Not Just a Banana: The Extent of Fruit Cross-Reactivity and Reaction Severity in Adults with Banana Allergy
Abstract
1. Introduction
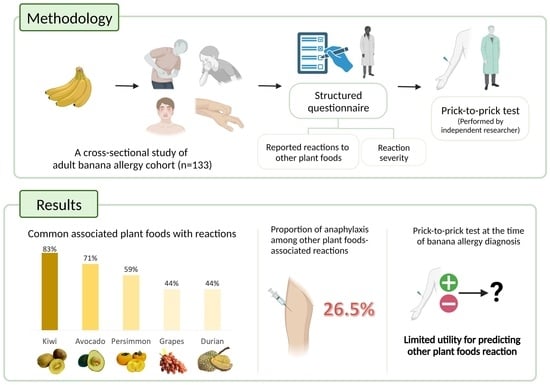
2. Materials and Methods
2.1. Study Design
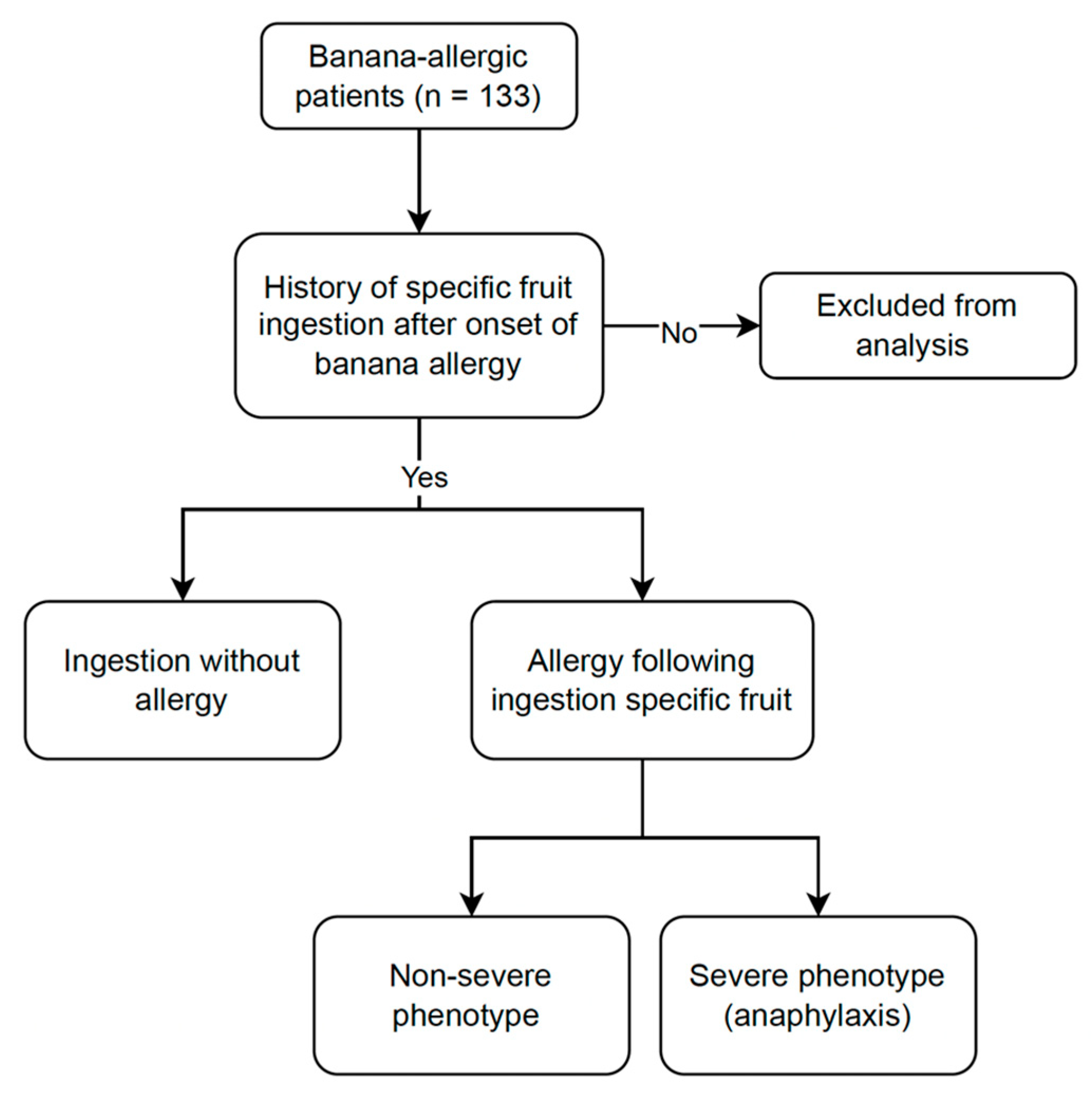
2.2. Participants
2.3. Laboratory Testing
2.4. Data Collection and Definitions
2.5. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. PTP and Banana-Specific IgE Results
3.3. Clinical Reactions to Other Plant Foods
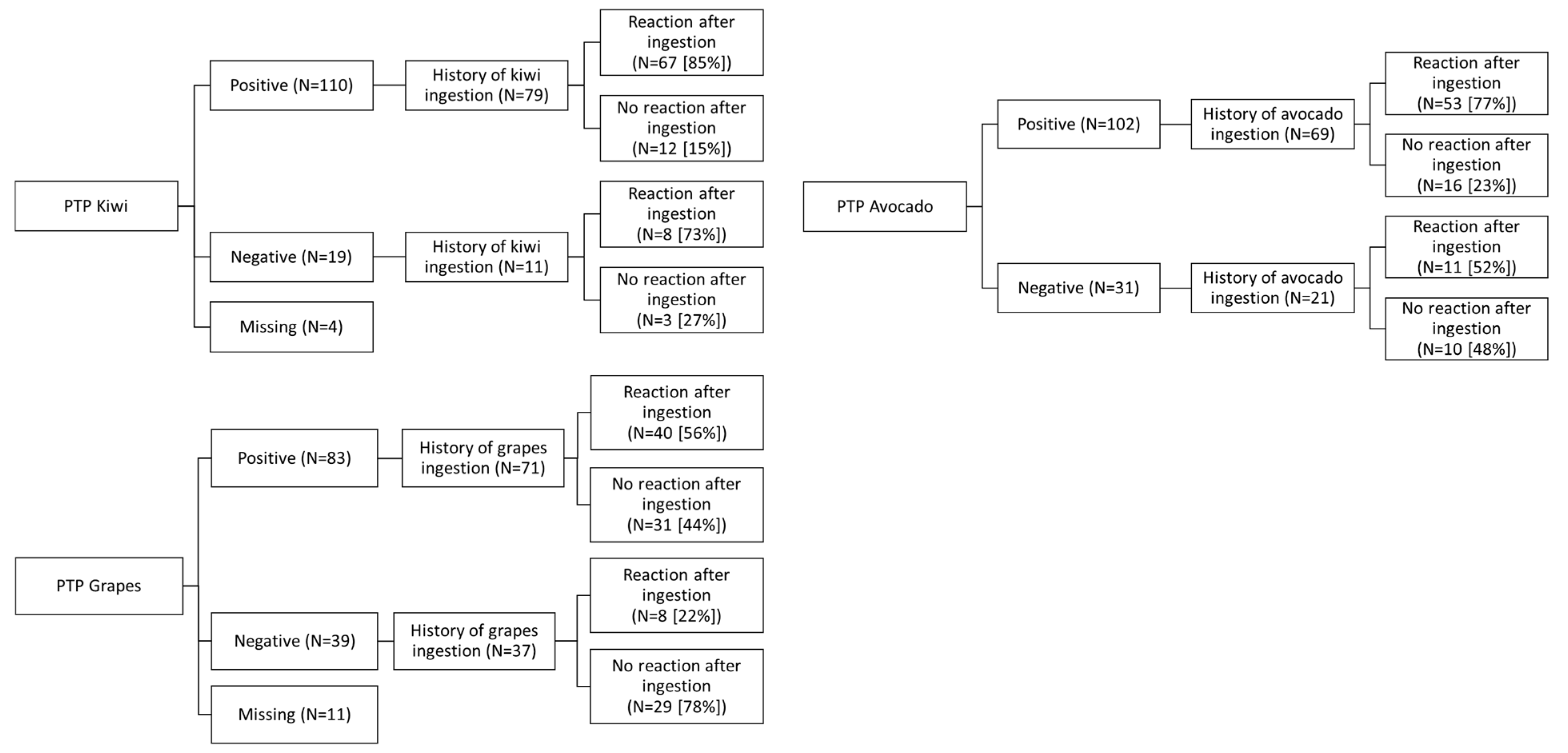
3.4. PTP for Possible Cross-Reactive or Co-Allergic Plant Foods
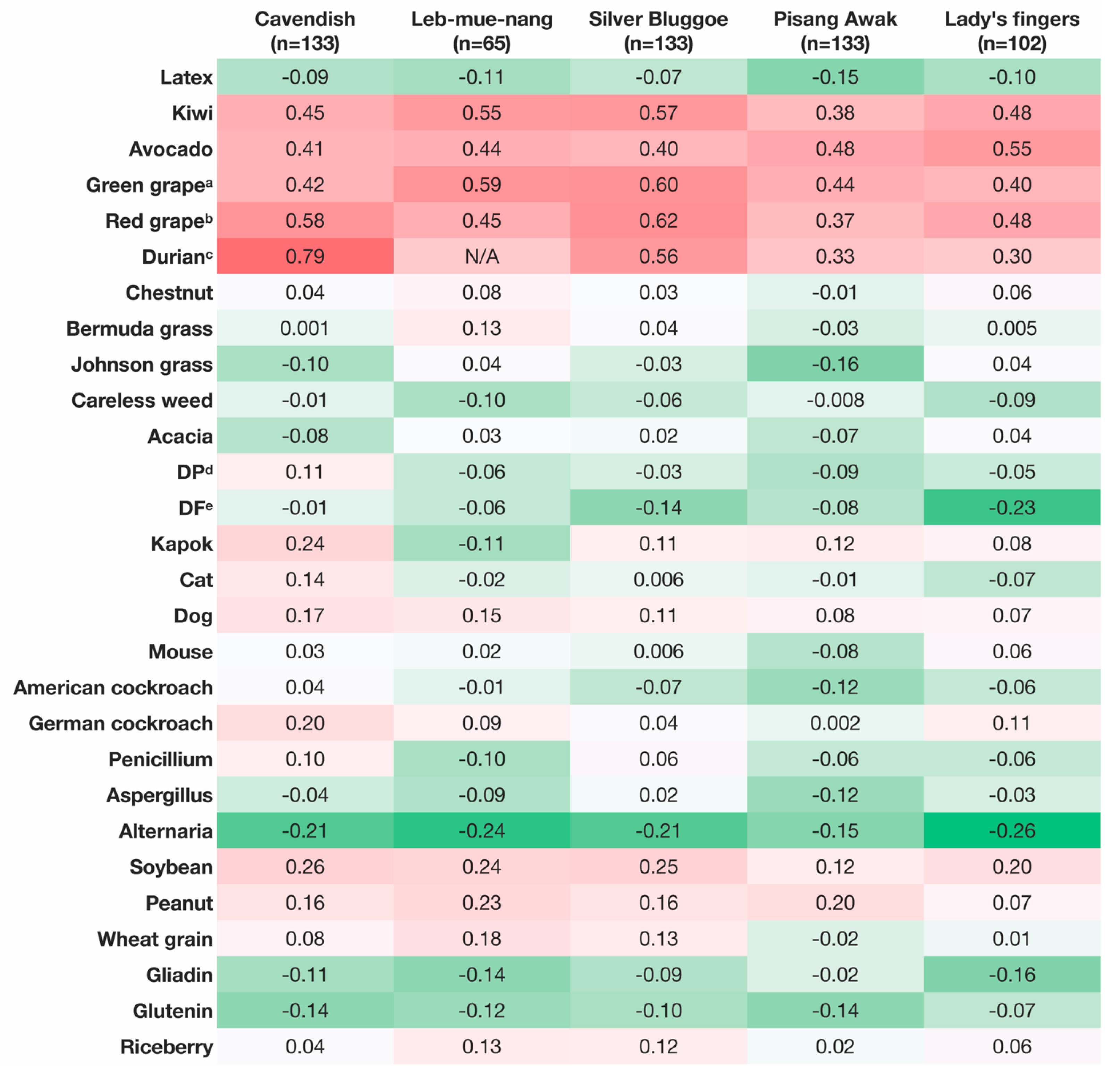
3.5. Correlation Analysis of PTP Mean Wheal Diameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Maraschin, M. Banana (Musa spp) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015, 160, 149–163. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Z.A.; El-Ghoneimy, D.H.; El-Shennawy, D.; Nasser, M.W. Evaluation of banana hypersensitivity among a group of atopic egyptian children: Relation to parental/self reports. Allergy Asthma Immunol. Res. 2013, 5, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ancillo, A.; Domínguez-Noche, C.; Gil-Adrados, A.C.; Cosmes, P.M. Allergy to banana in a 5-month-old infant. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2004, 15, 284–285. [Google Scholar] [CrossRef]
- Thongkhom, R.; Oncham, S.; Sompornrattanaphan, M.; Laisuan, W. Banana anaphylaxis in Thailand: Case series. Asia Pac. Allergy 2020, 10, e4. [Google Scholar] [CrossRef]
- Faber, M.A.; Van Gasse, A.L.; Decuyper, I.I.; Sabato, V.; Hagendorens, M.M.; Mertens, C.; Bridts, C.H.; De Clerck, L.S.; Ebo, D.G. Cross-Reactive Aeroallergens: Which Need to Cross Our Mind in Food Allergy Diagnosis? J. Allergy Clin. Immunol. Pract. 2018, 6, 1813–1823. [Google Scholar] [CrossRef]
