Antioxidants and Quality Changes of Thermally Processed Purple Corn (Zea mays L.) Milk Fortified with Low Sucrose Content during Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Microbial Media
2.2. Procurement and Preparation of Fresh Purple Corn Milk
- PCM-CON: Purple corn milk sample without any treatment
- PCM-B5, PCM-B10, PCM-B15: Purple corn milk samples without pasteurization, extracted from corn kernels boiled for 5, 10 and 15 min at 100 °C, respectively.
- PPCM-B5, PPCM-B10, PPCM-B15: Purple corn milk samples with pasteurization, extracted from corn kernels boiled for 5, 10 and 15 min at 100 °C, respectively.
- PCM-S5, PCM-S10, PCM-S15: Purple corn milk samples without pasteurization, extracted from corn kernels steamed for 5, 10 and 15 min at 100 °C, respectively.
- PPCM-S5, PPCM-S10, PPCM-S15: Purple corn milk samples with pasteurization, extracted from corn kernels steamed for 5, 10 and 15 min at 100 °C, respectively.
2.3. Analysis of Physicochemical, and Functional Quality Changes of Thermally Processed PCM
2.3.1. Color, pH, Total Soluble Solid and Viscosity
2.3.2. Anthocyanin Content
2.3.3. Total Phenolic Compound and Antioxidant Activity by 2, 2-diphenyl-1-picrylhydrazyl Assay
2.4. Preparation of PCM Samples Fortified with Sucrose without and with Pasteurization
- PCM5-S0: Unpasteurized PCM extracted from corn kernels after 5 min of steaming at 110 °C without addition of sucrose
- PCM5-S2, PCM5-S4, PCM5-S6: Pasteurized PCM samples extracted from kernels after 5 min of steaming at 110 °C and fortified with 2, 4 and 6% of sucrose.
2.5. Analysis of Physicochemical, Functional, Microbial and Sensory Quality Retention of PCM with Added Sucrose during Refrigerated Storage
2.6. Statistical Analysis
3. Results and Discussion
3.1. Impact of Thermal Processing on Physical Properties, Bioactive Compounds and Antioxidant Potential of PCM
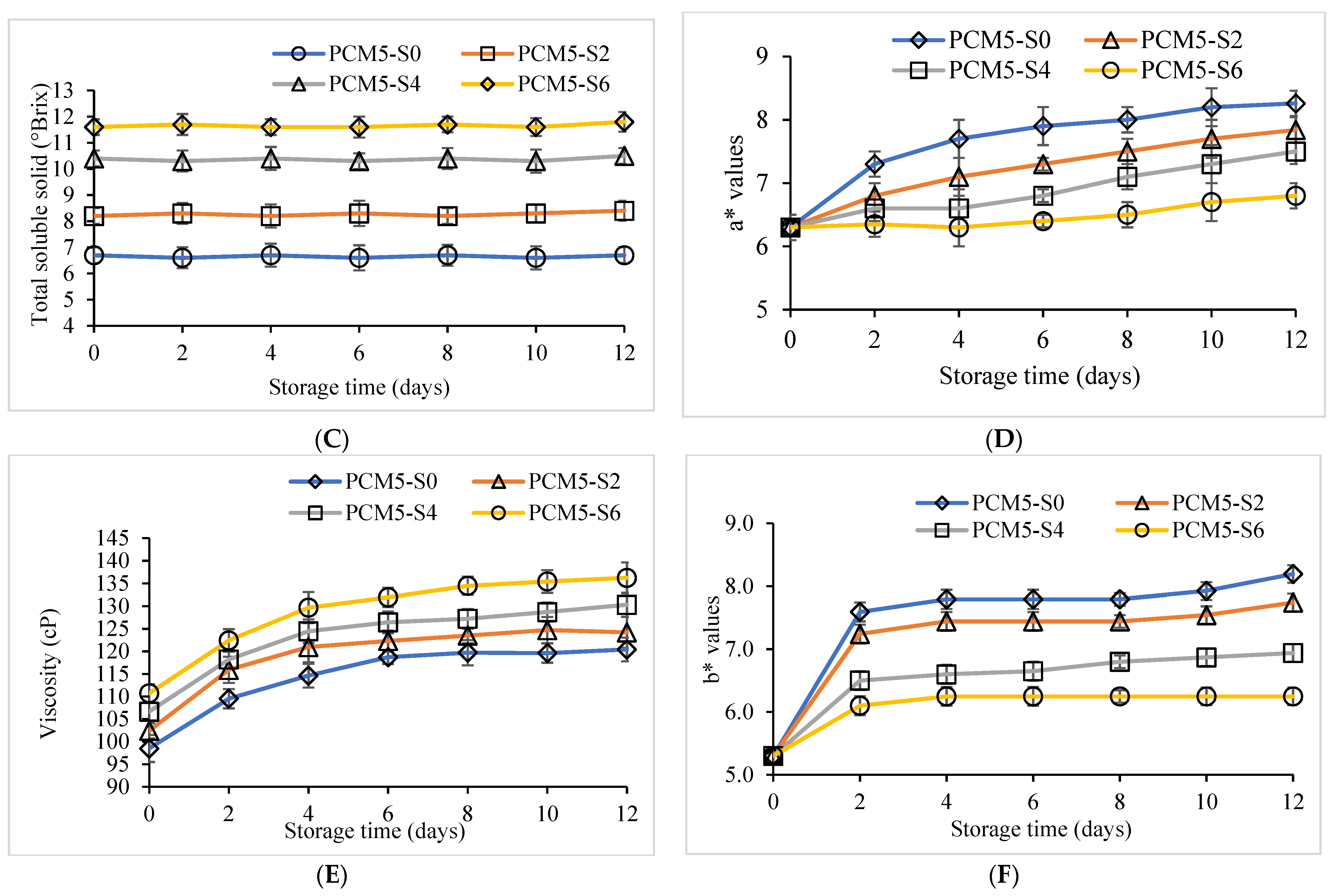
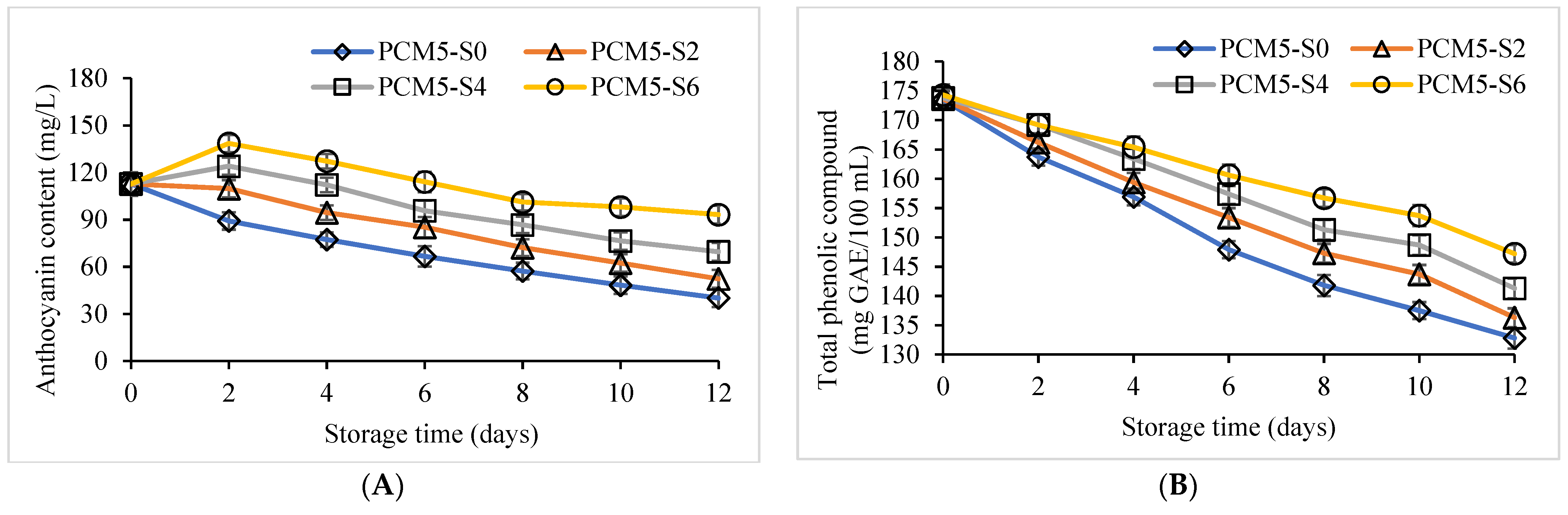
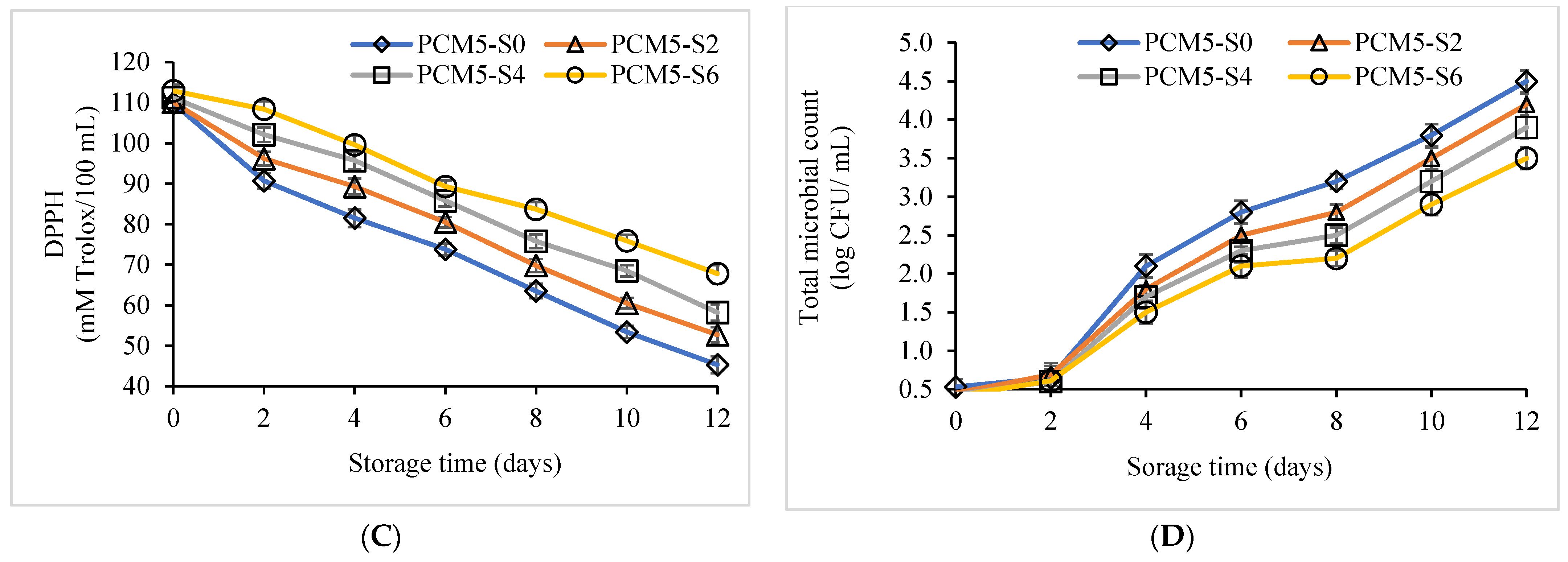
