Enzymatic Hydrolysis of Orange-Footed Sea Cucumber (Cucumaria frondosa)—Effect of Different Enzymes on Protein Yield and Bioactivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
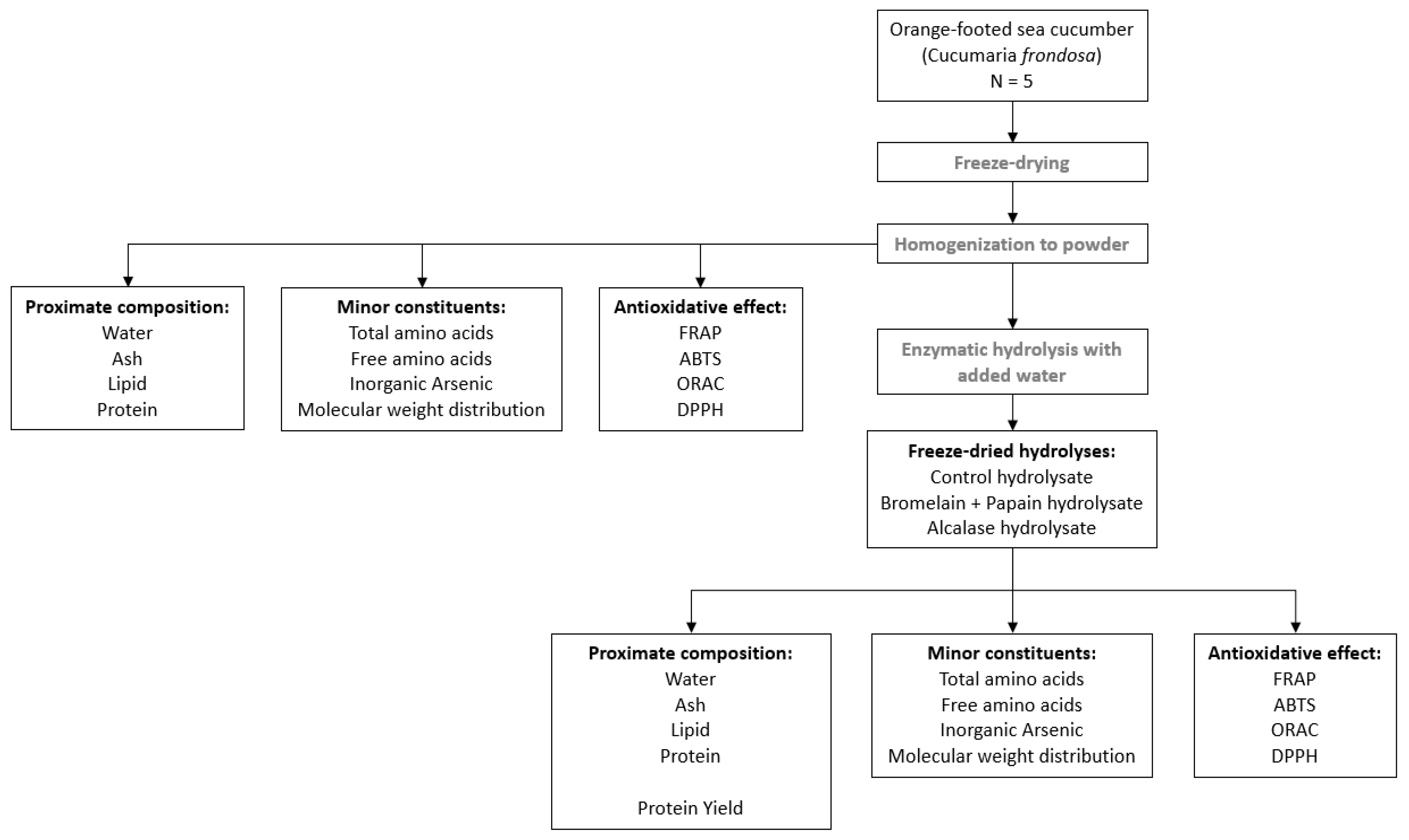
2.2. Study Design
2.3. Proximate Composition
2.4. Enzymatic Hydrolysis
2.5. Total Amino Acids
2.6. Free Amino Acids
2.7. Inorganic Arsenic Analysis
2.8. Molecular Weight Distribution
2.9. Antioxidant Assays
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of C. frondosa
3.2. Total and Free Amino Acid Composition of C. frondosa
3.3. Protein Content and Protein Yield of C. frondosa Hydrolysates
3.4. Total and Free Amino Acid Composition of C. frondosa Hydrolysates