- Suriyamoorthy, P.; Madhuri, A.; Tangirala, S.; Michael, K.R.; Sivanandham, V.; Rawson, A.; Anandharaj, A. Comprehensive Review on Banana Fruit Allergy: Pathogenesis, Diagnosis, Management, and Potential Modification of Allergens through Food Processing. Plant Foods Hum. Nutr. 2022, 77, 159–171. [Google Scholar] [CrossRef]
- Popescu, F.D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef]
- Hauser, M.; Roulias, A.; Ferreira, F.; Egger, M. Panallergens and their impact on the allergic patient. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2010, 6, 1. [Google Scholar] [CrossRef]
- Asero, R.; Mistrello, G.; Roncarolo, D.; Amato, S.; Zanoni, D.; Barocci, F.; Caldironi, G. Detection of clinical markers of sensitization to profilin in patients allergic to plant-derived foods. J. Allergy Clin. Immunol. 2003, 112, 427–432. [Google Scholar] [CrossRef]
- Westphal, S.; Kempf, W.; Foetisch, K.; Retzek, M.; Vieths, S.; Scheurer, S. Tomato profilin Lyc e 1: IgE cross-reactivity and allergenic potency. Allergy 2004, 59, 526–532. [Google Scholar] [CrossRef]
- Reindl, J.; Anliker, M.D.; Karamloo, F.; Vieths, S.; Wüthrich, B. Allergy caused by ingestion of zucchini (Cucurbita pepo): Characterization of allergens and cross-reactivity to pollen and other foods. J. Allergy Clin. Immunol. 2000, 106, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Anliker, M.D.; Reindl, J.; Vieths, S.; Wüthrich, B. Allergy caused by ingestion of persimmon (Diospyros kaki): Detection of specific IgE and cross-reactivity to profilin and carbohydrate determinants. J. Allergy Clin. Immunol. 2001, 107, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Palacin, A.; Quirce, S.; Sanchez-Monge, R.; Bobolea, I.; Diaz-Perales, A.; Martin-Muñoz, F.; Pascual, C.; Salcedo, G. Sensitization profiles to purified plant food allergens among pediatric patients with allergy to banana. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2011, 22, 186–195. [Google Scholar] [CrossRef]
