm. 2016, 7, 12767. [Google Scholar] [CrossRef]
- Sulpice, R. Closing the yield gap: Can metabolomics be of help? J. Exp. Bot. 2019, 71, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Verschoyle, R.D.; Greaves, P.; Cai, H.; Edwards, R.E.; Steward, W.P.; Gescher, A.J. Evaluation of the cancer chemopreventive efficacy of rice bran in genetic mouse models of breast, prostate and intestinal carcinogenesis. Br. J. Cancer 2007, 96, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Taira, H. Protein content of Japanese rice and its variation. Jp. Agric. Res. Quart. 1971, 6, 122–128. [Google Scholar]
- Chen, Y.; Wang, Z.; Wang, C.; Li, H.; Huang, D.; Zhou, D.; Zhao, L.; Pan, Y.; Gong, R.; Zhou, S. Comparison of metabolic profiles for carbohydrates, amino acids, lipids, fragrance and flavones during grain development in indica rice cultivars. Rice Sci. 2022, 29, 155–165. [Google Scholar]
- Wang, J.C.; Xu, H.; Zhu, Y.; Liu, Q.Q.; Cai, X.L. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J. Exp. Bot. 2013, 64, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.X.; Qian, Q.; Liu, Q.Q.; Yan, M.X.; Liu, X.F.; Yan, C.J.; Liu, G.F.; Gao, Z.Y.; Tang, S.Z.; Zeng, D.L.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.C. Glycemic load and chronic disease. Nutr. Rev. 2003, 61, S49–S55. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Hamaker, B.R. Slowly digestible starch: Concept, mechanism, and proposed extended glycemic index. Crit. Rev. Food Sci. 2009, 49, 852–867. [Google Scholar] [CrossRef]
- Lee, B.H.; Bello-Perez, L.A.; Lin, A.H.M.; Kim, C.Y.; Hamaker, B.R. Importance of location of digestion and colonic fermentation of starch related to its quality. Cereal Chem. 2013, 90, 335–343. [Google Scholar] [CrossRef]
- Shanita, S.N.; Hasnah, H.; Khoo, C.W. Amylose and amylopectin in selected Malaysian foods and its relationship to glycemic index. Sains Malays. 2011, 40, 865–870. [Google Scholar]
- Jeevetha, S.; Barakatun-Nisak, M.Y.; Ngan, H.-B.; Ismail, A.; Azlan, A. Relationship between amylose content and glycemic index of commonly consumed white rice. IOSR J. Agric. Veter. Sci. 2014, 7, 12–18. [Google Scholar] [CrossRef]
