The Molecular Mechanism of Yam Polysaccharide Protected H2O2-Induced Oxidative Damage in IEC-6 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of CYP
2.3. Cell Culture
2.4. RNA-Seq Analysis
2.5. Inhibition of MAPKs Using Specific Inhibitors
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results and Discussion
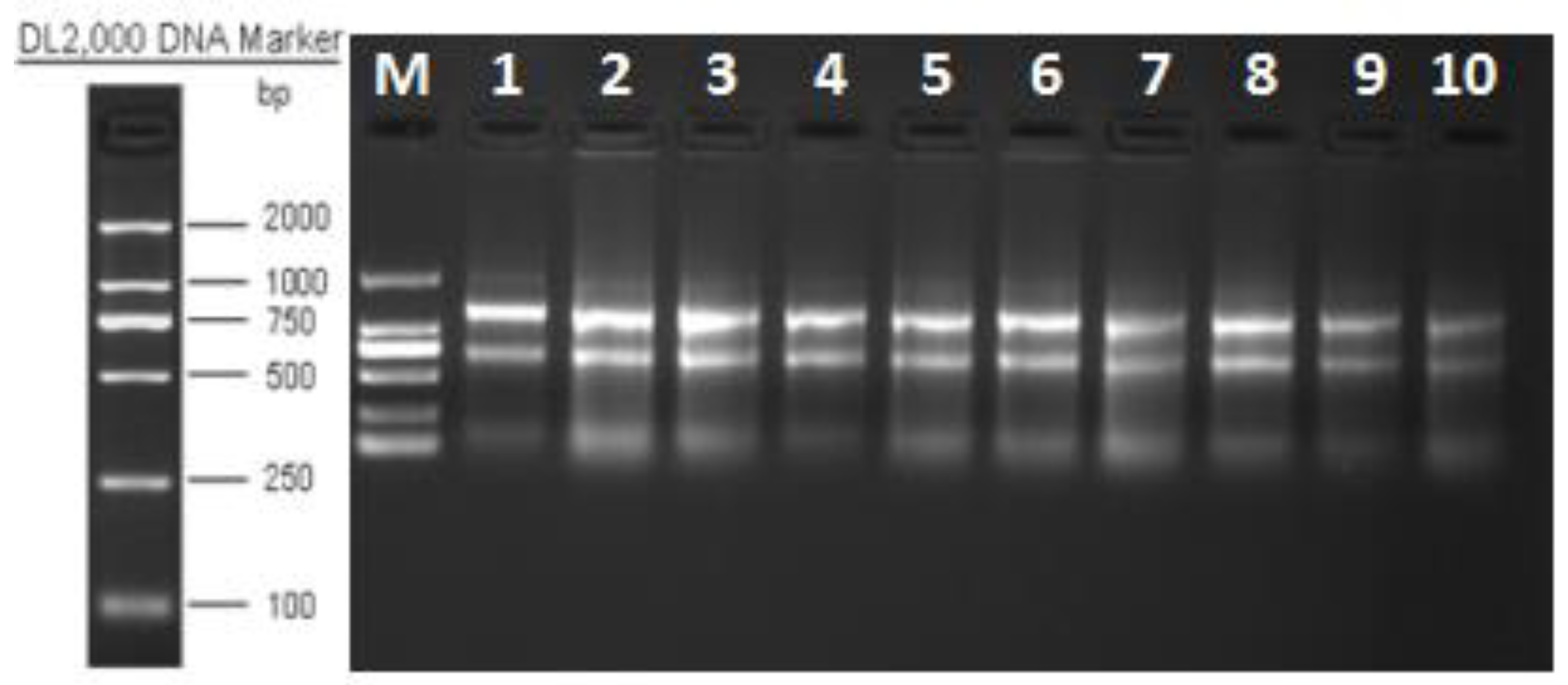
3.1. Purity and Quality of Isolated RNA
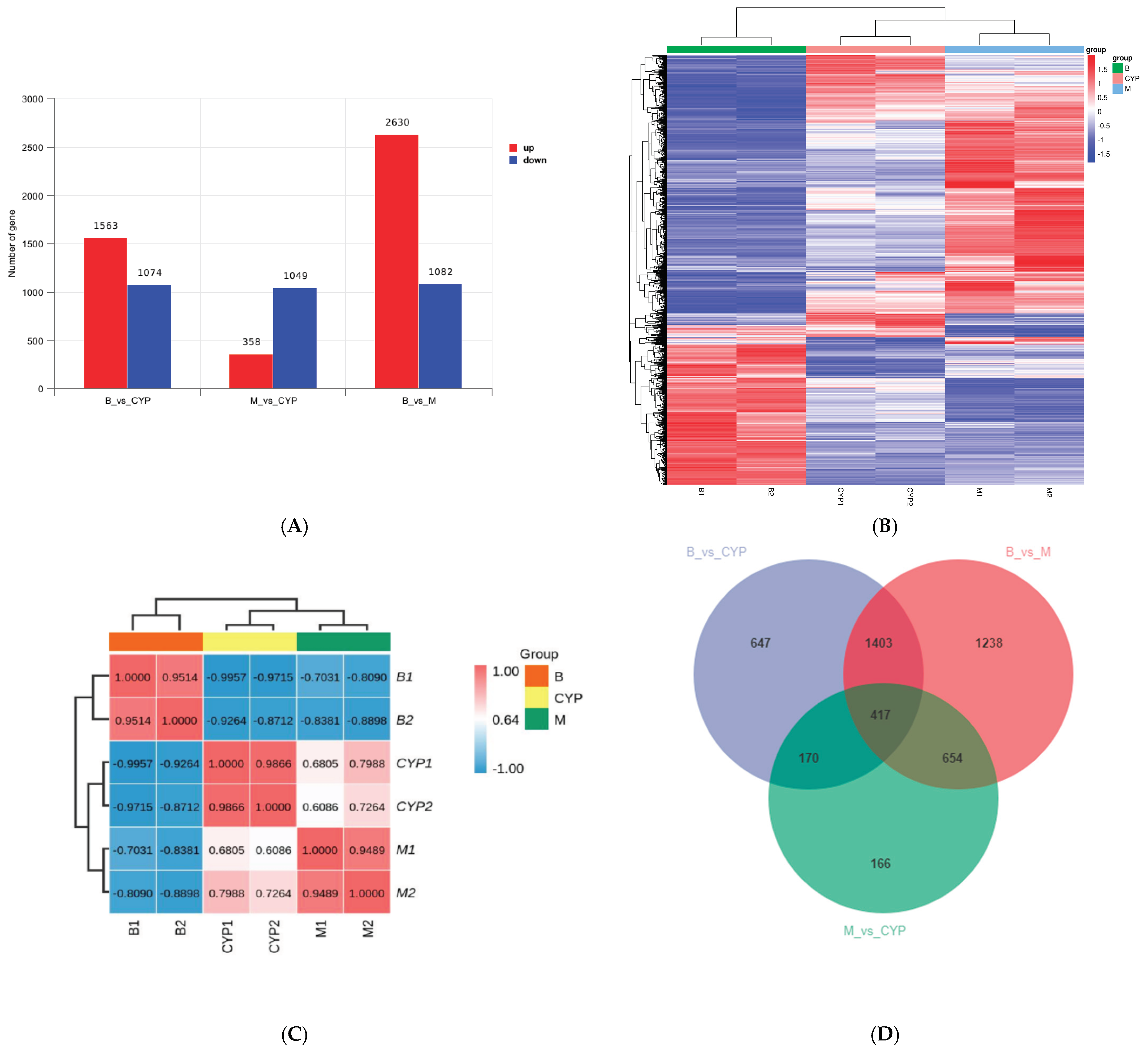
3.2. Expression Difference Result Statistics
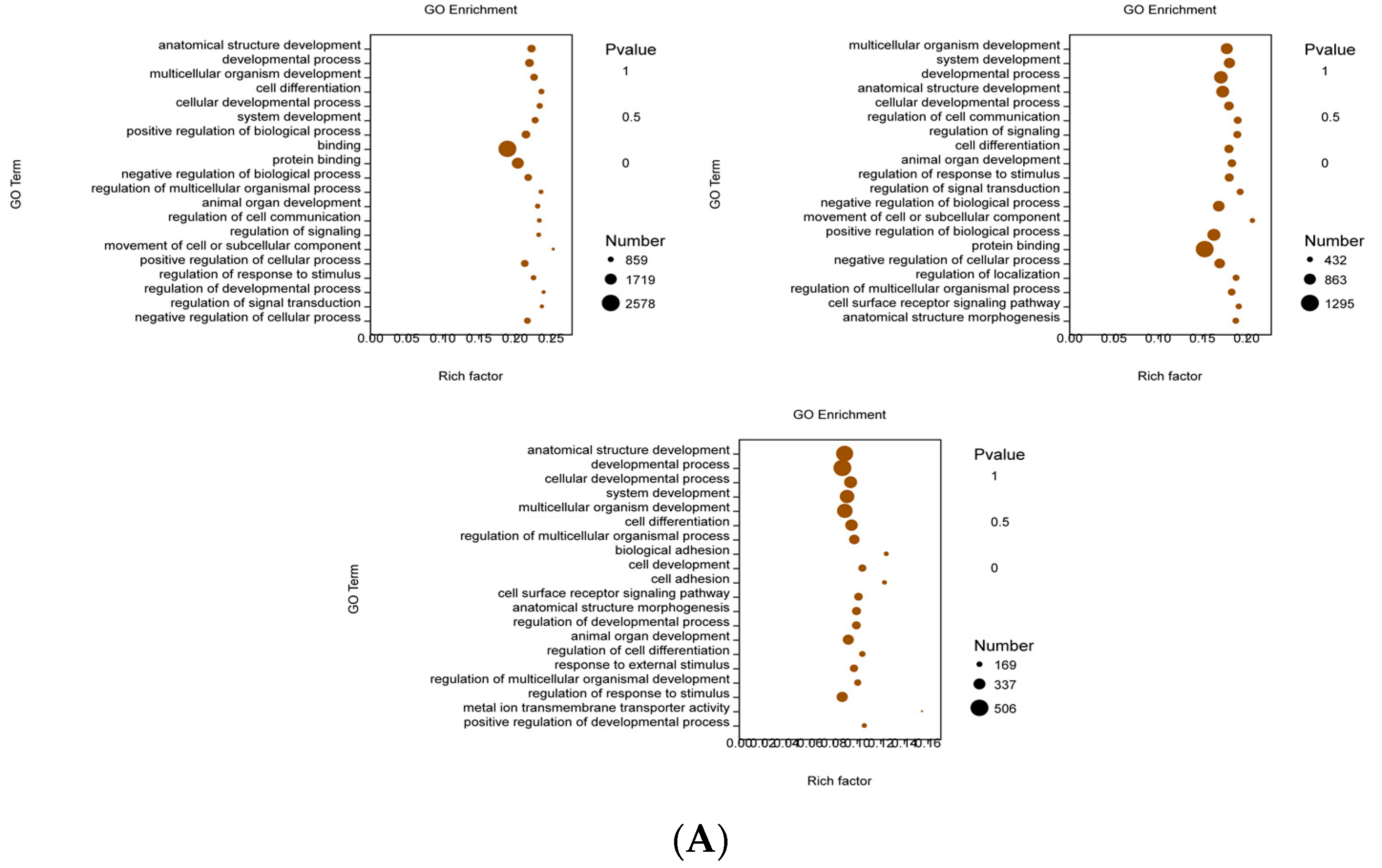
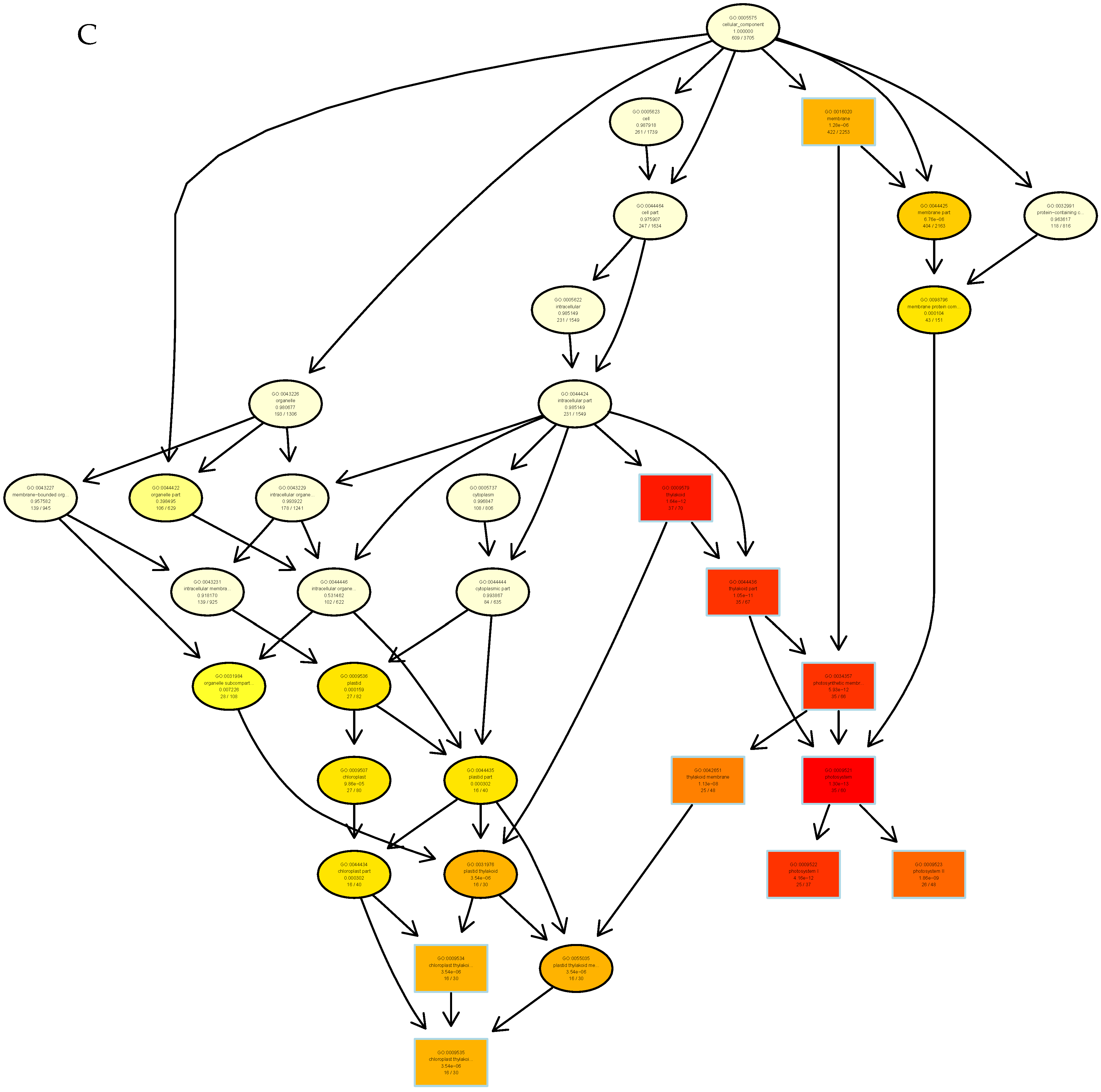
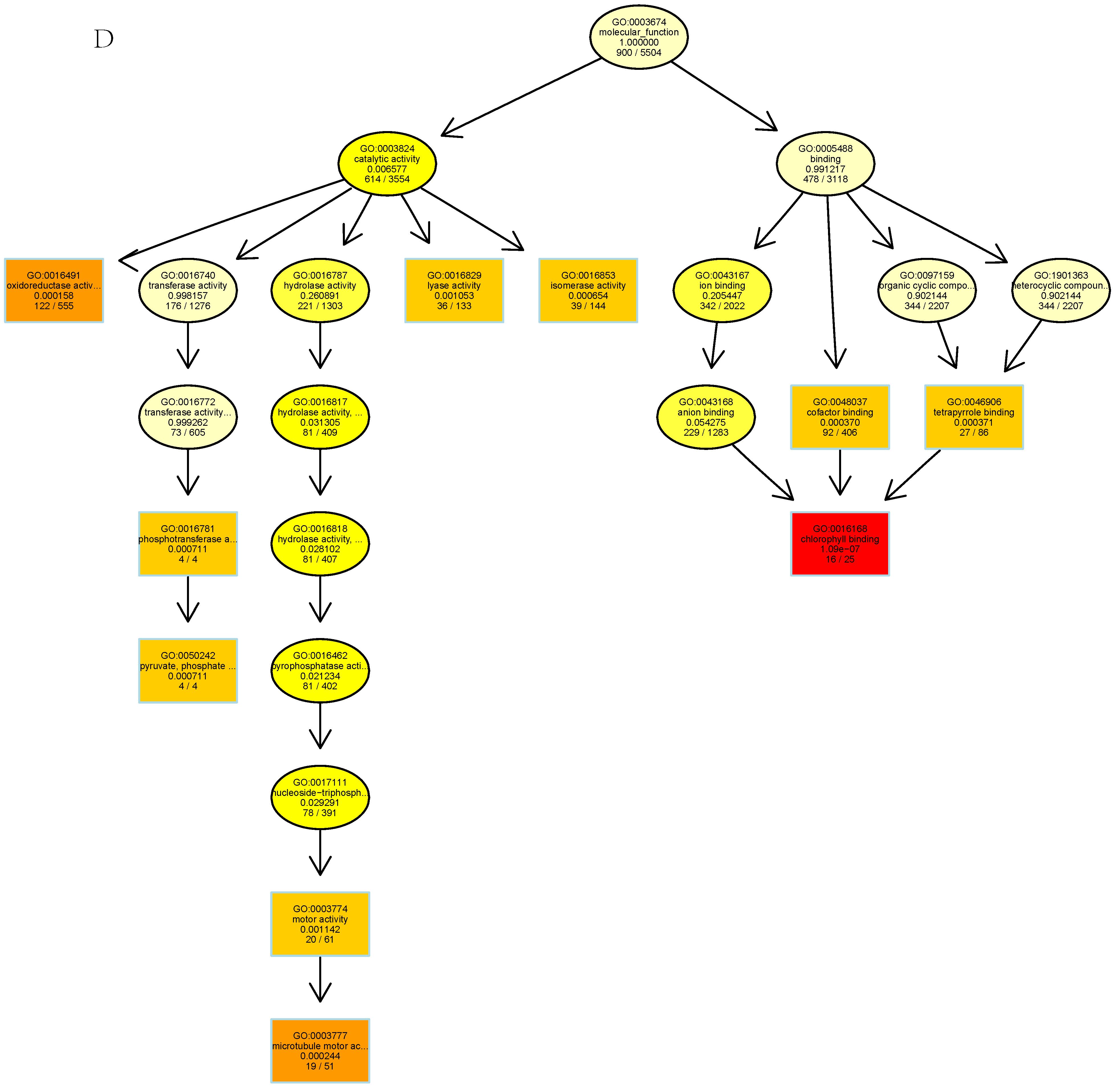
3.3. Functional Prediction Analysis by GO Enrichment
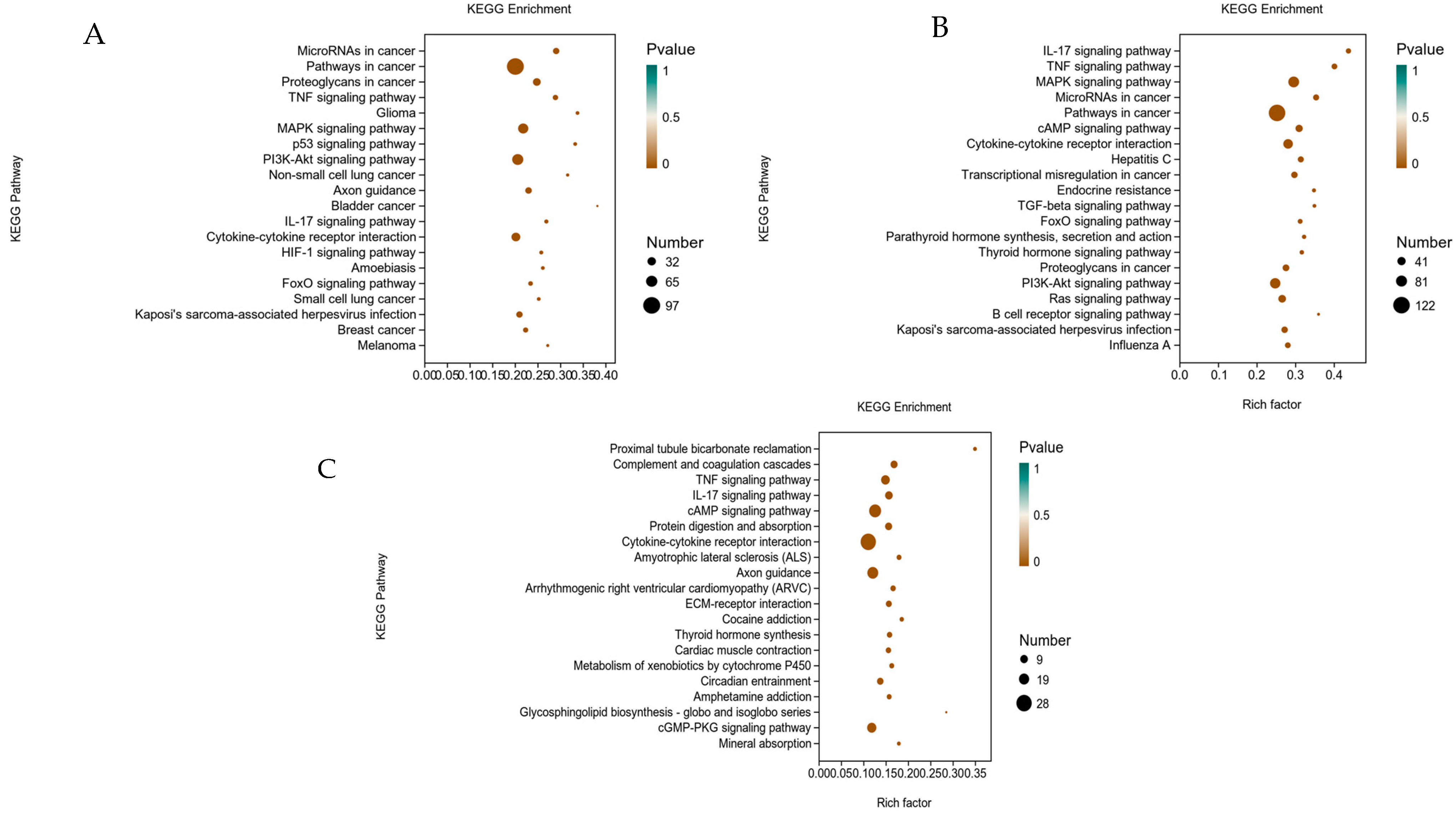
3.4. Signaling Pathway Analysis by KEGG Enrichment
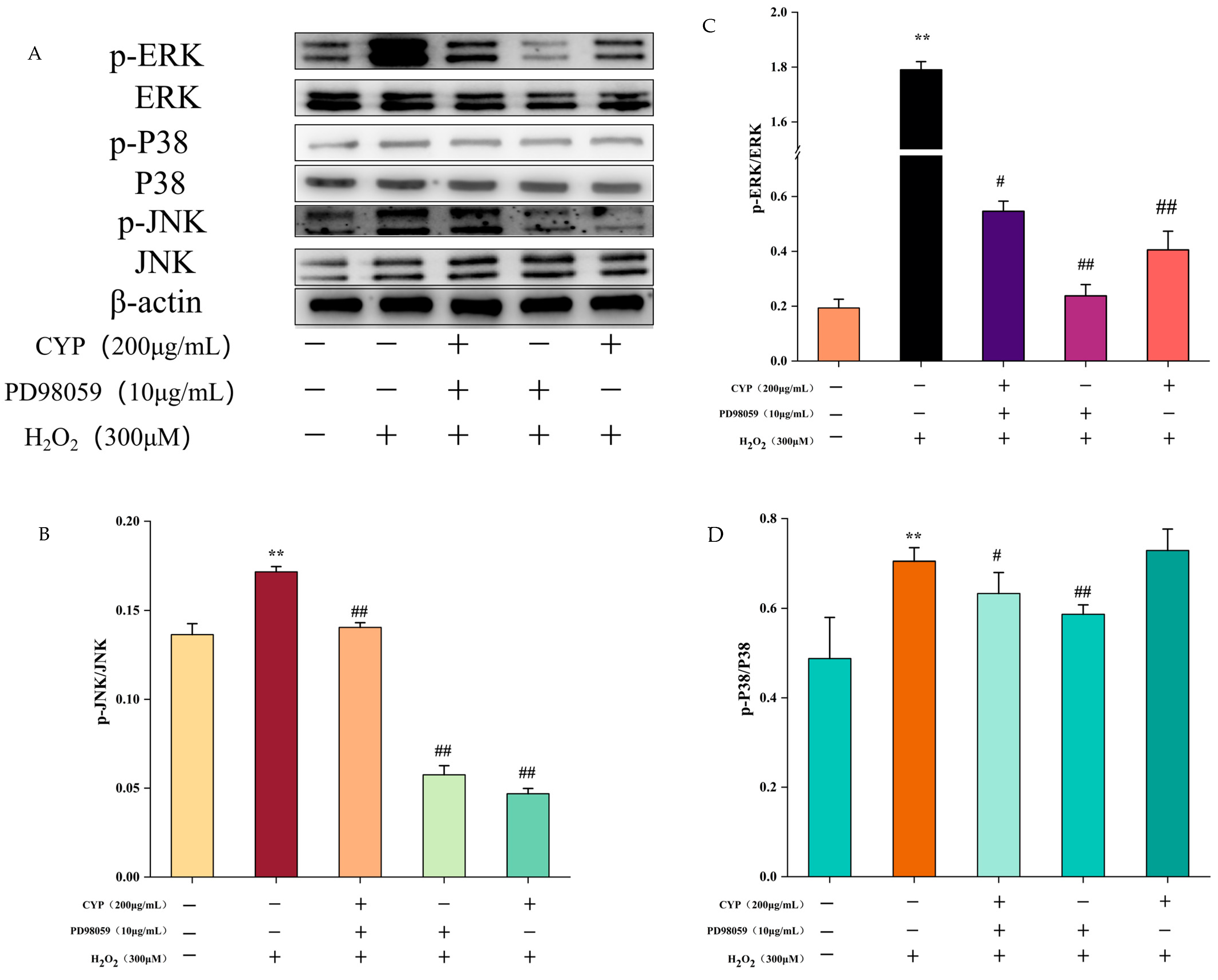
3.5. Effect of ERK Inhibitor on MAPK Pathway of H2O2-Induced Oxidative Damage in IEC-6 Cells
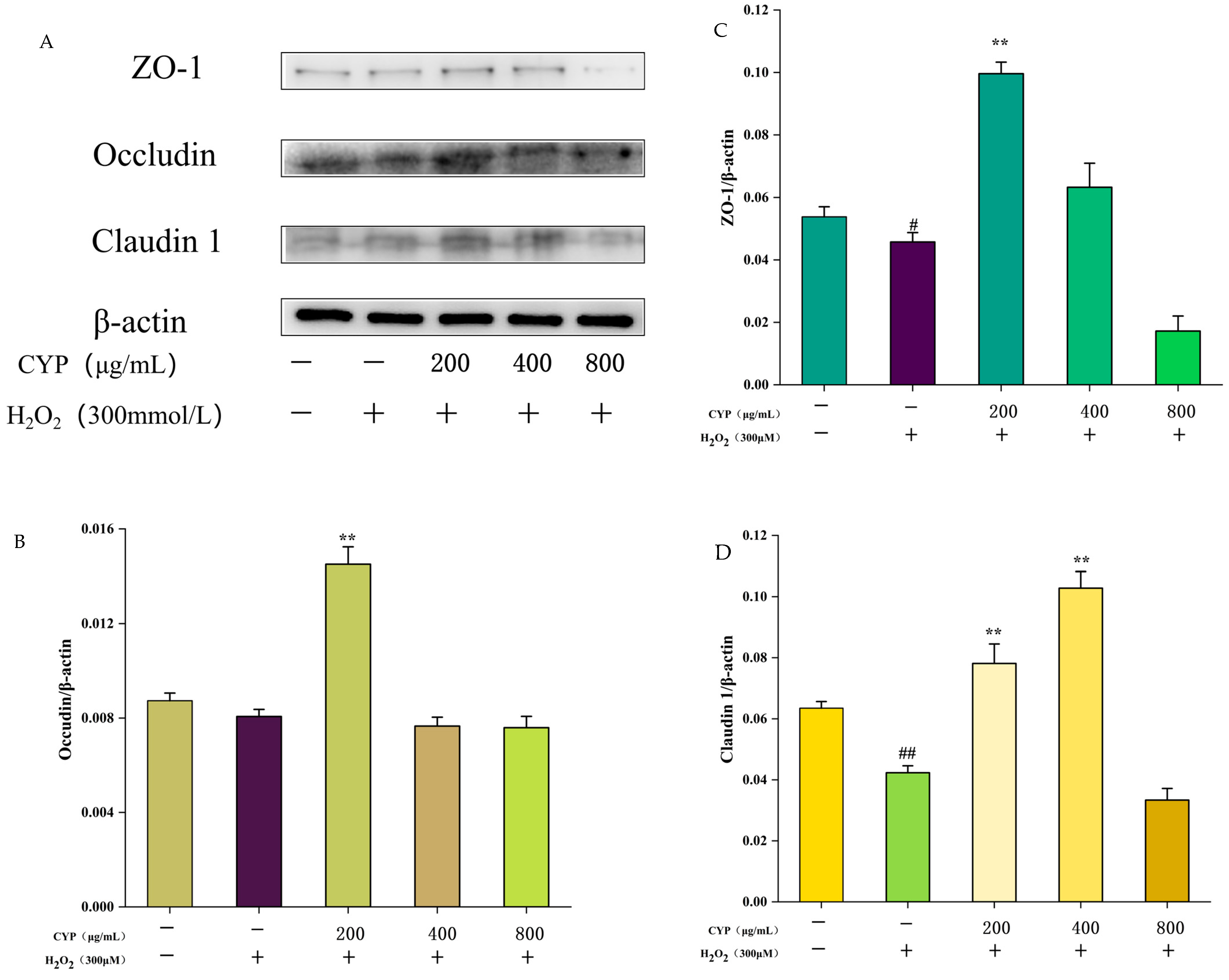
3.6. Effect of CYP on Intestinal Tight Junction Proteins ZO-1, Occludin, and Claudin-1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, Y.; Fan, F.; Fang, Y.; Li, P.; Xia, J.; Shen, X.; Liu, Q.; Hu, Q. Neuroprotective Effect of Alkylresorcinols from Wheat Bran in HT22 Cells: Correlation with in vitro Antioxidant Activity. eFood 2021, 2, 13–20. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.; King, M.; Draz, M.S.; Kline, T.; Rodriguez-Palacios, A. Oxidative reactivity across kingdoms in the gut: Host immunity, stressed microbiota and oxidized foods. Free. Radic. Biol. Med. 2022, 178, 97–110. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Chopyk, D.M.; Grakoui, A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology 2020, 159, 849–863. [Google Scholar] [CrossRef]
- Kataoka, K. The intestinal microbiota and its role in human health and disease. Med. Invest. 2016, 63, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.R.; Metz, L.; Meddings, J.B.; Sharkey, K.A.; Yong, V.W. The intestinal barrier in multiple sclerosis: Implications for pathophysiology and therapeutics. Brain 2018, 141, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Medini, F.; Bourgou, S.; Lalancette, K.; Snoussi, M.; Mkadmini, K.; Coté, I.; Abdelly, C.; Legault, J.; Ksouri, R. Phytochemical analysis, antioxidant, anti-inflammatory, and anticancer activities of the halophyte Limonium densiflorum extracts on human cell lines and murine macrophages. South Afr. J. Bot. 2015, 99, 158–164. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, M.; Ma, J.; Fang, Y.; Yang, W.; Ma, N.; Fang, D.; Hu, Q.; Pei, F. The antioxidant and antimicrobial activities of different phenolic acids grafted onto chitosan. Carbohydr. Polym. 2019, 225, 115238. [Google Scholar] [CrossRef]
