Characteristics of Oat and Buckwheat Malt Grains for Use in the Production of Fermented Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cereals
2.2. Cereals Characteristics
2.3. Micro-Malting Procedure
2.4. Malt Characteristics
2.5. Malt Mashing
2.6. Congress Wort Characteristics
2.7. Polyphenol Content and Antioxidant Activity of Malt
2.7.1. Extract Preparation
2.7.2. Determination of Total Polyphenols Content with Folin–Ciocalteu Reagent
2.7.3. Determination of Antioxidant Activity by DPPH Assay
- A1—absorbance of the DPPH radical with ethyl alcohol extract from the sample;
- A0—absorbance of the DPPH radical with ethyl alcohol (control sample).
2.8. Carotenoids and Chlorophylls Content in Malt
- CC—carotenoids content in acetone extract, (μg/mL);
- CA—chlorophyll content A in acetone extract, (μg/mL);
- CB—chlorophyll B content in the acetone extract, (μg/mL);
- CA+B—chlorophylls (A + B) content in the acetone extract, (μg/mL);
- A663—absorbance of acetone extract measured at the wavelength λ = 663 nm;
- A647—absorbance of acetone extract measured at the wavelength of λ = 647 nm;
- A470—absorbance of acetone extract measured at the wavelength λ = 470 nm.
2.9. Statistical Analysis
3. Results and Discussion
3.1. Cereals Characteristics
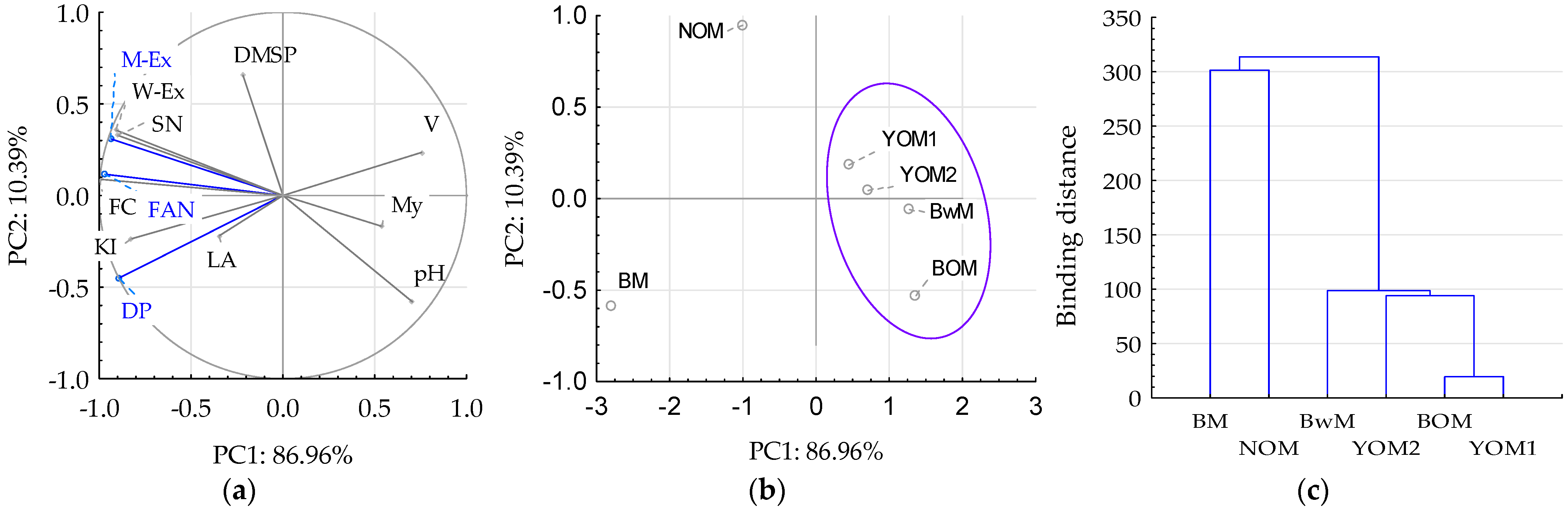
3.2. Malt and Congress Wort Characteristics
3.2.1. Malting Yield
3.2.2. Extractivity, Kolbach Index, and Diastatic Power of Malt
3.2.3. Saccharification Rate of Mash and pH Value of Congress Wort
3.2.4. Wort Viscosity and Time of Filtration of Mash
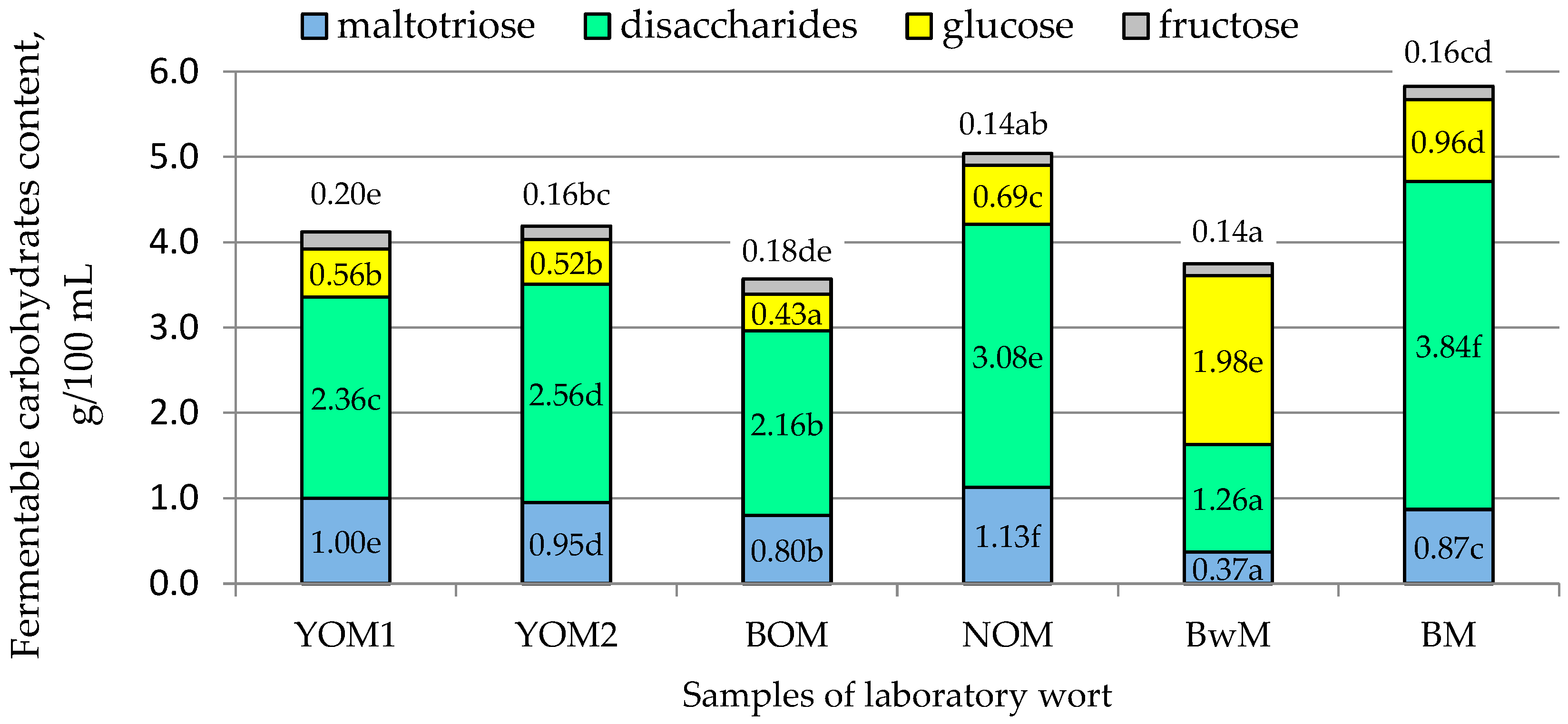
3.2.5. Wort Extract, Fermentable Carbohydrates, and Limit of Attenuation of Wort
3.2.6. Dimethyl Sulfide Precursors (DMS-P) in Malt
3.2.7. Technological Quality of Oat and Buckwheat Malt and the Possibilities of Their Use in the Production of Fermented Beverages
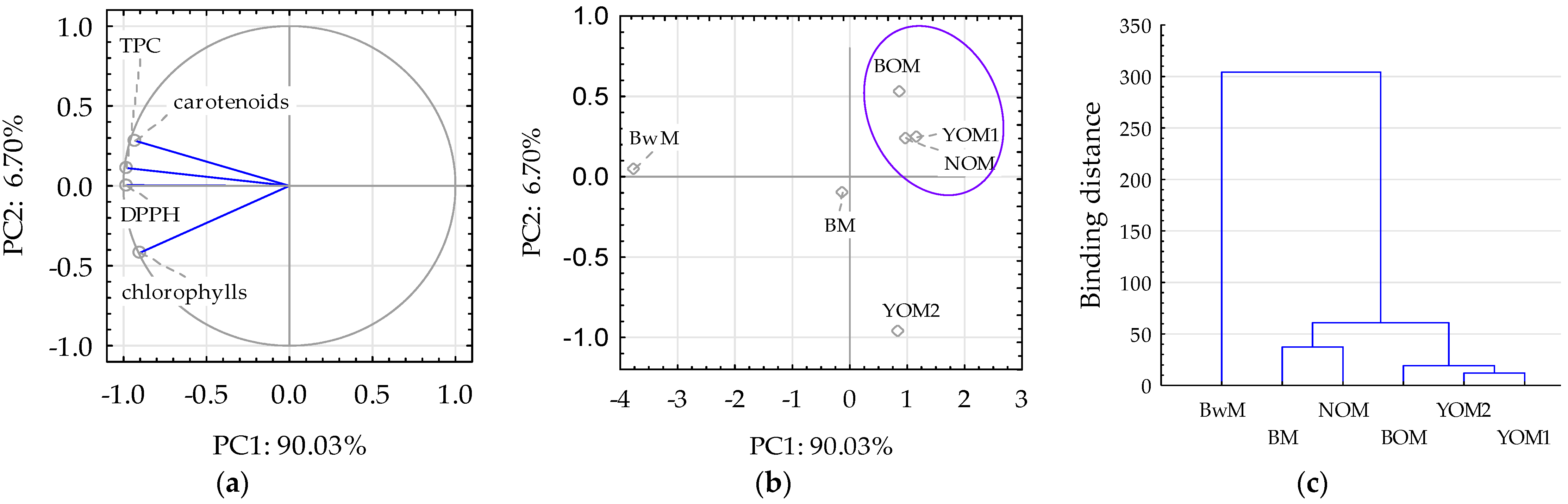
3.3. Bioactive Compounds and Antioxidant Activity of Malt
3.3.1. Carotenoids and Chlorophyll Content in Malt
3.3.2. Total Polyphenol Content in Malt
3.3.3. Antioxidant Activity of Malt
3.3.4. Antioxidant Properties of Malt and Directions of Its Use in the Production of Fermented Foods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kunze, W. Technology Brewing and Malting, 1st ed.; (8th ed. transl.); Piwochmiel Ltd.: Warsaw, Poland, 1999. [Google Scholar]
- Šimić, G.; Horvat, D.; Dvojković, K.; Abičić, I.; Viljevac Vuletić, M.; Tucak, M.; Lalić, A. Evaluation of total phenolic content and antioxidant activity of malting and hulless barley grain and malt extracts. Czech J. Food Sci. 2017, 35, 73–78. [Google Scholar] [CrossRef]