- Reindl, J.; Rihs, H.P.; Scheurer, S.; Wangorsch, A.; Haustein, D.; Vieths, S. IgE reactivity to profilin in pollen-sensitized subjects with adverse reactions to banana and pineapple. Int. Arch. Allergy Immunol. 2002, 128, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, W.; Focke, M.; Götz, M.; Jarisch, R. Sensitization to Ficus benjamina: Relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2004, 34, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.F.; Rodríguez, J.; James, J.M.; Daroca, P.; Reaño, M.; Vives, R. Reactivity to potential cross-reactive foods in fruit-allergic patients: Implications for prescribing food avoidance. Allergy 2002, 57, 946–949. [Google Scholar] [CrossRef]
- Unhapipatpong, C.; Julanon, N.; Krikeerati, T.; Vichara-Anont, I.; Sompornrattanaphan, M. Adult IgE-mediated food allergy is on the rise: A review of phenotypes, pathophysiologic mechanisms, diagnosis, and advances in management. Asian Pac. J. Allergy Immunol. 2022, 40, 308–320. [Google Scholar]
- Hoffmann-Sommergruber, K.; Santos, A.F.; Breiteneder, H. The Molecular Allergology User’s Guide version 2.0 is freely available! Allergy 2023, 78, 1139–1141. [Google Scholar] [CrossRef]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar] [PubMed]
- FAO. Banana Market Review 2021; FAO: Rome, Italy, 2022. [Google Scholar]
- Suvittawatt, A. Thailand’s Banana Supply Chain Management: Export Success Factors. Int. J. Manag. Sci. Bus. Res. 2014, 3, 6–11. [Google Scholar]
- Hassan, A.K.; Venkatesh, Y.P. An overview of fruit allergy and the causative allergens. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 180–187. [Google Scholar] [PubMed]
- Fernández-Rivas, M. Fruit and vegetable allergy. Chem. Immunol. Allergy 2015, 101, 162–170. [Google Scholar] [PubMed]
- Kocacık Uygun, D.F.; Filiz, S.; Bingöl, A. An evaluation of banana allergy in children living in the Mediterranean region. Turk. J. Med. Sci. 2018, 48, 469–475. [Google Scholar] [PubMed]