- Hao, W.; Chen, Z.; Yuan, Q.; Ma, M.; Gao, C.; Zhou, Y.; Zhou, H.; Wu, X.; Wu, D.; Farag, M.A.; et al. Ginger polysaccharides relieve ulcerative colitis via maintaining intestinal barrier integrity and gut microbiota modulation. Int. J. Biol. Macromol. 2022, 219, 730–739. [Google Scholar] [CrossRef]
- Feng, T.; Yang, X.; Kong, Q.; Lu, J. Editorial: Food Bioactive Polysaccharides and Their Health Functions. RSC Adv. 2021, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Xu, J.L.; Zhang, J.C.; Liu, Y.; Sun, H.J.; Zha, X. Physicochemical properties and antioxidant activities of polysaccharide from floral mushroom cultivated in Huangshan Mountain. Carbohydr. Polym. 2015, 131, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.H.; Hou, J.L.; Han, H.P.; Wu, C.Z.; Zhang, L.Q. Effects of Yam Polysaccharide on Blood Glucose and Serum Antioxidant Capacity in Type I Diabetic Mice. Food Res. Dev. 2014, 35, 17. [Google Scholar]
- Ju, Y.; Xue, Y.; Huang, J.; Zhai, Q.; Wang, X.H. Antioxidant Chinese yam polysaccharides and its pro-proliferative effect on endometrial epithelial cells. Int. J. Biol. Macromol. 2014, 66, 81–85. [Google Scholar] [CrossRef]