3.2. Quality Retention of PCM Samples Fortified with Sucrose Followed by Pasteurization, Stored for 12 Days at 4 °C
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsanasco, M.; Alonso, S.d.V. Physicochemical, functional, and sensory characterization of orange juice containing food additives with bioactive compounds under heat treatment and storage conditions. Food Biosci. 2021, 44, 101393. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Pacier, C.; Martirosyan, D.M. Vitamin C: Optimal dosages, supplementation and use in disease prevention. Funct. Foods Health Dis. 2015, 5, 89–107. [Google Scholar] [CrossRef]
- Xiao, S.; Li, J. Study on functional components of functional food based on food vitamins. J. Phys. Conf. Ser. 2020, 1549, 032002. [Google Scholar] [CrossRef]
- Ghassemi, S.; Delangiz, N.; Asgari Lajayer, B.; Saghafi, D.; Maggi, F. Review and future prospects on the mechanisms related to cold stress resistance and tolerance in medicinal plants. Acta Ecol. Sin. 2021, 41, 120–129. [Google Scholar] [CrossRef]
- Guo, X.; He, X.; Dai, T.; Liu, W.; Liang, R.; Chen, J.; Liu, C. The physicochemical and pasting properties of purple corn flour ground by a novel low temperature impact mill. Innov. Food Sci. Emerg. Technol. 2021, 74, 102825. [Google Scholar] [CrossRef]
- Díaz-García, A.; Salvá-Ruíz, B.; Bautista-Cruz, N.; Condezo-Hoyos, L. Optimization of a natural low-calorie antioxidant tea prepared from purple corn (Zea mays L.) cobs and stevia (Stevia rebaudiana Bert.). LWT 2021, 150, 111952. [Google Scholar] [CrossRef]
- Kapcum, C.; Uriyapongson, J. Effects of storage conditions on phytochemical and stability of purple corn cob extract powder. Food Sci. Technol. 2018, 38, 301–305. [Google Scholar] [CrossRef]
- Liu, Z.; Ying, H.; Chen, M.; Bai, J.; Xue, Y.; Yin, Y.; Batchelor, W.D.; Yang, Y.; Bai, Z.; Du, M.; et al. Optimization of China’s maize and soy production can ensure feed sufficiency at lower nitrogen and carbon footprints. Nat. Food 2021, 2, 426–433. [Google Scholar] [CrossRef]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health Benefits of Purple Corn (Zea mays L.) Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lu, Q.; Paengkoum, P.; Paengkoum, S. Short communication: Effect of purple corn pigment on change of anthocyanin composition and unsaturated fatty acids during milk storage. J. Dairy Sci. 2020, 103, 7808–7812. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, S.; Sun, B.; Wang, F.; Huang, J.; Wang, X.; Bao, Q. Effects of thermal properties and behavior of wheat starch and gluten on their interaction: A review. Int. J. Biol. Macromol. 2021, 177, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Preti, R.; Rapa, M.; Vinci, G. Effect of steaming and boiling on the antioxidant properties and biogenic amines content in green bean (Phaseolus vulgaris) varieties of different colours. J. Food Qual. 2017, 2017, 5329070. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E.; Olejnik, A.; Biegańska-Marecik, R. Effect of pretreatment on bioactive compounds in wild rocket juice. J Food Sci Technol. 2019, 56, 5234–5242. [Google Scholar] [CrossRef]
- Mazzeo, T.; N’Dri, D.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effect of two cooking procedures on phytochemical compounds, total antioxidant capacity and colour of selected frozen vegetables. Food Chem. 2011, 128, 627–633. [Google Scholar] [CrossRef]
- Chew, S.K.; Noor, N.A.; Murad, M.; Tan, T.-C.; Rusul, G. Effect of pasteurization treatment and calamansi (Fortunella japonica) juice on the physicochemical, microbiological, and sensory characteristics of black stem sugarcane juice. Int. Food Res. J. 2018, 25, 1007–1015. [Google Scholar]
- Jafari, S.; Pongsarn, K.; Srestasupana, C.; Wetchasart, N.; Assatarakul, K. Kinetic study of microbial inhibition by dimethyl dicarbonate and quality attributes of pomegranate juice during cold storage. LWT 2021, 152, 112309. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Hossain, M.B.; Brunton, N.; Lyng, J.; Valverde, J.; Rai, D.K. Pulsed electric fields pre-treatment of carrot purees to enhance their polyacetylene and sugar contents. Innov. Food Sci. Emerg. Technol. 2014, 23, 79–86. [Google Scholar] [CrossRef]