- Mastrorilli, C.; Cardinale, F.; Giannetti, A.; Caffarelli, C. Pollen-Food Allergy Syndrome: A not so Rare Disease in Childhood. Medicine 2019, 55, 641. [Google Scholar] [CrossRef]
- Worm, M.; Jappe, U.; Kleine-Tebbe, J.; Schäfer, C.; Reese, I.; Saloga, J.; Treudler, R.; Zuberbier, T.; Waßmann, A.; Fuchs, T.; et al. Food allergies resulting from immunological cross-reactivity with inhalant allergens: Guidelines from the German Society for Allergology and Clinical Immunology (DGAKI), the German Dermatology Society (DDG), the Association of German Allergologists (AeDA) and the Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J. Int. 2014, 23, 1–16. [Google Scholar]
- Focke, M.; Hemmer, W.; Wöhrl, S.; Götz, M.; Jarisch, R. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin. Exp. Allergy 2003, 33, 971–977. [Google Scholar] [CrossRef]
- Brehler, R.; Theissen, U.; Mohr, C.; Luger, T. “Latex-fruit syndrome”: Frequency of cross-reacting IgE antibodies. Allergy 1997, 52, 404–410. [Google Scholar] [CrossRef]
- Anderson, L.B., Jr.; Dreyfuss, E.M.; Logan, J.; Johnstone, D.E.; Glaser, J. Melon and banana sensitivity coincident with ragweed pollinosis. J. Allergy 1970, 45, 310–319. [Google Scholar] [CrossRef]
- Bold, G.; Gorb, S.; Klein, M.-C.; Nellesen, A.; von Tapavicza, M.; Speck, T. Comparative Study on Plant Latex Particles and Latex Coagulation in Ficus benjamina, Campanula glomerata and Three Euphorbia species. PLoS ONE 2014, 9, e113336. [Google Scholar]
- Asero, R.; Tripodi, S.; Dondi, A.; Di Rienzo Businco, A.; Sfika, I.; Bianchi, A.; Candelotti, P.; Caffarelli, C.; Povesi Dascola, C.; Ricci, G.; et al. Prevalence and Clinical Relevance of IgE Sensitization to Profilin in Childhood: A Multicenter Study. Int. Arch. Allergy Immunol. 2015, 168, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Alessandri, C.; Palazzo, P.; Pomponi, D.; Liso, M.; Bernardi, M.L.; Ferrara, R.; Zennaro, D.; Santoro, M.; Rasi, C.; et al. IgE Recognition Patterns of Profilin, PR-10, and Tropomyosin Panallergens Tested in 3,113 Allergic Patients by Allergen Microarray-Based Technology. PLoS ONE 2011, 6, e24912. [Google Scholar] [CrossRef]