- Deng, Y.; Lim, J.; Lee, G.H.; Hanh Nguyen, T.T.; Xiao, Y.; Piao, M.; Kim, D. Brewing rutin-enriched lager beer with buckwheat malt as adjuncts. J. Microbiol. Biotechnol. 2019, 29, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Zdaniewicz, M.; Pater, A.; Hrabia, O.; Duliński, R.; Cioch-Skoneczny, M. Tritordeum malt: An innovative raw material for beer production. J. Cereal Sci. 2020, 96, 103095. [Google Scholar] [CrossRef]
- Ispiryan, L.; Kuktaite, R.; Zannini, E.; Arendt, E.K. Fundamental study on changes in the FODMAP profile of cereals, pseudo-cereals, and pulses during the malting process. Food Chem. 2021, 343, 128549. [Google Scholar] [CrossRef] [PubMed]
- Gasiński, A.; Błażewicz, J.; Kawa-Rygielska, J.; Śniegowska, J.; Zarzecki, M. Analysis of Physicochemical Parameters of Congress Worts Prepared from Special Legume Seed Malts, Acquired with and without Use of Enzyme Preparations. Foods 2021, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Agu, R.C.; Chiba, Y.; Goodfellow, V.; MacKinlay, J.; Brosnan, J.M.; Bringhurst, T.A.; Jack, F.R.; Harrison, B.; Pearson, S.Y.; Bryce, J.H. Effect of Germination Temperatures on Proteolysis of the Gluten-Free Grains Rice and Buckwheat during Malting and Mashing. J. Agric. Food Chem. 2012, 60, 10147–10154. [Google Scholar] [CrossRef] [PubMed]
- Hübner, F.; Arendt, E.K. Studies on the Influence of Germination Conditions on Protein Breakdown in Buckwheat and Oats. J. Inst. Brew. 2010, 116, 3–13. [Google Scholar] [CrossRef]
- Hosseini, E.; Kadivar, M.; Shahedi, M. Optimization of Enzymatic Activities in Malting of Oat. In Proceedings of the WASET Summer Congress, Bali, Indonesia, 14–16 June 2010; World Academy of Science, Engineering and Technology: Chicago, IL, USA, 2010; Volume 67, pp. 766–771. [Google Scholar]
- Taylor, J.R.N.; Dlamini, B.C.; Kruger, J. 125th Anniversary Review: The science of the tropical cereals sorghum, maize and rice in relation to lager beer brewing. J. Inst. Brew. 2013, 119, 1–14. [Google Scholar] [CrossRef]
- Deželak, M.; Zarnkow, M.; Becker, T.; Košir, I.J. Processing of bottom-fermented gluten-free beer-like beverages based on buckwheat and quinoa malt with chemical and sensory characterization. J. Inst. Brew. 2014, 120, 360–370. [Google Scholar] [CrossRef]
- Mikyška, A.; Matoulková, D.; Slabý, M.; Kubizniaková, P.; Hartman, I. Characterization of the Strains Isolated from Kefir Grains and their Use for the Production of Beer-based Fermented Beverages from Nontraditional Cereals. Kvasny Prum. 2015, 61, 311–319. [Google Scholar] [CrossRef]
- Kreisz, S.; Zarnkow, M.; Keβler, M.; Burberg, F.; Krahl, M.; Back, W.; Kurz, T. Beer and innovative (functional) drinks based on malted cereals and pseudo-cereals. In Proceedings of the 30th EBC Congress, Prague, Czech Republic, 14–19 May 2005; European Brewery Convention: Brussels, Belgium, 2005; pp. 925–932. [Google Scholar]