- Pokhrel, P.R.; Boulet, C.; Yildiz, S.; Sablani, S.; Tang, J.; Barbosa-Cánovas, G.V. Effect of high hydrostatic pressure on microbial inactivation and quality changes in carrot-orange juice blends at varying pH. LWT 2022, 159, 113219. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Ge, Y.; Sun, Q.; Hu, X. Clarification and sterilization of raw depectinized apple juice by ceramic ultrafiltration membranes. J. Sci. Food Agric. 2006, 86, 148–155. [Google Scholar] [CrossRef]
- Yıldız, D.; Gürel, D.B.; Çağındı, Ö.; Kayaardı, S. Heat treatment and microwave applications on homemade sour cherry juice: The effect on anthocyanin content and some physicochemical properties. Curr. Plant Biol. 2022, 29, 100242. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nedelkova, M.; Delova, A.; Petreska Ivanovska, T.; Zhivikj, Z.; Tozi, L. Assessment of microbial contamination of drinking water with total coliform bacteria and Escherichia coli in the Bitola region. Maced. Pharm. Bull. 2021, 65. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, J.; Li, J.; Zhang, P.; Tang, F.; Shan, C. Influence of lactic acid bacteria on physicochemical indexes, sensory and flavor characteristics of fermented sea buckthorn juice. Food Biosci. 2022, 46, 101519. [Google Scholar] [CrossRef]
- Kachhadiya, S.; Kumar, N.; Seth, N. Process kinetics on physico-chemical and peroxidase activity for different blanching methods of sweet corn. J. Food Sci. Technol. 2018, 55, 4823–4832. [Google Scholar] [CrossRef]
- Feng, X.; Pan, L.; Wang, Q.; Liao, Z.; Wang, X.; Zhang, X.; Guo, W.; Hu, E.; Li, J.; Xu, J.; et al. Nutritional and physicochemical characteristics of purple sweet corn juice before and after boiling. PLoS ONE 2020, 15, e0233094. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Murador, D.C.; da Cunha, D.T.; de Rosso, V.V. Effects of cooking techniques on vegetable pigments: A meta-analytic approach to carotenoid and anthocyanin levels. Food Res. Int. 2014, 65, 177–183. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Extraction of purple corn (Zea mays L.) cob pigments and phenolic compounds using food-friendly solvents. J. Cereal Sci. 2018, 80, 87–93. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins using spectrophotometric and HPLC approaches: Method comparison and correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Charmongkolpradit, S.; Somboon, T.; Phatchana, R.; Sang-aroon, W.; Tanwanichkul, B. Influence of drying temperature on anthocyanin and moisture contents in purple waxy corn kernel using a tunnel dryer. Case Stud. Therm. Eng. 2021, 25, 100886. [Google Scholar] [CrossRef]
- Yang, J.; Gadi, R.L. Effects of steaming and dehydration on anthocyanins, antioxidant activity, total phenols and color characteristics of purple-fleshed sweet potatoes(Ipomoea batatas). Am. J. Food Technol. 2008, 3, 224–234. [Google Scholar] [CrossRef]
- Ursu, M.S.; Aprodu, I.; Milea, Ș.A.; Enachi, E.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Thermal degradation kinetics of anthocyanins extracted from purple maize flour extract and the effect of heating on selected biological functionality. Foods 2020, 9, 1593. [Google Scholar] [CrossRef]
- Akdaş, Z.Z.; Bakkalbaşı, E. Influence of different cooking methods on color, bioactive compounds, and antioxidant activity of kale. Int. J. Food Prop. 2017, 20, 877–887. [Google Scholar] [CrossRef]
- Vázquez-Román, S.; Escuder-Vieco, D.; Martín-Pelegrina, M.D.; Muñoz-Amat, B.; Fernández-Álvarez, L.; Brañas-García, P.; Lora-Pablos, D.; Beceiro-Mosquera, J.; Pallás-Alonso, C.R. Short communication: Effect of refrigerated storage on the pH and bacterial content of pasteurized human donor milk. J. Dairy Sci. 2018, 101, 10714–10719. [Google Scholar] [CrossRef] [PubMed]
- Serpen, J. Comparison of sugar content in bottled 100% fruit juice versus extracted juice of fresh fruit. Food Nutr. Sci. 2012, 3, 1509–1513. [Google Scholar] [CrossRef]