- Kivity, S. Adult-onset food allergy. Isr. Med. Assoc. J. 2012, 14, 70–72. [Google Scholar]
- McKenna, O.E.; Asam, C.; Araujo, G.R.; Roulias, A.; Goulart, L.R.; Ferreira, F. How relevant is panallergen sensitization in the development of allergies? Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2016, 27, 560–568. [Google Scholar] [CrossRef]
- Grob, M.; Reindl, J.; Vieths, S.; Wüthrich, B.; Ballmer-Weber, B.K. Heterogeneity of banana allergy: Characterization of allergens in banana-allergic patients. Ann. Allergy Asthma Immunol. 2002, 89, 513–516. [Google Scholar] [CrossRef]
- Rodríguez Del Río, P.; Díaz-Perales, A.; Sánchez-García, S.; Escudero, C.; Ibáñez, M.D.; Méndez-Brea, P.; Barber, D. Profilin, a Change in the Paradigm. J. Investig. Allergol. Clin. Immunol. 2018, 28, 1–12. [Google Scholar] [CrossRef]
- Skypala, I.J.; Asero, R.; Barber, D.; Cecchi, L.; Diaz Perales, A.; Hoffmann-Sommergruber, K.; Pastorello, E.A.; Swoboda, I.; Bartra, J.; Ebo, D.G.; et al. Non-specific lipid-transfer proteins: Allergen structure and function, cross-reactivity, sensitization, and epidemiology. Clin. Transl. Allergy 2021, 11, e12010. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Mistrello, G.; Roncarolo, D.; de Vries, S.C.; Gautier, M.F.; Ciurana, C.L.; Verbeek, E.; Mohammadi, T.; Knul-Brettlova, V.; Akkerdaas, J.H.; et al. Lipid transfer protein: A pan-allergen in plant-derived foods that is highly resistant to pepsin digestion. Int. Arch. Allergy Immunol. 2001, 124, 67–69. [Google Scholar] [CrossRef]
- Eriksson, N.E.; Werner, S.; Foucard, T.; Möller, C.; Berg, T.; Kiviloog, J.; Norrlind, K.; Söderberg, M.; Wihl, J.-Å. Self-reported hypersensitivity to exotic fruit in birch pollen-allergic patients. Allergol. Int. 2003, 52, 199–206. [Google Scholar] [CrossRef]