- Shopska, V.; Teneva, D.; Denkova-Kostova, R.; Ivanova, K.; Denev, P.; Kostov, G. Modelling of Malt Mixture for the Production of Wort with Increased Biological Value. Beverages 2022, 8, 44. [Google Scholar] [CrossRef]
- Fanari, M.; Forteschi, M.; Sanna, M.; Zinellu, M.; Porcu, M.K.; Pretti, L. Comparison of enzymatic and precipitation treatments for gluten-free craft beers production. Innov. Food Sci. Emerg. Technol. 2018, 49, 76–81. [Google Scholar] [CrossRef]
- Phiarais, B.P.N.; Mauch, A.; Schehl, B.D.; Zarnkow, M.; Gastl, M.; Herrmann, M.; Zannini, E.; Arendt, E.K. Processing of a Top Fermented Beer Brewed form 100% Buckwheat Malt with Sensory and Analytical Characterization. J. Inst. Brew. 2010, 116, 265–274. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Błażewicz, J.; Leszczyńska, D. Malting procedure and its impact on the composition of volatiles and antioxidative potential of naked and covered oat varieties. J. Cereal Sci. 2022, 107, 103537. [Google Scholar] [CrossRef]
- Hartmann, G.; Koehler, P.; Wieser, H. Rapid degradation of gliadin peptides toxic for coeliac disease patients by proteases from germinating cereals. J. Cereal Sci. 2006, 44, 68–371. [Google Scholar] [CrossRef]
- Tanner, G.J.; Colgrave, M.L.; Howitt, C.A. Gluten, Celiac Disease, and Gluten Intolerance and the Impact of Gluten Minimization Treatments with Prolylendopeptidase on the Measurement of Gluten in Beer. J. Am. Soc. Brew. Chem. 2014, 72, 36–50. [Google Scholar] [CrossRef]
- Harasym, J.; Podeszwa, T. Towards sustainable de-growth—Medical survey data as predictors for estimation of niche market value—Gluten-free beer market case. J. Clean. Prod. 2015, 108 Pt A, 1232–1238. [Google Scholar] [CrossRef]
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated grains—Sources of bioactive compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Stempińska, K.; Soral-Śmietana, M. Chemical compounds and physicochemical estimation of buckwheat grains—Comparison of three polish varieties. Food Sci. Technol. Qual. 2006, 2, 348–357. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) No 828/2014 of 30 July 2014 on the Requirements for the Provision of Information to Consumers on the Absence or Reduced Presence of Gluten in Food (OJ L 228, 31.7.2014, p. 5). Available online: http://data.europa.eu/eli/reg_impl/2014/828/oj (accessed on 15 September 2023).
- CXS 118-1979; Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten. Codex Alimentarius Commission: Rome, Italy, 1979.