- Brand-Miller, J.; Pang, E.; Bramall, L. Rice: A high or low glycemic index food. Am. J. Clin. Nutr. 1992, 56, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.; Galili, G.; Cohen, H. The metabolic roles of free amino acids during seed development. Plant Sci. 2018, 275, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Moriyama, M. Studies on the nitrogen metabolism of rice plant. I. The protein content and quality of white rice as affected by latest nitrogen application after heading. J. Sci. Soil Manu. Jpn. 1953, 23, 146–148. (In Japanese) [Google Scholar]
- An, L.; Tao, Y.; Chen, H.; He, M.J.; Xiao, F.; Li, G.H.; Ding, Y.F.; Liu, Z.H. Embryo-endosperm interaction and its agronomic relevance to rice quality. Front. Plant Sci. 2020, 11, 587–641. [Google Scholar] [CrossRef] [PubMed]
- Taira, H.; Taira, H. Effect of irrigation on protein content of upland, lowland and their hybrid brown rice. Proc. Crop Sci. Jpn. 1971, 40, 294–298. [Google Scholar] [CrossRef]
- Kido, M.; Yanatori, S. Histochemical studies of protein accumulating process in rice grain. Proc. Crop Sci. Jpn. 1965, 34, 204–209. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Mawal, Y.R.; Mawal, M.R.; Sainani, M.N.; Ranjekar, P.K. Rice seed storage proteins: A structural insight. Plant Sci. 1990, 70, 73–80. [Google Scholar] [CrossRef]
- Ogawa, M.; Kumamaru, T.; Satoh, H.; Iwata, N.; Omura, T.; Kasai, Z.; Tanaka, K. Purification of protein body-I of rice seed and its polypeptide composition. Plant Cell Physiol. 1987, 28, 1517–1527. [Google Scholar]
- Yang, L.; Kumagai, T.; Kawamura, H.; Watanabe, T.; Kubota, M.; Fujimura, S.; Watanabe, R.; Kadowaki, M. Effects of rice proteins from two cultivars, Koshihikari and Shunyo, on cholesterol and triglyceride metabolism in growing and adult rats. Biosci. Biotechnol. Biochem. 2007, 71, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Kobayashi, A.; Ohta, H.; Shimizu, H.; Fukui, K.; Miura, K.; Otsuki, H.; Komaki, Y.; Sasahara, H. A new rice variety “Shunyou”. Bull. Natl. Agric. Res. Cent. 2002, 1, 1–21. (In Japanese) [Google Scholar]