- Benítez, E.I.; Genovese, D.B.; Lozano, J.E. Effect of typical sugars on the viscosity and colloidal stability of apple juice. Food Hydrocoll. 2009, 23, 519–525. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Zou, B. Effect of high pressure homogenization and dimethyl dicarbonate (DMDC) on microbial and physicochemical qualities of mulberry juice. J. Food Sci. 2016, 81, M702-8. [Google Scholar] [CrossRef]
- Dias, N.; Lara, S.; Miranda, L.; Pires, I.; Pires, C.; Halboth, N. Influence of color on acceptance and identification of flavor of foods by adults. Food Sci. Technol. 2012, 32, 296–301. [Google Scholar] [CrossRef]
- Junpatiw, A.; Mitmungkorn, Y.; Montri, N. Effects of heat and storage treatments on the anthocyanin contents in selected purple vegetables. Khon Kaen Agric. J. 2017, 45, 1278–1282. [Google Scholar]
- Moon, K.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lu, Q.; Zhao, S.; Li, J.; Luo, Q.; Wang, X.; Zhang, Y.; Zheng, N. Purple corn anthocyanin affects lipid mechanism, flavor compound profiles, and related gene expression of Longissimus Thoracis et Lumborum muscle in goats. Animals 2021, 11, 2407. [Google Scholar] [CrossRef] [PubMed]
- Jirattanarangsri, W. the effect of traditional thermal cooking processes on anthocyanin, total phenolic content, antioxidant activity and glycemic index in purple waxy corn. Food Appl. Biosci. J. 2018, 6, 154–166. [Google Scholar] [CrossRef]
- Paredes, D.; Ortiz, C.; Torres, R. Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Nanomed. 2014, 9, 1717–1729. [Google Scholar] [CrossRef]
- Noor, R.; Uddin, M.; Haq, M.; Munshi, S.; Acharjee, M.; Rahman, M. Microbiological study of vendor and packed fruit juices locally available in Dhaka city, Bangladesh. Int. Food Res. J. 2012, 20, 1011–1015. [Google Scholar]
- Tian, X.-Z.; Wang, X.; Ban, C.; Luo, Q.-Y.; Li, J.-X.; Lu, Q. Effect of purple corn anthocyanin on antioxidant activity, volatile compound and sensory property in milk during storage and light prevention. Front. Nutr. 2022, 9, 862689. [Google Scholar] [CrossRef]


| Sample Treatments | L* | a* | b* | ΔE | pH | Total Soluble Solid (°Brix) | Viscosity (cP) |