- Olivieri, J.; Quiliquini-Chambard, A.M.; Hauser, C. Allergy to durian. Allergy 2002, 57, 263. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; Eigenmann, P.A.; Sicherer, S.H. Clinical Relevance of Cross-Reactivity in Food Allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Lopes, C.; Castro, E.; Ferraz de Oliveira, J.; Bartolomé, B.; Castel-Branco, M.G. Anaphylaxis to mango fruit and crossreactivity with Artemisia vulgaris pollen. J. Investig. Allergol. Clin. Immunol. 2009, 19, 420–422. [Google Scholar]
- Bolhaar, S.T.H.P.; van Ree, R.; Bruijnzeel-Koomen, C.A.F.M.; Knulst, A.C.; Zuidmeer, L. Allergy to jackfruit: A novel example of Bet v 1-related food allergy. Allergy 2004, 59, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Dramburg, S.; Hilger, C.; Santos, A.F.; de Las Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2023, 34 (Suppl. S28), e13854. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Ballmer-Weber, B.; Beyer, K.; Conti, A.; Dubakiene, R.; Fernandez-Rivas, M.; Hoffmann-Sommergruber, K.; Lidholm, J.; Mustakov, T.; Oude Elberink, J.N.G.; et al. IgE-Mediated food allergy diagnosis: Current status and new perspectives. Mol. Nutr. Food Res. 2007, 51, 135–147. [Google Scholar] [CrossRef]
- Vlieg-Boerstra, B.J.; van de Weg, W.E.; van der Heide, S.; Dubois, A.E. Where to prick the apple for skin testing? Allergy 2013, 68, 1196–1198. [Google Scholar] [CrossRef]
- Bolhaar, S.T.; van de Weg, W.E.; van Ree, R.; Gonzalez-Mancebo, E.; Zuidmeer, L.; Bruijnzeel-Koomen, C.A.; Fernandez-Rivas, M.; Jansen, J.; Hoffmann-Sommergruber, K.; Knulst, A.C.; et al. In vivo assessment with prick-to-prick testing and double-blind, placebo-controlled food challenge of allergenicity of apple cultivars. J. Allergy Clin. Immunol. 2005, 116, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Patients (N = 133) |
|---|---|
| Female | 96 (72.7) |