- Smulders, M.J.M.; van de Wiel, C.C.M.; van den Broeck, H.C.; van der Meer, I.M.; Israel-Hoevelaken, T.P.M.; Timmer, R.D.; van Dinter, B.-J.; Braun, S.; Gilissen, L.J.W.J. Oats in healthy gluten-free and regular diets: A perspective. Food Res. Int. 2018, 110, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gilissen, L.J.W.J.; van der Meer, I.M.; Smulders, M.J.M. Why Oats Are Safe and Healthy for Celiac Disease Patients. Med. Sci. 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Comino, I.; Moreno, M.L.; Sousa, C. Role of oats in celiac disease. World J. Gastroenterol. 2015, 41, 11825–11831. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.H.; Arendt, E.K. Optimization of a mashing program for 100% malted buckwheat. J. Inst. Brew. 2006, 112, 57–65. [Google Scholar] [CrossRef]
- De Meo, B.; Freeman, G.; Marconi, O.; Booer, C.; Perretti, G.; Fantozzi, P. Behavior of Malted Cereals and Pseudo-Cereals for Gluten-Free Beer Production. J. Inst. Brew. 2011, 117, 541–546. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E.; Bogdan, P.; Diowksz, A. Malted and unmalted oats in brewing. J. Inst. Brew. 2014, 120, 390–398. [Google Scholar] [CrossRef]
- Marcos, A.; Serra-Majem, L.; Pérez-Jiménez, F.; Pascual, V.; Tinahones, F.J.; Estruch, R. Moderate Consumption of Beer and Its Effects on Cardiovacular and Metabolic Health: An Updated Review on Recent Scientific Evidence. Nutrients 2021, 13, 879. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Paz, I.; Brunauer, R.; Alavez, S. Beer and its non-alcoholic compounds in health and disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 3492–3505. [Google Scholar] [CrossRef] [PubMed]
- Shafreen, R.M.B.; Lakshmi, S.A.; Pandian, S.K.; Park, Y.S.; Kim, Y.M.; Paśko, P.; Deutsch, J.; Katrich, E.; Gorinstein, S. Unraveling the Antioxidant, Binding and Health-Protecting Properties of Phenolic Compounds of Beers with Main Human Serum Proteins: In Vitro and In Silico Approaches. Molecules 2020, 25, 4962. [Google Scholar] [CrossRef]
- Hager, A.-S.; Taylor, J.P.; Waters, D.M.; Arendt, E.K. Gluten free beer—A review. Trends Food Sci. Technol. 2014, 36, 44–54. [Google Scholar] [CrossRef]
- Dostálek, P.; Hochel, I.; Méndez, E.; Hernando, A.; Garbovská, D. Immunochemical determination of gluten in malts and beers. Food Addit. Contam. 2006, 23, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Cela, N.; Condelli, N.; Caruso, M.C.; Perretti, G.; Di Cairano, M.; Tolve, R.; Galgano, F. Gluten-Free Brewing: Issues and Perspectives. Fermentation 2020, 6, 53. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its processing as value added foods—A review. J. Food Sci. Technol. 2015, 52, 662–675. [Google Scholar] [CrossRef]
- Ledley, A.J.; Elias, R.J.; Hopfer, H.; Cockburn, D.W. A Modified Brewing Procedure Informed by the Enzymatic Profiles of Gluten-Free Malts Significantly Improves Fermentable Sugar Generation in Gluten-Free Brewing. Beverages 2021, 7, 53. [Google Scholar] [CrossRef]
- Podeszwa, T.; Harasym, J.; Czerniecki, P.; Kopacz, M. Top and Bottom Fermentation Beer Brewed with Commercial Buckwheat Malt. Eng. Sci. Technol. 2016, 3, 90–100. [Google Scholar]