- Shao, Y.; Kang, Q.; Zhu, J.; Zhao, C.C. Antioxidant properties and digestion behaviors of polysaccharides from Chinese yam fermented by Saccharomyces boulardii. LWT 2022, 154, 112752. [Google Scholar] [CrossRef]
- Ahmed, W. RNA-seq resolving host-pathogen interactions: Advances and applications. Ecol. Genet. Genom. 2020, 15, 100057. [Google Scholar] [CrossRef]
- Ding, X.; Yu, Q.; Hou, K.; Hu, X.; Wang, Y.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Indirectly stimulation of DCs by Ganoderma atrum polysaccharide in intestinal-like Caco-2/DCs co-culture model based on RNA-seq. J. Funct. Foods 2020, 67, 103850. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, R.; Cheng, H.; Li, Y.; Sui, S. Analysis of the resistance of small peptides from Periplaneta americana to hydrogen peroxide-induced apoptosis in human ovarian granular cells based on RNA-seq. Gene 2022, 813, 146120. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Shen, M.; Chen, Y. Combined RNA-seq and molecular biology technology revealed the protective effect of Cyclocarya paliurus polysaccharide on H2O2-induced oxidative damage in L02 cells thought regulating mitochondrial function, oxidative stress and PI3K/Akt and MAPK signaling pathways. Food Res. Int. 2022, 155, 111080. [Google Scholar]