| Current age at recruitment, median (IQR), y | 36.0 (32.0, 41.0) |
| Age of banana allergy onset, median (IQR), y | 33.0 (29.0, 38.0) |
| Personal history of atopic-related disorders | 88 (66.2) |
| Allergic rhinitis | 78 (58.6) |
| Asthma | 9 (6.8) |
| Atopic dermatitis | 4 (3.0) |
| Chronic urticaria | 13 (9.8) |
| Contact dermatitis | 2 (1.5) |
| Other eczema | 2 (1.5) |
| Reported banana-associated first reaction | |
| Anaphylaxis | 83 (62.9) |
| Non-anaphylaxis | 49 (37.1) |
| At least one anaphylactic episode related to banana ingestion | 116 (87.9) |
| Systems involved in worst-reported reactions | |
| Oro-mucosal system | 114 (85.7) |
| Generalized skin involvement (≥3 sites) | 89 (66.9) |
| Cardiovascular system | 22 (16.5) |
| Respiratory system | 80 (60.1) |
| Gastrointestinal system | 83 (62.4) |
| Positive banana prick-to-prick test 1 | 130 (97.7) |
| Positive banana-specific IgE 2 | 80 (65) |
| History of latex-related immediate reactions among latex-exposed participants 3 | 8 (8.1) |
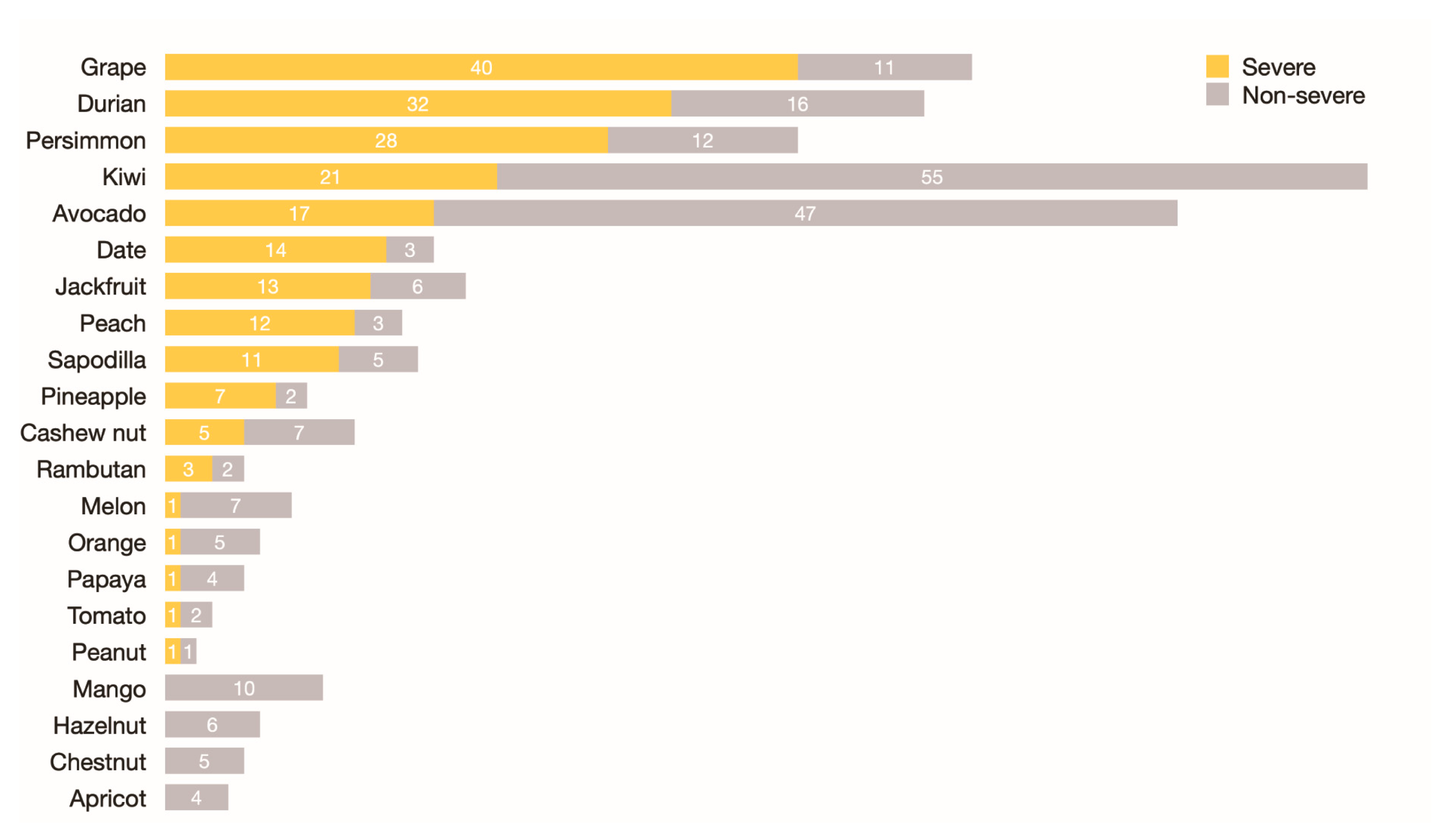
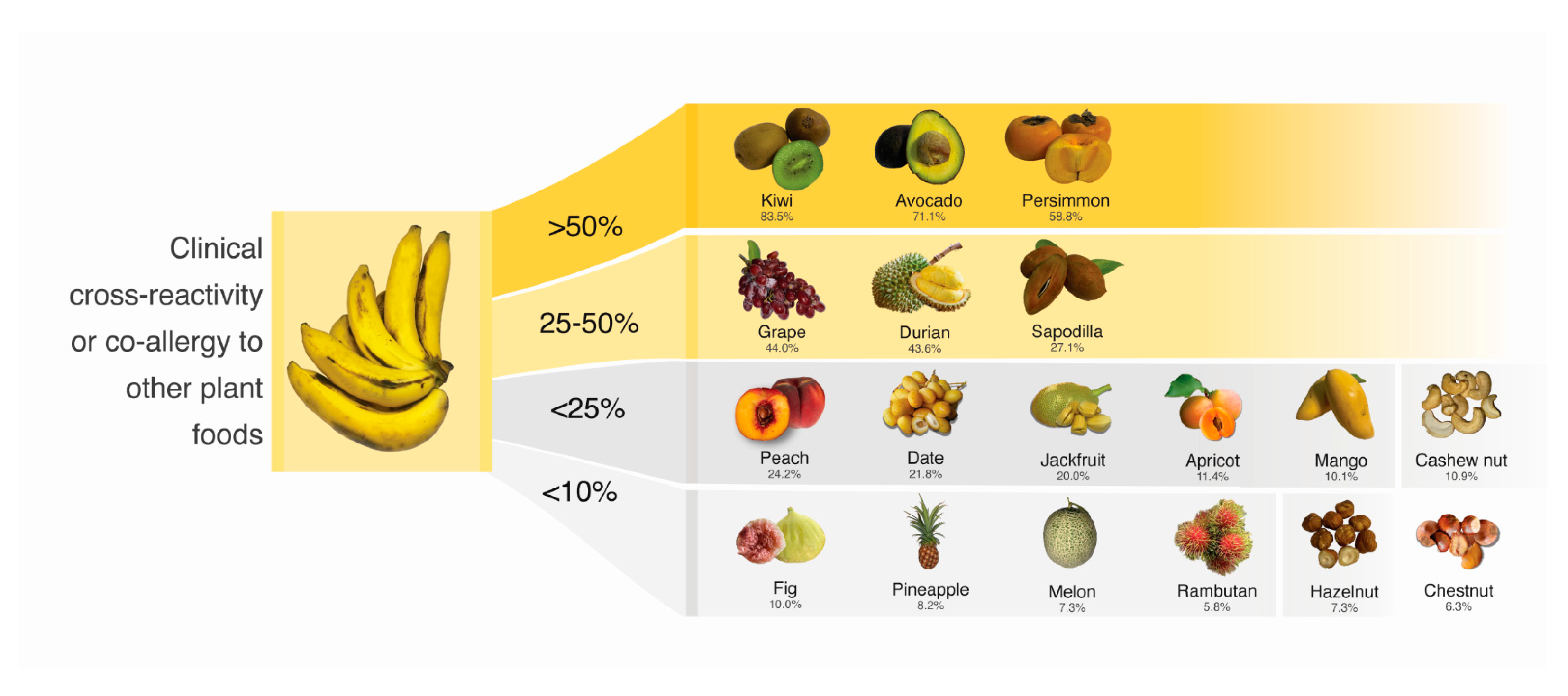
| Type of Plant Foods | Family | Without History of Ingestion (n) | Ingestion, but No Reaction (n) | Ingestion with Convincing Allergic Symptoms (n) | Proportion of Cross-Reactivity/Co-Allergy (%) 1 |
|---|---|---|---|---|---|
| Fruits | |||||
| Kiwi | Actinidiaceae | 42 | 15 | 76 | 83.5 |
| Avocado | Lauraceae | 43 | 26 | 64 | 71.1 |
| Persimmon | Ebenaceae | 65 | 28 | 40 | 58.8 |
| Grape | Vitaceae | 17 | 65 | 51 | 44.0 |
| Durian | Malvaceae | 23 | 62 | 48 | 43.6 |
| Sapodilla | Sapotaceae | 74 | 43 | 16 | 27.1 |
| Peach | Rosaceae | 71 | 47 | 15 | 24.2 |
| Date | Arecaceae | 55 | 61 | 17 | 21.8 |
| Jackfruit | Moraceae | 38 | 76 | 19 | 20.0 |
| Apricot | Rosaceae | 98 | 31 | 4 | 11.4 |
| Mango | Anacardiaceae | 34 | 89 | 10 | 10.1 |
| Fig | Moraceae | 123 | 9 | 1 | 10.0 |
| Pineapple | Bromeliaceae | 23 | 101 | 9 | 8.2 |