- Klose, C.; Mauch, A.; Wunderlich, S.; Thiele, F.; Zarnkow, M.; Jacob, F.; Arendt, E.K. Brewing with 100% Oat Malt. J. Inst. Brew. 2011, 117, 411–421. [Google Scholar] [CrossRef]
- Analytica EBC. Approved Methods of Analysis by EBC. Methods: 3.11.1—Sieving Test of Barley; 3.2—Moisture Content of Barley; 3.3.1—Total Nitrogen of Barley: Kjeldahl Method; 3.6.3—Germinative Energy of Barley: Schönfeld Method; 4.2—Moisture Content of Malt; 4.16.1—High Molecular Weight β-Glucan Content of Malt: Enzymatic Method; 8.4—Viscosity of Wort (IM); 8.7—Fermentable Carbohydrates in Wort by HPLC (IM). Ed.; European Brewery Convention: Brussels, Belgium, 2023; Available online: https://brewup.eu/ebc-analytica (accessed on 15 September 2023).
- PN-A-79011-4; Food Concentrates—Test Methods—Determination of Fat Content. Polish Standardization Committee: Warsaw, Poland, 1998.
- PN-A-79083-10; Brewing Malt—Test Methods—Determination of Diastatic Power. Polish Standardization Committee: Warsaw, Poland, 1998.
- MEBAK. Collection of Brewing Analysis Methods of the Mitteleuropäische Brautechnische Analysenkommission. Methods: 1.5.3—Micromalting; 3.1.4.2.1—Congress Mash. DLFU Mill (Setting and Grinding) (EBC); 3.1.4.2.2—Congress Mash. Extract (EBC); 3.1.4.2.4—Congress Mash. Iodine Test/Saccharification Rate (EBC); 3.1.4.2.5—Congress Mash. Filtration (EBC); 3.1.4.2.7—Congress Mash. pH (EBC); 3.1.4.5.1.1—Total Nitrogen. Kjeldahl Method; 3.1.4.5.2.1—Soluble Nitrogen. Kjeldahl Method; 3.1.4.5.3—Kolbach Index (EBC); 3.1.4.5.5.1—Free Amino Nitrogen (FAN) (EBC); 3.1.4.10.1.3—Limit of Attenuation of Congress Wort (Rapid Method—EBC); 3.1.4.17—Dimethyl Sulfide and Its Precursors in Malt; 3.3—Wheat Malt. Ed.; MEBAK—Raw Materials, Barley, Adjuncts, Malt, Hops and Hop Products; Published by Jacob, F.; MEBAK: Freising-Weihenstephan, Germany, 2011. [Google Scholar]
- Urbańska, B.; Szafrański, T.; Kowalska, H.; Kowalska, J. Study of Polyphenol Content and Antioxidant Properties of Various Mix of Chocolate Milk Masses with Different Protein Content. Antioxidants 2020, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Salamon, A. Evaluation of quality of malted grain gluten-free cereals and pseudo-cereals compared to brewery barley malt. Adv. Sci. Technol. Agric. Food Ind. 2016, 71, 59–71. [Google Scholar]
- Pawłowska, P.; Diowksz, A.; Kordialik-Bogacka, E. Gluten-free oat malt as brewing raw material. Food Sci. Technol. Qual. 2013, 86, 181–190. [Google Scholar]
- Schnitzenbaumer, B.; Arendt, E.K. Brewing with up to 40% unmalted oats (Avena sativa) and sorghum (Sorghum bicolor): A review. J. Inst. Brew. 2014, 120, 315–330. [Google Scholar] [CrossRef]
- PN-A-79082; Brewing Malt. Polish Standardization Committee: Warsaw, Poland, 1997.