- Hao, B.H.; Yang, X.; Ma, Y. Study on deproteinization in extraction of polysaccharides from Patentillaunserina by Sevage. Sci. Technol. Food Ind. 2011, 32, 254–258. [Google Scholar]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, W.; Xie, J.; Chen, Y.; Yu, Q.; Zhang, W.; Shen, M. Isolation, Characterization and Antioxidant Activity of Yam Polysaccharides. Foods 2022, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Kaneko, N.; Okudaira, K.; Matsumoto, T.; Kobayashi, T. Endothelial dysfunction caused by circulating microparticles from diabetic mice is reduced by PD98059 through ERK and ICAM-1. Eur. J. Pharmacol. 2021, 913, 174630. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Yang, W.-X. Kinesins in MAPK cascade: How kinesin motors are involved in the MAPK pathway? Gene 2019, 684, 1–9. [Google Scholar] [CrossRef]
- Su, X.; Zhao, M.; Fu, X.; Ma, X.; Xu, W.; Hu, S. Immunomodulatory activity of purified polysaccharides from Rubus chingii Hu fruits in lymphocytes and its molecular mechanisms. J. Funct. Foods 2021, 87, 104785. [Google Scholar] [CrossRef]
- Zhao, M.; Hou, J.; Zheng, S.; Ma, X.; Fu, X.; Hu, S.; Zhao, K.; Xu, W. Peucedanum praeruptorum Dunn polysaccharides regulate macrophage inflammatory response through TLR2/TLR4-mediated MAPK and NF-kappaB pathways. Biomed Pharm. 