| Melon | Cucurbitaceae | 23 | 102 | 8 | 7.3 |
| Rambutan | Sapindaceae | 46 | 82 | 5 | 5.8 |
| Orange | Rutaceae | 6 | 121 | 6 | 4.7 |
| Papaya | Caricaceae | 25 | 103 | 5 | 4.6 |
| Tomato | Solanaceae | 14 | 116 | 3 | 2.5 |
| Non-orange citrus fruits | Rutaceae | 10 | 123 | 0 | 0.0 |
| Tree nuts | |||||
| Cashew nut | Anacardiaceae | 23 | 98 | 12 | 10.9 |
| Hazelnut | Betulaceae | 51 | 76 | 6 | 7.3 |
| Chestnut | Fagaceae | 51 | 75 | 5 | 6.3 |
| Peanut | Fabaceae | 10 | 121 | 2 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julanon, N.; Thiravetyan, B.; Unhapipatpong, C.; Xanthavanij, N.; Krikeerati, T.; Thongngarm, T.; Wongsa, C.; Songnuan, W.; Naiyanetr, P.; Sompornrattanaphan, M. Not Just a Banana: The Extent of Fruit Cross-Reactivity and Reaction Severity in Adults with Banana Allergy. Foods 2023, 12, 2456. https://doi.org/10.3390/foods12132456
Julanon N, Thiravetyan B, Unhapipatpong C, Xanthavanij N, Krikeerati T, Thongngarm T, Wongsa C, Songnuan W, Naiyanetr P, Sompornrattanaphan M. Not Just a Banana: The Extent of Fruit Cross-Reactivity and Reaction Severity in Adults with Banana Allergy. Foods. 2023; 12(13):2456. https://doi.org/10.3390/foods12132456
Chicago/Turabian StyleJulanon, Narachai, Ben Thiravetyan, Chanita Unhapipatpong, Nutchapon Xanthavanij, Thanachit Krikeerati, Torpong Thongngarm, Chamard Wongsa, Wisuwat Songnuan, Phornnop Naiyanetr, and Mongkhon Sompornrattanaphan. 2023. "Not Just a Banana: The Extent of Fruit Cross-Reactivity and Reaction Severity in Adults with Banana Allergy" Foods 12, no. 13: 2456. https://doi.org/10.3390/foods12132456
APA StyleJulanon, N., Thiravetyan, B., Unhapipatpong, C., Xanthavanij, N., Krikeerati, T., Thongngarm, T., Wongsa, C., Songnuan, W., Naiyanetr, P., & Sompornrattanaphan, M. (2023). Not Just a Banana: The Extent of Fruit Cross-Reactivity and Reaction Severity in Adults with Banana Allergy. Foods, 12(13), 2456. https://doi.org/10.3390/foods12132456