- Wijngaard, H.H.; Ulmer, H.M.; Neumann, M.; Arendt, E.K. The Effect of Steeping Time on the Final Malt Quality of Buckwheat. J. Inst. Brew. 2005, 111, 275–281. [Google Scholar] [CrossRef]
- Błażewicz, J.; Kawa-Rygielska, J.; Leszczyńska, D.; Grabiński, J.; Gasiński, A. Influence of Variety and Nitrogen Fertilization on the Technological Parameters of Special Malts Prepared from Naked and Hulled Oat Varieties. Agronomy 2021, 11, 2566. [Google Scholar] [CrossRef]
- Podeszwa, T.; Rutkowska, W. The impact of buckwheat seed germination conditions on the content of extract, colour and viscosity in congress mash. Res. Pap. Wrocław Univ. Econ. 2015, 411, 115–123. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Pater, A.; Knapik, A.; Duliński, R. The effect of different oat (Avena sativa L.) malt contents in a top-fermented beer recipe on the brewing process performance and product quality. J. Cereal Sci. 2021, 101, 103301. [Google Scholar] [CrossRef]
- Salamon, A. The Application of Enzyme Preparations in the Process of Mashing of Buckwheat Malt as a Raw Material for the Production of Gluten-Free Fermented Beverages. Ferment. Fruit-Veg. Process. Ind. 2016, 60, 26–28. [Google Scholar] [CrossRef]
- Ciołek, A.; Makarska, E.; Makarski, B. The content of some selected nutrients components in black and yellow hull oats. Food Sci. Technol. Qual. 2008, 3, 80–88. [Google Scholar]
- Li, Y.; Lu, J.; Gu, G.; Shi, Z.; Mao, Z. Studies on water-extractable arabinoxylans during malting and brewing. Food Chem. 2005, 93, 33–38. [Google Scholar] [CrossRef]
- Piddocke, M.P.; Kreisz, S.; Heldt-Hansen, H.P.; Nielsen, K.F.; Olsson, L. Physiological characterization of brewer’s yeast in high-gravity beer fermentation with glucose or maltose syrups as adjuncts. Appl. Microbiol. Biotechnol. 2009, 84, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Buiatti, S. Beer Composition: An Overview. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: London, UK, 2009; pp. 213–225. [Google Scholar]
- Bamforth, C. Dimethyl Sulfide—Significance, Origins, and Control. J. Am. Soc. Brew. Chem. 2014, 72, 165–168. [Google Scholar] [CrossRef]
- Sebestyén, A.; Kiss, Z.; Vecseri-Hegyes, B.; Kun-Farkas, G.; Hoschke, Á. The experiences with laboratory and pilot plant preparation of millet and buckwheat beer. Acta Aliment. 2013, 42, 81–89. [Google Scholar] [CrossRef]
- Hassani, A.; Procopio, S.; Becker, T. Influence of malting and lactic acid fermentation on functional bioactive components in cereal-bades raw materials: A review. Int. J. Food Sci. Technol. 2016, 51, 14–22. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Pandiella, S.S.; Webb, C. Growth studies of potentially probiotic lactic acid bacteria in cereal-based substrates. Int. J. Food Microbiol. 2002, 92, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of substrate composition and inoculum on the fermentation kinetics and flavour compound profiles of potentially non-dairy probiotic formulations. LWT-Food Sci. Technol. 2014, 55, 240–247. [Google Scholar] [CrossRef]
- Zhai, S.; Xia, X.; He, Z. Carotenoids in staple cereals: Metabolism, regulation, and genetic manipulation. Front. Plant Sci. 2016, 7, 1197. [Google Scholar] [CrossRef]
- Gani, A.; Wani, S.; Masoodi, F.; Hameed, G. Whole-grain cereal bioactive compounds and their health benefits: A review. J. Food Process. Technol. 2012, 3, 146. [Google Scholar] [CrossRef]
- Ndolo, V.U.; Beta, T. Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels. Food Chem. 2013, 139, 663–671. [Google Scholar] [CrossRef]
- Trono, G. Carotenoids in Cereal Food Crops: Composition and Retention throughout Grain Storage and Food Processing. Plants 2019, 8, 551. [Google Scholar] [CrossRef]