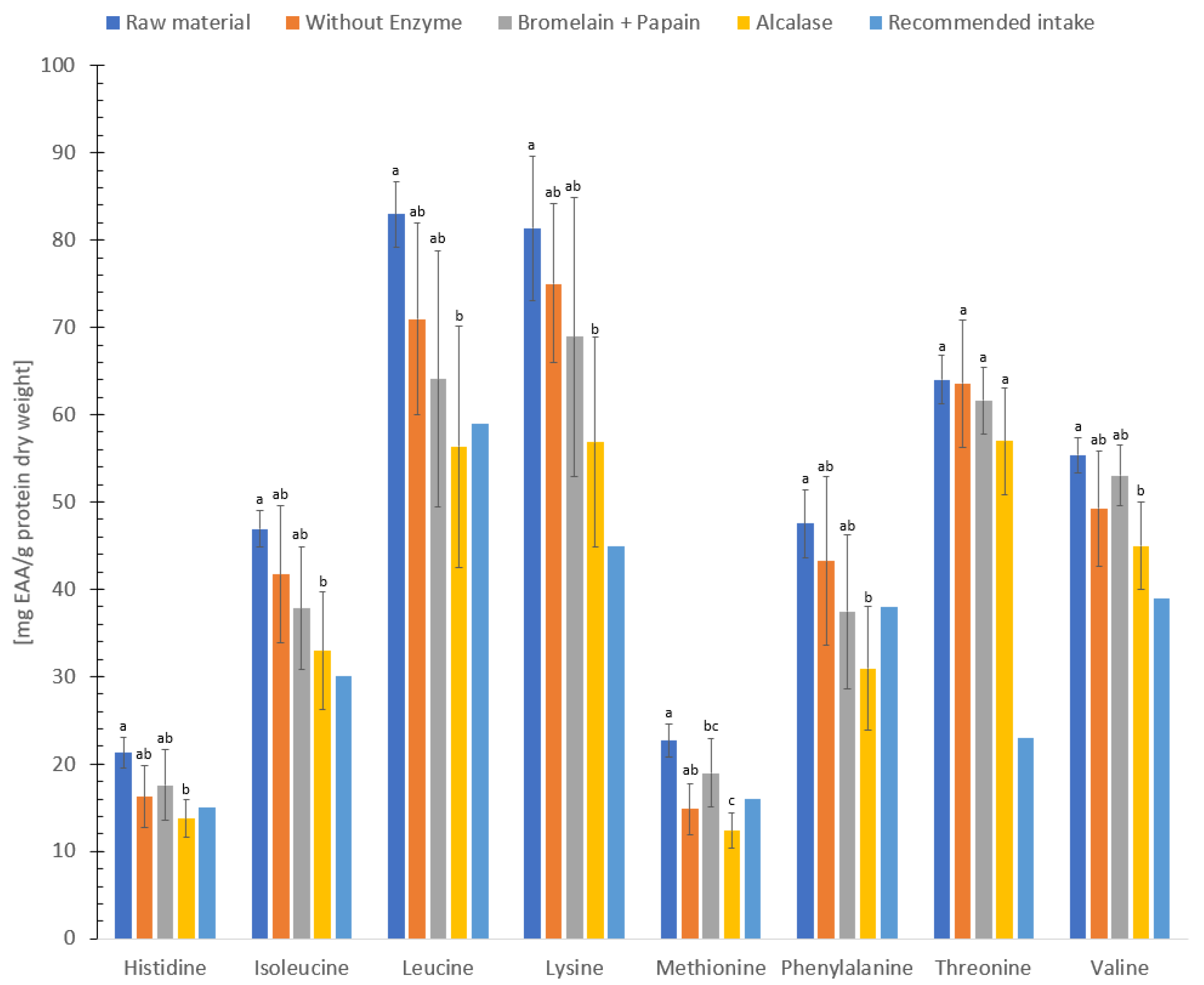
3.5. Protein Quality
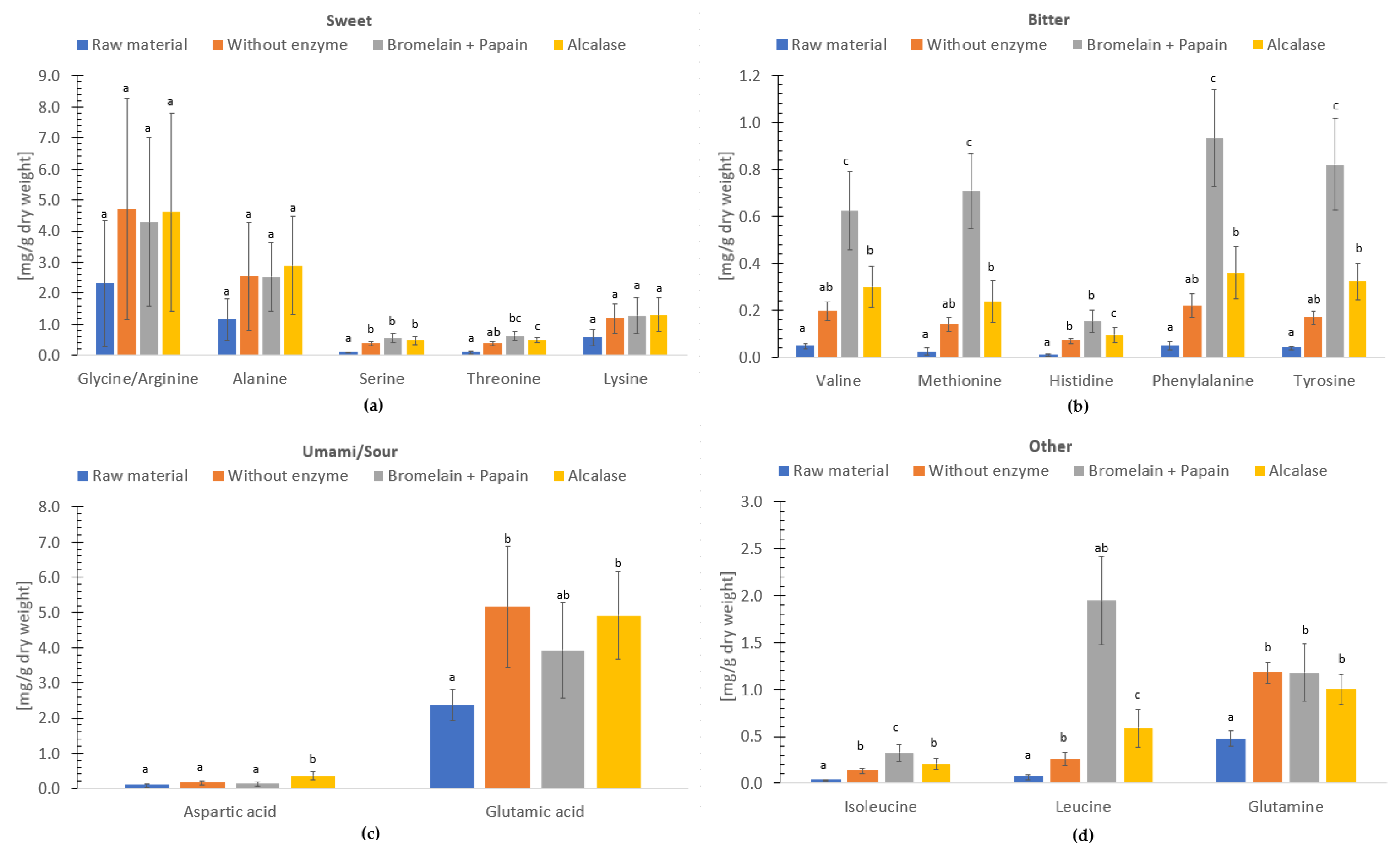
3.6. Umami
3.7. Total Inorganic Arsenic Concentration
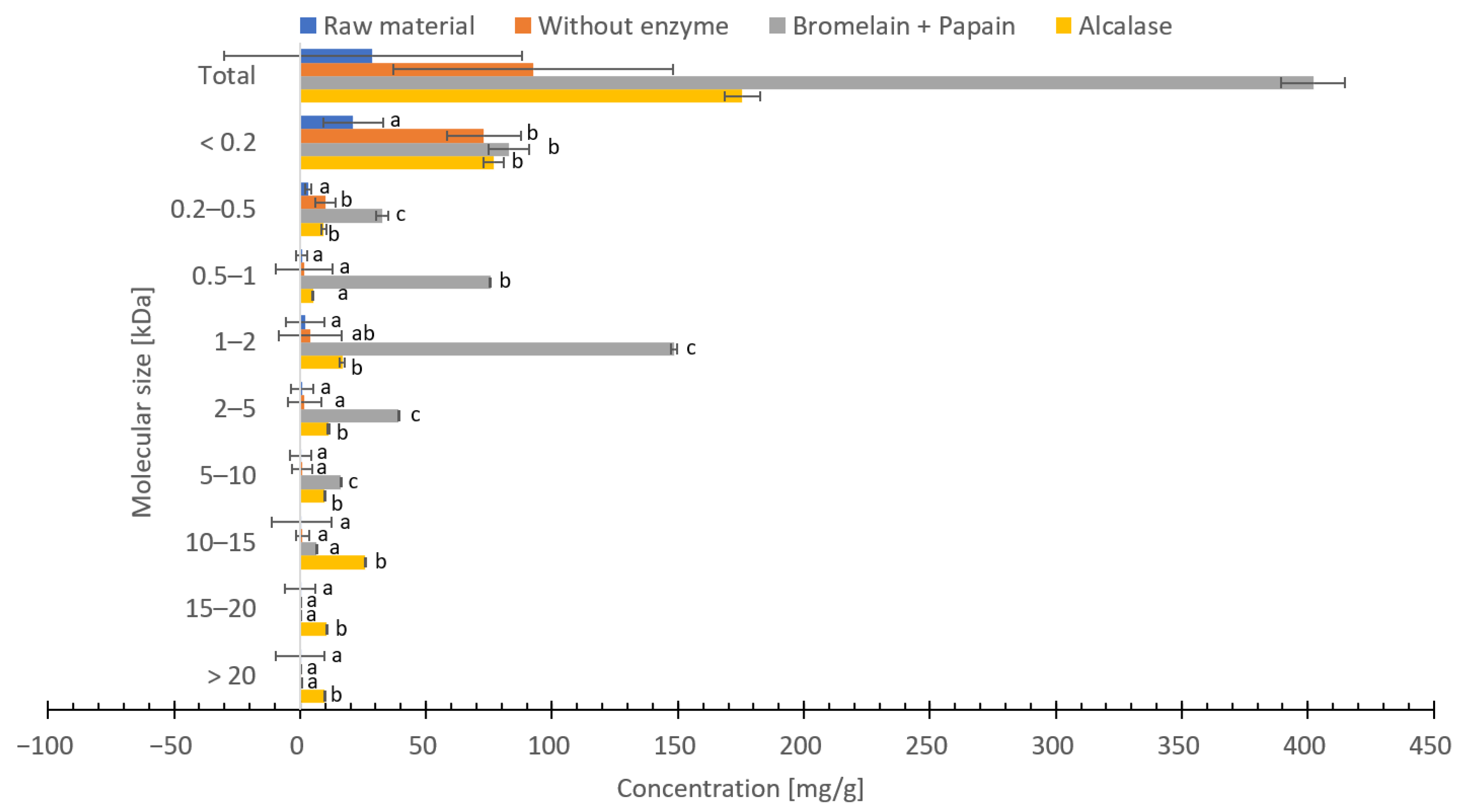
3.8. Molecular Weight Distribution
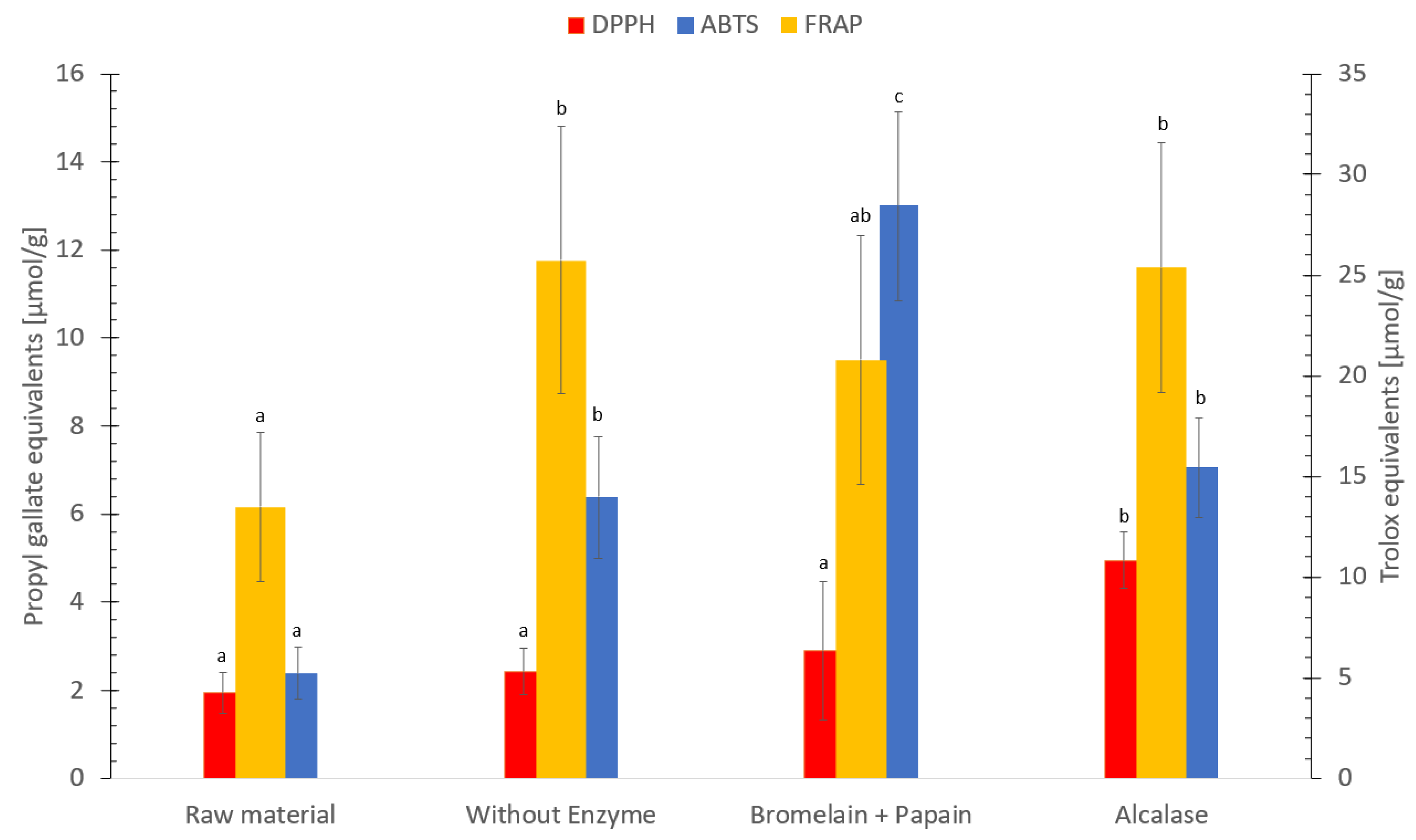
3.9. Antioxidative Capacity Measured by FRAP, ABTS, DPPH and ORAC Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golden, C.D.; Koehn, J.Z.; Shepon, A.; Passarelli, S.; Free, C.M.; Viana, D.F.; Matthey, H.; Eurich, J.G.; Gephart, J.A.; Fluet-Chouinard, E. Aquatic foods to nourish nations. Nature 2021, 598, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zamborain-Mason, J.; Viana, D.; Nicholas, K.; Jackson, E.D.; Koehn, J.Z.; Passarelli, S.; Yoo, S.