|---|---|---|---|---|---|---|---|
| PCM-CON | 52.6 ± 0.6 c | 8.2 ± 0.3 a | 7.8 ± 0.6 f | 6.5± 0.2 a | 5.6 ± 0.4 a | 28.1 ± 2.3 g | |
| PCM-B5 | 59.7 ± 3.2 bc | 7.1 ± 0.4 b | 12.3 ± 0.5 e | 8.47 | 6.6 ± 0.3 a | 5.7 ± 0.2 a | 76.8 ± 3.9 f |
| PPCM-B5 | 60.5 ± 0.6 bc | 6.4 ± 0.3 bc | 13.8 ± 0.6 d | 10.08 | 6.7 ± 0.2 a | 5.5 ± 0.3 a | 88.7 ± 2.9 e |
| PCM-B10 | 61.4 ± 0.7 b | 5.8 ± 0.2 c | 15.1 ± 0.3 c | 11.68 | 6.7 ± 0.2 a | 5.5 ± 0.2 a | 98.8 ± 2.8 d |
| PPCM-B10 | 61.9 ± 0.4 b | 4.1 ± 0.4 d | 16.5 ± 0.7 bc | 13.37 | 6.7 ± 0.3 a | 5.5 ± 0.2 a | 105.3 ± 3.9 c |
| PCM-B15 | 62.7 ± 0.6 a | 3.4 ± 0.2 e | 16.8 ± 0.5 b | 14.35 | 6.7 ± 0.2 a | 5.5 ± 0.3 a | 113.8 ± 2.6 b |
| PPCM-B15 | 62.9 ± 0.5 a | 2.7 ± 0.2 f | 18.3 ± 0.4 a | 15.70 | 6.8 ± 0.3 a | 5.5 ± 0.4 a | 136.9 ± 3.5 a |
| Sample Treatments | L* | a* | b* | ΔE | pH | Total Soluble Solid (°Brix) | Viscosity (cP) |
|---|---|---|---|---|---|---|---|
| PCM-CON | 52.6 ± 0.6 cd | 8.2 ± 0.3 a | 7.8 ± 0.6 b | 6.5± 0.2 a | 5.6 ± 0.4 b | 28.1 ± 2.3 f | |
| PCM-S5 | 53.6 ± 0.3 cd | 8.1 ± 0.3 a | 7.4 ± 2.6 b | 1.08 | 6.5± 0.2 a | 6.7 ± 0.1 a | 79.5 ± 2.1 e |
| PPCM-S5 | 52.1 ± 0.4 d | 7.2 ± 0.4 b | 5.1 ± 0.3 c | 2.92 | 6.6 ± 0.2 a | 6.6 ± 0.2 a | 98.4 ± 2.9 d |
| PCM-S10 | 55.5 ± 0.8 bc | 6.5 ± 0.2 bc | 7.3 ± 0.4 b | 3.39 | 6.6 ± 0.1 a | 6.6 ± 0.3 a | 103.3 ± 2.7 c |
| PPCM-S10 | 56.4 ± 0.5 b | 6.1 ± 0.4 bc | 6.5 ± 0.6 bc | 4.53 | 6.7 ± 0.1 a | 6.6 ± 0.2 a | 107.8 ± 2.3 bc |
| PCM-S15 | 57.9 ± 0.7 ab | 5.2 ± 0.4 c | 8.4 ± 0.8 a | 6.11 | 6.7 ± 0.1 a | 6.6 ± 0.2 a | 110.4 ± 2.6 b |
| PPCM-S15 | 58.3 ± 0.3 a | 4.3 ± 0.3 d | 6.7 ± 0.3 bc | 6.99 | 6.7± 0.1 a | 6.6 ± 0.3 a | 115.8 ± 2.4 a |
| Storage Time (Days) | Sample Treatments | Appearance | Color | Odor | Viscosity | Taste | Overall Preference |
|---|---|---|---|---|---|---|---|
| 0 | PCM5-S0 | 6.5 ± 0.4 Ac | 6.7 ± 0.3 Ac | 6.6 ± 0.4 Ac | 6.5 ± 0.3 Ac | 6.7 ± 0.4 Ac | 6.7 ± 0.3 Ac |
| PCM5-S2 | 7.6 ± 0.3 Ab | 7 ± 0.4 Ab | 7.7 ± 0.2 Ab | 7.5 ± 0.3 Ab | 7.3 ± 0.3 Ab | 7.6 ± 0.2 Ab | |