2022, 152, 113258. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.R.; Chang, H.Y. Long Noncoding RNAs in Cell-Fate Programming and Reprogramming. Cell Stem Cell 2014, 14, 752–761. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, J.; Xie, H.; Cai, M.; Yao, L.; Li, J.; Han, L.; Chen, W.; Yu, N.; Peng, D. Dendrobium huoshanense polysaccharides ameliorate ulcerative colitis by improving intestinal mucosal barrier and regulating gut microbiota. J. Funct. Foods 2022, 96, 105231. [Google Scholar] [CrossRef]
- Xu, W.; Du, A.; Hu, S. Transcriptome analysis of bovine lymphocytes stimulated by Atractylodis macrocephalae Koidz. polysaccharides in vitro. Vet. Immunol. Immunopathol. 2018, 196, 30–34. [Google Scholar] [CrossRef]
- Abdelzaher, W.Y.; Bahaa, H.A.; Elkhateeb, R.; Atta, M.; Fawzy, M.A.; Ahmed, A.F.; Rofaeil, R.R. Role of JNK, ERK, and p38 MAPK signaling pathway in protective effect of sildenafil in cyclophosphamide-induced placental injury in rats. Life Sci. 2022, 293, 120354. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.T.; Yan, T.J.; Cavanaugh, J.E.; Flaherty, P.T.; Beckman, B.S.; Burow, M.E. Oncogenic signaling of MEK5-ERK5. Cancer Lett. 2017, 392, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chien, C.