-H.; Zhang, A.W.; Davin, H.C.; Duggan, C.P. A Decision Framework for Selecting Critically Important Nutrients from Aquatic Foods. Curr. Environ. Health Rep. 2023, 10, 172–183. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- SAPEA. Science Advice for Policy by European Academies—Food from the Oceans, 1st ed.; SAPEA: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Gephart, J.A.; Henriksson, P.J.; Parker, R.W.; Shepon, A.; Gorospe, K.D.; Bergman, K.; Eshel, G.; Golden, C.D.; Halpern, B.S.; Hornborg, S. Environmental performance of blue foods. Nature 2021, 597, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Styrbæk, K. Design and ‘umamification’ of vegetable dishes for sustainable eating. Int. J. Food Des. 2020, 5, 9–42. [Google Scholar] [CrossRef]
- Schmidt, C.V.; Mouritsen, O.G. The solution to sustainable eating is not a one-way street. Front. Psychol. 2020, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O. Seaweeds—Edible, Available, and Sustainable; The University of Chicago Press: Chicago, IL, USA, 2013; pp. 1–304. [Google Scholar]
- Mouritsen, O.G.; Duelund, L.; Petersen, M.A.; Hartmann, A.L.; Frøst, M.B. Umami taste, free amino acid composition, and volatile compounds of brown seaweeds. J. Appl. Phycol. 2019, 31, 1213–1232. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef]
- Purcell, S.W.; Samyn, Y.; Conand, C. Commercially Important Sea Cucumbers of the World, FAO Species Catalogue for Fishery Purposes No. 6 ed.; FAO: Rome, Italy, 2012. [Google Scholar]
- Purcell, S.W.; Conand, C.; Uthicke, S.; Byrne, M. Ecological roles of exploited sea cucumbers. In Oceanography and Marine Biology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 375–394. [Google Scholar]
- Department of Fisheries and Aquaculture—Sea Cucumber (Cucumaria frondosa). Available online: https://www.gov.nl.ca/ffa/files/research-development-fdp-pdf-sea-cumcumber.pdf (accessed on 20 September 2023).