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Atkins, R.C.; Zimmet, P. World kidney day 2010: Diabetic kidney disease—Act now or pay later. Am. J. Kidney Dis. 2010, 55, 205–208. [Google Scholar] [CrossRef] [PubMed]
- El Nahas, M. The global challenge of chronic kidney disease. Kidney Int. 2005, 68, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Bilous, R. Microvascular disease: What does the UKPDS tell us about diabetic nephropathy? Diabet. Med. 2003, 20, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.-L.; Rhee, C.M.; Streja, E.; et al. Plant-dominant low-protein diet for conservative management of chronic kidney disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Watanabe, N.; Nakajo, M. Low-Protein Rice (LPR) product: Processing method and product safety. Adv. Food Technol. Nutr. Sci. Open J. 2017, 3, 33–41. [Google Scholar] [CrossRef]
- Watanabe, S.; Nobori, M. Low-Protein Diet Recipe to Preserve Renal Function; Shufu-No-Tomo: Tokyo, Japan, 2010; p. C2047. [Google Scholar]
- Shirota, N.; Aoki, R.; Sato, Y.; Mineki, M. Characteristic of the smell of the low protein cooking rice, and relationship of taste. J. Integr. Study Diet. Habits 2017, 28, 35–40. [Google Scholar] [CrossRef][Green Version]
- Watanabe, S.; Ohtsubo, K. Low-protein diet: History and use of processed low-protein rice for the treatment of chronic kidney disease. Foods 2021, 10, 2255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Liang, M.; Yang, L. Glutelin and prolamin, different components of rice protein, exert differently in vitro antioxidant activities. J. Cereal Sci. 2016, 72, 108–116. [Google Scholar] [CrossRef]
- Ma, Z.-H.; Wang, Y.-B.; Cheng, H.-T.; Zhang, G.-C.; Lyu, W.-Y. Biochemical composition distribution in different grain layers is associated with the edible quality of rice cultivars. Food Chem. 2020, 311, 125–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Anthony, L.D.; Tesfamichael, H.K.; Lloyd, W.S. Biomarker metabolites capturing the metabolite variance present in a rice plant developmental period. BMC Plant Biol. 2005, 5, 8. [Google Scholar]