-C.; Yang, L.-Y.; Huang, G.-C.; Cheng, M.-C.; Lin, C.-T.; Shen, S.-C.; Chen, Y.-C. Vitamin K3-2,3-epoxide induction of apoptosis with activation of ROS-dependent ERK and JNK protein phosphorylation in human glioma cells. Chem. -Biol. Interact. 2011, 193, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Montes-Alvarado, J.B.; Barragán, M.; Larrauri-Rodríguez, K.A.; Perez-Gonzalez, A.; Delgado-Magallón, A.; Millán-Perez-Peña, L.; Rosas-Murrieta, N.H.; Maycotte, P. ERK activation modulates invasiveness and Reactive Oxygen Species (ROS) production in triple negative breast cancer cell lines. Cell. Signal. 2023, 101, 110487. [Google Scholar]
- Ding, H.; Wang, F.; Su, L.; Zhao, L.; Hu, B.; Zheng, W.; Yao, S.; Li, Y. Involvement of MEK5/ERK5 signaling pathway in manganese-induced cell injury in dopaminergic MN9D cells. J. Trace Elem. Med. Biol. 2020, 61, 126546. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Park, S.-Y.; Choung, S.-Y. Enhancing effects of myricetin on the osteogenic differentiation of human periodontal ligament stem cells via BMP-2/Smad and ERK/JNK/p38 mitogen-activated protein kinase signaling pathway. Eur. J. Pharmacol. 2018, 834, 84–91. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Jia, L. Polysaccharides from Agrocybe cylindracea residue alleviate type 2-diabetes-induced liver and colon injuries by p38 MAPK signaling pathway. Food Biosci. 2022, 47, 101690. [Google Scholar] [CrossRef]