- Kjerstad, M. Sjøpølse Har et Stort Potensial [Sea cucumber Has a Great Potential]. Fiskeribladet [The Fishery Newspaper]. Available online: https://www.fiskeribladet.no/meninger/-sjopolse-har-et-stort-potensial/8-1-62678 (accessed on 19 December 2022).
- Langdal, A.; Eilertsen, K.-E.; Kjellevold, M.; Heimstad, E.S.; Jensen, I.-J.; Elvevoll, E.O. Climate Performance, Environmental Toxins and Nutrient Density of the Underutilized Norwegian Orange-Footed Sea Cucumber (Cucumaria frondosa). Foods 2023, 12, 114. [Google Scholar] [CrossRef]
- HI. Sjøpølse [Sea Cucumber]. Available online: https://www.hi.no/hi/temasider/hav-og-kyst/nye-marine-ressurser-til-mat-og-for/sjopolse (accessed on 20 December 2022).
- EFSA. Dietary exposure to inorganic arsenic in the European population (European Food Safety Authority). EFSA J. 2014, 12, 3597. [Google Scholar] [CrossRef]
- Gajdosechova, Z.; Palmer, C.H.; Dave, D.; Jiao, G.; Zhao, Y.; Tan, Z.; Chisholm, J.; Zhang, J.; Stefanova, R.; Hossain, A. Arsenic speciation in sea cucumbers: Identification and quantitation of water-extractable species. Environ. Pollut. 2020, 266, 115190. [Google Scholar] [CrossRef] [PubMed]
- Elvevoll, E.O.; James, D.; Toppe, J.; Gamarro, E.G.; Jensen, I.-J. Food Safety Risks Posed by Heavy Metals and Persistent Organic Pollutants (POPs) related to Consumption of Sea Cucumbers. Foods 2022, 11, 3992. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, T.R.; Dave, D.; Shahidi, F. Antioxidant potential and physicochemical properties of protein hydrolysates from body parts of North Atlantic sea cucumber (Cucumaria frondosa). Food Prod. Process. Nutr. 2021, 3, 1–22. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Nutritional composition and antioxidant properties of protein hydrolysates prepared from echinoderm byproducts. Int. J. Food Sci. Technol. 2010, 45, 147–154. [Google Scholar] [CrossRef]
- Nanda, R.F.; Bahar, R.; Syukri, D.; Thu, N.N.A.; Kasim, A. A review: Application of bromelain enzymes in animal food products. Andalasian Int. J. Agric. Nat. Sci. (AIJANS) 2020, 1, 33–44. [Google Scholar] [CrossRef]
- Shouket, H.; Ameen, I.; Tursunov, O.; Kholikova, K.; Pirimov, O.; Kurbonov, N.; Ibragimov, I.; Mukimov, B. Study on industrial applications of papain: A succinct review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Tashkent, Uzbekistan, 2020; p. 012171. [Google Scholar]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavano, O.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef] [PubMed]
- Hjellnes, V.; Rustad, T.; Jensen, I.-J.; Eiken, E.; Pettersen, S.M.; Falch, E. Ultrafiltration of Saithe (Pollachius virens) Protein Hydrolysates and Its Effect on Antioxidative Activity. Catalysts 2021, 11, 1053. [Google Scholar] [CrossRef]
- Yan, M.; Tao, H.; Qin, S. Effect of enzyme type on the antioxidant activities and functional properties of enzymatic hydrolysates from sea cucumber (Cucumaria frondosa) viscera. J. Aquat. Food Prod. 2016, 25, 940–952. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Hashim, R.B.; Taher, M.; Daud, J.M.; Ikeda, M.-A.; Zali, B. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur. J. Sci. Res. 2009, 37, 376–387. [Google Scholar]
- Hamaguchi, P.; Geirsdottir, M.; Vrac, A.; Kristinsson, H.; Sveinsdottir, H.; Fridjonsson, O.; Hreggvidsson, G. In vitro antioxidant and antihypertensive properties of Icelandic sea cucumber (Cucumaria frondosa). Proc. IFT 2010, 10, 282-04. [Google Scholar]