- Das, P.; Adak, S.; Majumder, A.L. Genetic manipulation for improved nutritional quality in rice. Front. Genet. 2020, 11, 776. [Google Scholar] [CrossRef]
- Yamada, T.; Matsuda, F.; Kasai, K.; Fukuoka, S.; Kitamura, K.; Tozawa, Y.; Miyagawa, H.; Wakasa, K. Mutation of a rice gene encoding a phenylalanine biosynthetic enzyme results in accumulation of phenylalanine and tryptophan. Plant Cell 2008, 20, 1316–1329. [Google Scholar] [CrossRef]


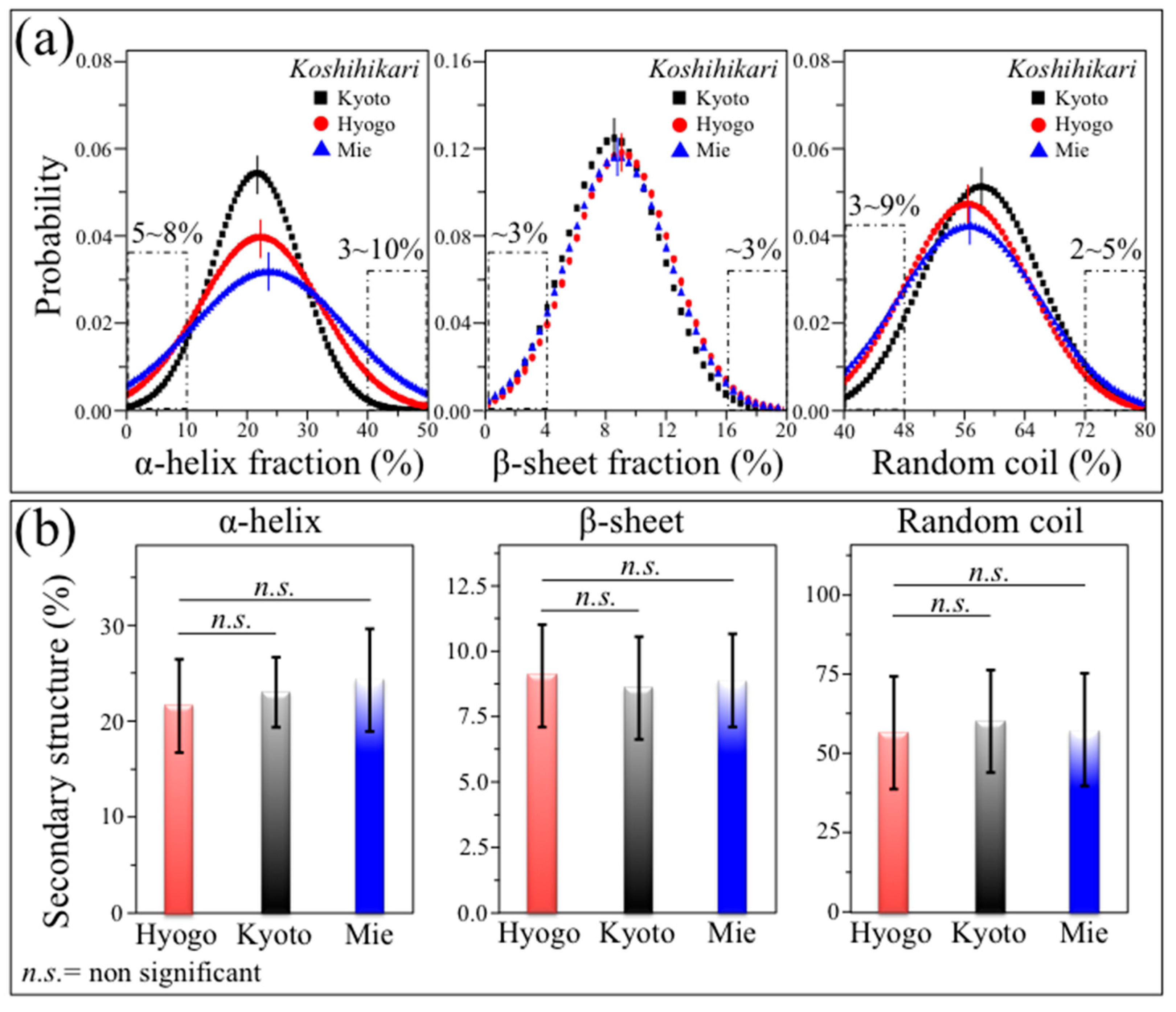
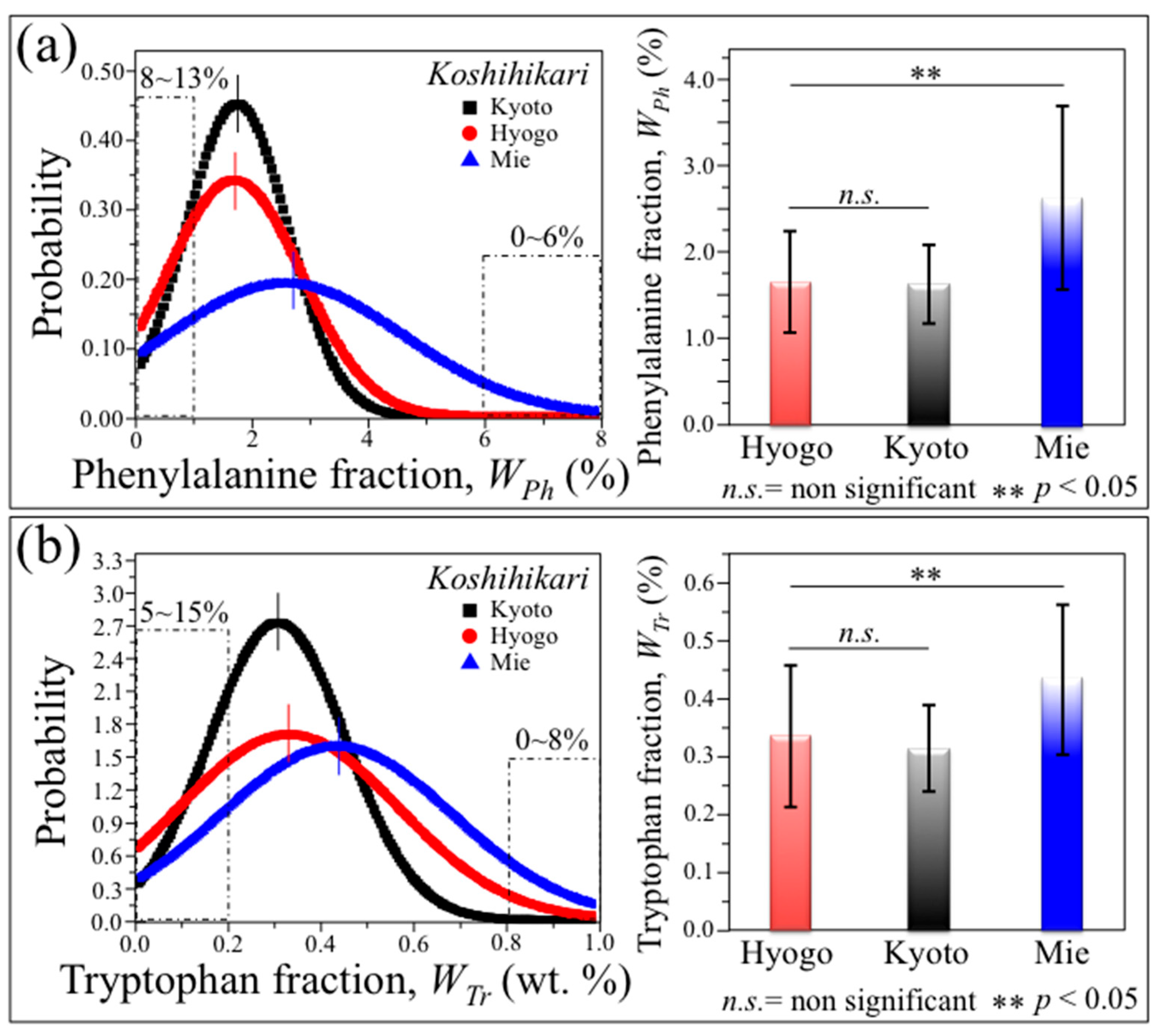

| Molecules | K vs. H | K vs. M | H vs. M |
|---|---|---|---|
| Amylopectin | 3.24 | 6.05 | 2.82 |
| Protein fraction | 2.27 | 14.47 | 12.20 |
| α-helix | 2.77 | 3.94 | 1.17 |
| β-sheets | 1.70 | 1.30 | 0.40 |
| Random coil | 1.72 | 0.02 | 1.70 |
| Phenylalanine | 0.69 | 12.13 | 12.82 |
| Tryptophan | 3.23 | 9.32 | 12.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzotti, G.; Tsubota, Y.; Zhu, W.; Marin, E.; Masumura, T.; Kobayashi, T.; Nakazaki, T. Raman Multi-Omic Snapshots of Koshihikari Rice Kernels Reveal Important Molecular Diversities with Potential Benefits in Healthcare. Foods 2023, 12, 3771. https://doi.org/10.3390/foods12203771
Pezzotti G, Tsubota Y, Zhu W, Marin E, Masumura T, Kobayashi T, Nakazaki T. Raman Multi-Omic Snapshots of Koshihikari Rice Kernels Reveal Important Molecular Diversities with Potential Benefits in Healthcare. Foods. 2023; 12(20):3771. https://doi.org/10.3390/foods12203771
Chicago/Turabian StylePezzotti, Giuseppe, Yusuke Tsubota, Wenliang Zhu, Elia Marin, Takehiro Masumura, Takuya Kobayashi, and Tetsuya Nakazaki. 2023. "Raman Multi-Omic Snapshots of Koshihikari Rice Kernels Reveal Important Molecular Diversities with Potential Benefits in Healthcare" Foods 12, no. 20: 3771. https://doi.org/10.3390/foods12203771
APA StylePezzotti, G., Tsubota, Y., Zhu, W., Marin, E., Masumura, T., Kobayashi, T., & Nakazaki, T. (2023). Raman Multi-Omic Snapshots of Koshihikari Rice Kernels Reveal Important Molecular Diversities with Potential Benefits in Healthcare. Foods, 12(20), 3771. https://doi.org/10.3390/foods12203771