- Özcan, M.M.; Aljuhaimi, F.; Uslu, N. Effect of malt process steps on bioactive properties and fatty acid composition of barley, green malt and malt grains. J. Food Sci. Technol. 2018, 55, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Kun, S.; Vecseri, B.H.; Kun-Farkas, G. Study of antioxidant activity during the malting and brewing process. J. Food Sci. Technol. 2019, 56, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Kaukovirta-Norja, A.; Wilhelmson, A.; Poutanen, K. Germination: A means to improve the functionality of oat. Agric. Food Sci. 2004, 13, 100–112. [Google Scholar] [CrossRef]
- Krahl, M.; Back, W.; Zarnkow, M.; Kreisz, S. Determination of Optimised Malting Conditions for the Enrichment of Rutin, Vitexin and Orientin in Common Buckwheat (Fagopyrum esculentum Moench). J. Inst. Brew. 2008, 114, 294–299. [Google Scholar] [CrossRef]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and melanoidins as natural antioxidants in beer. Structure, reactivity and antioxidant activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Guido, L.F. A review on the fate of fenolic compounds during malting and brewing: Technological strategies and beer styles. Food Chem. 2022, 372, 131093. [Google Scholar] [CrossRef] [PubMed]
- Vanbeneden, N.; Van Roey, T.; Willems, F.; Delvaux, F.; Delvaux, F.R. Release of phenolic flavor precursors during wort productions: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008, 111, 83–91. [Google Scholar] [CrossRef]
- Ciocan, M.E.; Salamon, R.V.; Ambrus, Á.; Codină, G.G.; Chetrariu, A.; Dabija, A. Use of Unmalted and Malted Buckwheat in Brewing. Appl. Sci. 2023, 13, 2199. [Google Scholar] [CrossRef]
- Molinari, R.; Costantini, L.; Timperio, A.M.; Lelli, V.; Bonafaccia, F.; Bonafaccia, G.; Merendino, N. Tartary buckwheat malt as ingredient of gluten-free cookies. J. Cereal Sci. 2018, 80, 37–43. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Arendt, E.K. Nonbrewing Applications of Malted Cereals, Pseudocereals, and Legumes: A Review. J. Am. Soc. Brew. Chem. 2015, 73, 223–227. [Google Scholar] [CrossRef]
- Ninfali, P.; Mari, M.; Meli, M.A.; Roselli, C.; Antonini, E. In vitro bioaccessibility of avenanthramides in cookies made with malted oat flours. Int. J. Food Sci. Technol. 2019, 54, 1558–1565. [Google Scholar] [CrossRef]
| Sample Code | Indicator Code | ||||
|---|---|---|---|---|---|
| YO1—hulled yellow oat (cultivar 1) YO2—hulled yellow oat (cultivar 2) BO—hulled brown oat NO—naked oat Bw—buckwheat B—brewing barley | M-Ex—Extractivity (extract content in the malt), % d.m. SN—Soluble nitrogen in the wort, mg/100 g d.m. of the malt FAN—Free amino nitrogen in the wort, mg/100 g d.m. of the malt DP—Diastatic power, °WK (Windisch–Kolbach units) W-Ex—Wort extract, % w/v V—Wort viscosity (calculated to 8.6 % w/w), mPa⋅s FC—Fermentable carbohydrates of wort (calculated), g/100 mL DMS-P—Dimethyl Sulfide Precursors (DMS-P), mg/kg KI—Kolbach Index (calculated), % My—Malting yield, % pH—pH value LA—limit of attenuation of congress wort, % | ||||
| YOM1—yellow oat malt (cultivar 1) YOM2—yellow oat malt (cultivar 2) BOM—brown oat malt NOM—naked oat malt BwM—buckwheat malt BM—barley malt | |||||
| YOM1 | YOM2 | BOM | NOM | BwM | BM |
 |  |  |  |  |  |
| Quality Parameters | YO1 | YO2 | BO | NO | Bw | B |
|---|---|---|---|---|---|---|
| Germinative energy after 72 h, % | 76 ± 8 b | 75 ± 3 a,b | 73 ± 3 a,b | 80 ± 2 b,* | 60 ± 2 a | 97 ± 1 c,* |
| Germination capacity after 120 h, % | 79 ± 9 a,b,c | 80 ± 5 a,b,c | 78 ± 6 a,b | 87 ± 2 b,c,* | 67 ± 2 a | 98 ± 1 c,* |
| Husk content, % | 24.7 | 20.7 | 29.3 | ns | ns | ns |
| Sizing of grains (≥2.5 mm), % | 34.7 | 85.6 | 26.4 | 1.6 * | ns | 98.9 * |
| Moisture content, % | 13.2 ± 0.1 d | 13.1 ± 0.1 d | 11.4 ± 0.1 a | 12.6 ± 0.1 c,* | 13.5 ± 0.1 e | 12.2 ± 0.1 b,* |
| Protein content (N × 6.25), % d.m. | 8.8 ± 0.1 a | 10.9 ± 0.1 b | 11.7 ± 0.5 b | 14.4 ± 0.2 c,* | 11.8 ± 0.1 b | 11.3 ± 0,1 b,* |