- AOCS. Official Methods of Analysis of AOAC, 18th ed.; Association of Official Analytical Chemist: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Latimer, G. Association of Official Analytical Chemists International, Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Maclean, W.; Harnly, J.; Chen, J.; Chevassus-Agnes, S.; Gilani, G.; Livesey, G.; Warwick, P. Food energy–Methods of analysis and conversion factors. In Food and Agriculture Organization of the United Nations Technical Workshop Report; The Food and Agriculture Organization: Rome, Italy, 2003; Volume 77, pp. 8–9. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S. Amino Acid Determination: Methods and Techniques, 2nd ed.; Marcel Dekker: New York, NY, USA, 1978. [Google Scholar]
- Osnes, K.K.; Mohr, V. Peptide hydrolases of Antartic krill, Euphausia superba. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1985, 82, 599–606. [Google Scholar] [CrossRef]
- Standard-Norge. Foodstuffs—Determination of Elements and Their Chemical Species—Determination of Inorganic Arsenic in Foodstuffs of Marine and Plant Origin by Anion-Exchange HPLC-ICP-MS; Norsk Standard: Oslo, Norway, 2016. [Google Scholar]
- Standard-Norge. Foodstuffs—Determination of Trace Elements—Determination of Iodine by ICP-MS (Inductively Coupled Plasma Mass Spectrometry); Norsk Standard: Oslo, Norway, 2007. [Google Scholar]
- Wiech, M.; Silva, M.; Meier, S.; Tibon, J.; Berntssen, M.H.; Duinker, A.; Sanden, M. Undesirables in mesopelagic species and implications for food and feed safety—Insights from Norwegian Fjords. Foods 2020, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Wang, L.-F.; Tsimidou, M.; Zhang, H.-Y. Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J. Agric. Food Chem 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Lazaridou, O.; Tsimidou, M.Z. Use of reference compounds in antioxidant activity assessment. J. Agric. Food Chem. 2007, 55, 5452–5460. [Google Scholar] [CrossRef]
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme. J. Food Biochem. 2007, 31, 266–287. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Jensen, I.-J.; Abrahamsen, H.; Maehre, H.K.; Elvevoll, E.O. Changes in antioxidative capacity of saithe (Pollachius virens) and shrimp (Pandalus borealis) during in vitro digestion. J. Agric. Food Chem. 2009, 57, 10928–10932. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC− fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Khan, M.A.; Shahidi, F. Compositional characteristics and antioxidant properties of fresh and processed sea cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2007, 55, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Rasyid, A.; Yasman, Y.; Putra, M.Y. Current prospects of nutraceutical and pharmaceutical use of sea cucumbers. Pharmacia 2021, 68, 561. [Google Scholar]
- Al Azad, S.; Shaleh, S.R.M.; Siddiquee, S. Comparison of fatty acid and proximate composition between Holothuria edulis and Holothuria scabra collected from coastal water of Sabah, Malaysia. Adv. Biol. Biotechnol. 2017, 8, 91. [Google Scholar]
- Song, Z.; Li, H.; Wen, J.; Zeng, Y.; Ye, X.; Zhao, W.; Xu, T.; Xu, N.; Zhang, D. Consumers’ attention on identification, nutritional compounds, and safety in heavy metals of Canadian sea cucumber in Chinese food market. Food Sci. Nutr. 2020, 8, 5962–5975. [Google Scholar] [CrossRef] [PubMed]
- Sroyraya, M.; Hanna, P.J.; Siangcham, T.; Tinikul, R.; Jattujan, P.; Poomtong, T.; Sobhon, P. Nutritional components of the sea cucumber Holothuria scabra. Funct. Foods Health Dis. 2017, 7, 168–181. [Google Scholar] [CrossRef]
- Abuzaytoun, R.; Budge, S.M.; Xia, W.; MacKinnon, S. Unusual ether lipids and branched chain fatty acids in sea cucumber (Cucumaria frondosa) viscera and their seasonal variation. Mar. Drugs 2022, 20, 435. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Papain from Carica Papaya. Available online: https://www.sigmaaldrich.com/NO/en/product/sigma/76216 (accessed on 23 January 2023).