| PCM5-S4 | 8.5 ± 0.2 Aa | 8.6. ± 0.2 Aa | 8.4 ± 0.3 Aa | 8.2 ± 0.2 Aa | 8.5 ± 0.3 Aa | 8.6 ± 0.4 Aa | |
| PCM5-S6 | 8.5 ± 0.3 Aa | 8.3 ± 0.2 Aa | 7.5 ± 0.2 Ab | 7.1 ± 0.4 Ab | 7.2 ± 0.3 Ab | 7.6 ± 0.4 Ab | |
| 12 | PCM5-S0 | - | - | - | - | - | - |
| PCM5-S2 | 5.4 ± 0.4 Bb | 5.6 ± 0.2 Bb | 5.6 ± 0.3 Bb | 5.7 ± 0.3 Bb | 5.3 ± 0.3 Bb | 5.6 ± 0.2 Bb | |
| PCM5-S4 | 6.7 ± 0.2 Ba | 6.8 ± 0.2 Ba | 6.3 ± 0.2 Ba | 6.1 ± 0.2 Ba | 6.4 ± 0.2 Ba | 6.8 ± 0.3 Ba | |
| PCM5-S6 | 6.3 ± 0.3 Ba | 6.5 ± 0.3 Ba | 5.3 ± 0.4 Bb | 5.2 ± 0.3 Bb | 5.4 ± 0.4 Bb | 5.5 ± 0.4 Bb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiekh, K.A.; Luanglaor, T.; Hanprerakriengkrai, N.; Jafari, S.; Kijpatanasilp, I.; Asadatorn, N.; Worobo, R.W.; Bekhit, A.E.-D.A.; Assatarakul, K. Antioxidants and Quality Changes of Thermally Processed Purple Corn (Zea mays L.) Milk Fortified with Low Sucrose Content during Cold Storage. Foods 2023, 12, 277. https://doi.org/10.3390/foods12020277
Shiekh KA, Luanglaor T, Hanprerakriengkrai N, Jafari S, Kijpatanasilp I, Asadatorn N, Worobo RW, Bekhit AE-DA, Assatarakul K. Antioxidants and Quality Changes of Thermally Processed Purple Corn (Zea mays L.) Milk Fortified with Low Sucrose Content during Cold Storage. Foods. 2023; 12(2):277. https://doi.org/10.3390/foods12020277
Chicago/Turabian StyleShiekh, Khursheed Ahmad, Thitirat Luanglaor, Natchaya Hanprerakriengkrai, Saeid Jafari, Isaya Kijpatanasilp, Nicha Asadatorn, Randy W. Worobo, Alaa El-Din Ahmed Bekhit, and Kitipong Assatarakul. 2023. "Antioxidants and Quality Changes of Thermally Processed Purple Corn (Zea mays L.) Milk Fortified with Low Sucrose Content during Cold Storage" Foods 12, no. 2: 277. https://doi.org/10.3390/foods12020277
APA StyleShiekh, K. A., Luanglaor, T., Hanprerakriengkrai, N., Jafari, S., Kijpatanasilp, I., Asadatorn, N., Worobo, R. W., Bekhit, A. E.-D. A., & Assatarakul, K. (2023). Antioxidants and Quality Changes of Thermally Processed Purple Corn (Zea mays L.) Milk Fortified with Low Sucrose Content during Cold Storage. Foods, 12(2), 277. https://doi.org/10.3390/foods12020277