| Fat content, % d.m. | 3.4 ± 0.1 b | 3.9 ± 0.1 c | 5.2 ± 0.1 d | 7.3 ± 0.1 e,* | 2.0 ± 0.1 a | 1.7 ± 0.2 a,* |
| Diastatic power, °WK | 9 | 6 | ns | 10 | 4 | 79 |
| Quality Parameters | YOM1 | YOM2 | BOM | NOM | BwM | BM |
|---|---|---|---|---|---|---|
| Malting yield, % | 90.4 ± 0.5 b | 90.8 ± 1.0 b | 90.2 ± 0.1 b | 85.0 ± 0.9 a | 84.8 ± 0.6 a | 85.0 ± 2.1 a |
| Moisture content, % | 4.4 ± 0.1 b | 4.7 ± 0.1 b,c | 4.5 ± 0.1b,c | 4.3 ± 0.6 b,* | 3.2 ± 0.1 a | 5.4 ± 0.1 c,* |
| Extractivity, % d.m. | 62.2 ± 0.2 b | 64.4 ± 0.4 c | 51.9 ± 0.1 a | 79.5 ± 0.2 d,* | 61.8 ± 1.0 b | 82.8 ± 0.2 e,* |
| Kolbach Index, % | 40 ± 1 c | 33 ± 1 b | 29 ± 2 a,b | 42 ± 1 c,* | 25 ± 1 a | 53 ± 2 d,* |
| Soluble nitrogen, mg/100 g d.m. | 531 ± 2 b | 625 ± 36 c | 536 ± 4 b | 933 ± 18 d,* | 446 ± 19 a | 896 ± 14 d,* |
| Free amino nitrogen, mg/100 g d.m. | 122 ± 6 a,b | 134 ± 2 b | 108 ± 1 a,b | 186 ± 18 c,* | 82 ± 1 a | 219 ± 25 c,* |
| Diastatic power of malt, °WK | 45 ± 1 a | 47 ± 3 a,b | 50 ± 3 a,b | 72 ± 2 b,* | 30 ± 1 a | 369 ± 15 c,* |
| Saccharification time of mash, min | 10–15 | 15–20 | 20–25 | 10–15 * | >60 | <10 * |
| Time of wort filtration, h | >3 | >3 | >3 | >3 * | >2 | 1 * |
| pH | 6.02 ± 0.02 c,d | 5.98 ± 0.02 c | 6.02 ± 0.01 c,d | 5.71 ± 0.01 a,* | 6.10 ± 0.02 d | 5.88 ± 0.02 b,* |
| Wort viscosity (8.6% w/w), mPa·s | 1.66 ± 0.05 b | 1.65 ± 0.04 b | 1.76 ± 0.01 b | 1.75 ± 0.03 b,* | 1.90 ± 0.04 c | 1.50 ± 0.04 a,* |
| Beta-glucan in malt, % d.m. | 0.06 ± 0.01 a,b | 0.04 ± 0.01 a | 0.10 ± 0.01 c | 0.06 ± 0.01 a,b | 0.06 ± 0.01 a,b | 0.42 ± 0.02 d |
| Wort extract, g/100 mL | 6.82 ± 0.07 b | 7.29 ± 0.01 c | 5.74 ± 0.08 a | 8.92 ± 0.03 d,* | 6.90 ± 0.09 b | 9.17 ± 0.02 d,* |
| Fermentable carbohydrates of wort, g/100 mL | 4.10 ± 0.02 c | 4.20 ± 0.01 d | 3.56 ± 0.01 a | 5.05 ± 0.01 e,* | 3.75 ± 0.01 b | 5.83 ± 0.04 f,* |
| Limit of attenuation of wort, % | 85.1 ± 0.6 c | 84.8 ± 0.6 c | 85.0 ± 1.3 c | 80.4 ± 2.1 b,* | 69.2 ± 0.1 a | 85.9 ± 0.6 c,* |
| DMS-P content in malt, mg/kg | 7.4 ± 0.8 d | 7.8 ± 0.1 d | 4.1 ± 0.1 c | 9.5 ± 0.1 e | <0.1 a | 3.9 ± 0.1 b |
| Quality Parameters | YOM1 | YOM2 | BOM | NOM | BwM | BM |
|---|---|---|---|---|---|---|
| Carotenoids content, µg/g d.m. | 2.90 ± 0.61 a,b | 1.47 ± 0.66 a | 4.04 ± 0.48 b | 1.76 ± 0.21 a,b | 8.73 ± 0.18 c | 3.22 ± 1.01 a,b |
| Chlorophylls content, µg/g d.m. | 4.51 ± 0.62 a | 11.01 ± 2.03 a,b | 4.36 ± 0.43 a | 3.65 ± 0.24 a | 18.83 ± 7.73 b | 8.66 ± 2.11 a,b |
| Total polyphenol content, mg GA/100 g d.m. | 225 ± 8 a | 215 ± 11 a | 245 ± 11 a | 305 ± 12 a | 646 ± 93 b | 342 ± 4 a |
| Antioxidant activity (DPPH), µM Trolox/g d.m. | 1.20 ± 0.13 a | 1.63 ± 0.07 a | 1.45 ± 0.32 a | 2.33 ± 0.09 b | 8.09 ± 0.05 d | 3.89 ± 0.18 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamon, A.; Kowalska, H.; Ignaczak, A.; Marzec, A.; Kowalska, J.; Szafrańska, A. Characteristics of Oat and Buckwheat Malt Grains for Use in the Production of Fermented Foods. Foods 2023, 12, 3747. https://doi.org/10.3390/foods12203747
Salamon A, Kowalska H, Ignaczak A, Marzec A, Kowalska J, Szafrańska A. Characteristics of Oat and Buckwheat Malt Grains for Use in the Production of Fermented Foods. Foods. 2023; 12(20):3747. https://doi.org/10.3390/foods12203747
Chicago/Turabian StyleSalamon, Agnieszka, Hanna Kowalska, Anna Ignaczak, Agata Marzec, Jolanta Kowalska, and Anna Szafrańska. 2023. "Characteristics of Oat and Buckwheat Malt Grains for Use in the Production of Fermented Foods" Foods 12, no. 20: 3747. https://doi.org/10.3390/foods12203747
APA StyleSalamon, A., Kowalska, H., Ignaczak, A., Marzec, A., Kowalska, J., & Szafrańska, A. (2023). Characteristics of Oat and Buckwheat Malt Grains for Use in the Production of Fermented Foods. Foods, 12(20), 3747. https://doi.org/10.3390/foods12203747