- Huang, R.; Xie, J.; Liu, X.; Shen, M. Sulfated modification enhances the modulatory effect of yam polysaccharide on gut microbiota in cyclophosphamide-treated mice. Food Res. Int. 2021, 145, 110393. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, M.; Cai, R.; Li, Z.; Chen, X.; Xie, J. The Molecular Mechanism of Yam Polysaccharide Protected H2O2-Induced Oxidative Damage in IEC-6 Cells. Foods 2023, 12, 262. https://doi.org/10.3390/foods12020262
Shen M, Cai R, Li Z, Chen X, Xie J. The Molecular Mechanism of Yam Polysaccharide Protected H2O2-Induced Oxidative Damage in IEC-6 Cells. Foods. 2023; 12(2):262. https://doi.org/10.3390/foods12020262
Chicago/Turabian StyleShen, Mingyue, Ruixin Cai, Zhedong Li, Xiaodie Chen, and Jianhua Xie. 2023. "The Molecular Mechanism of Yam Polysaccharide Protected H2O2-Induced Oxidative Damage in IEC-6 Cells" Foods 12, no. 2: 262. https://doi.org/10.3390/foods12020262
APA StyleShen, M., Cai, R., Li, Z., Chen, X., & Xie, J. (2023). The Molecular Mechanism of Yam Polysaccharide Protected H2O2-Induced Oxidative Damage in IEC-6 Cells. Foods, 12(2), 262. https://doi.org/10.3390/foods12020262