- Drenth, J.; Jansonius, J.; Koekoek, R.; Wolthers, B. The structure of papain. Adv. Protein Chem. Struct. Biol. 1971, 25, 79–115. [Google Scholar]
- Singh, P.K.; Shrivastava, N.; Ojha, B.K. Chapter 8—Enzymes in the Meat Industry—Production, Application, and Future Prospects. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 111–128. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Bromelain from Pineapple Stem. Available online: https://www.sigmaaldrich.com/NO/en/product/sigma/b4882?gclid=CjwKCAiA2rOeBhAsEiwA2Pl7Q1HyezwxjtzyJ6rCuXpoBrU7sXzACnjzTs5RiGsVpRTZeKnZ4bCnGhoCUJ4QAvD_BwE&gclsrc=aw.ds (accessed on 23 January 2023).
- Biocon. Bromelain—Purified Enzymatic Preparation. Available online: http://biocon.es/wp-content/uploads/2016/12/TDS-Bromelain_eng.pdf (accessed on 23 January 2023).
- Sigma-Aldrich. Protease from Bacillus licheniformis. Available online: https://www.sigmaaldrich.com/NO/en/product/sigma/p4860?context=product (accessed on 23 January 2023).
- Adamson, N.J.; Reynolds, E.C. Characterization of casein phosphopeptides prepared using Alcalase: Determination of enzyme specificity. Enzyme Microb. Technol. 1996, 19, 202–207. [Google Scholar] [CrossRef]
- Slizyte, R.; Rommi, K.; Mozuraityte, R.; Eck, P.; Five, K.; Rustad, T. Bioactivities of fish protein hydrolysates from defatted salmon backbones. Biotechnol. Rep. 2016, 11, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Tripoteau, L.; Bedoux, G.; Gagnon, J.; Bourgougnon, N. In vitro antiviral activities of enzymatic hydrolysates extracted from byproducts of the Atlantic holothurian Cucumaria frondosa. Process Biochem. 2015, 50, 867–875. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.-J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- WHO. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation. WHO Technical Report Series; no. 935. Available online: https://apps.who.int/iris/handle/10665/43411 (accessed on 20 September 2022).
- Kendler, S.; Thornes, F.W.; Jakobsen, A.N.; Lerfall, J. Nutritional profiling and contaminant levels of five underutilized fish species in Norway. Front. Nutr. 2023, 10, 1118094. [Google Scholar] [CrossRef] [PubMed]
- Esaiassen, M.; Jensen, T.K.; Edvinsen, G.K.; Otnæs, C.H.; Ageeva, T.N.; Mæhre, H.K. Nutritional value and storage stability in commercially produced organically and conventionally farmed Atlantic salmon (Salmo salar L.) in Norway. Appl. Food Res. 2022, 2, 100033. [Google Scholar] [CrossRef]
- Wang, H.; Ma, F.; Cheng, L. Metabolism of organic acids, nitrogen and amino acids in chlorotic leaves of ‘Honeycrisp’apple (Malus domestica Borkh) with excessive accumulation of carbohydrates. Planta 2010, 232, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.G.; Jadhav, J.P. A novel source of biofertilizer from feather biomass for banana cultivation. Environ. Sci. Pollut. Res. 2013, 20, 4532–4539. [Google Scholar] [CrossRef]
- Boukid, F.; Baune, M.-C.; Gagaoua, M.; Castellari, M. Seafood alternatives: Assessing the nutritional profile of products sold in the global market. Eur. Food Res. Technol. 2022, 248, 1777–1786. [Google Scholar] [CrossRef]
- Sarower, M.G.; Hasanuzzaman, A.F.M.; Biswas, B.; Abe, H. Taste producing components in fish and fisheries products: A review. Int. J. Food Ferment. Technol. 2012, 2, 113–121. [Google Scholar]
- Kirimura, J.; Shimizu, A.; Kimizuka, A.; Ninomiya, T.; Katsuya, N. Contribution of peptides and amino acids to the taste of foods. J. Agric. Food Chem. 1969, 17, 689–695. [Google Scholar] [CrossRef]
- Fuke, S.; Konosu, S. Taste-active components in some foods: A review of Japanese research. Physiol. Behav. 1991, 49, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, X.; Liu, Y. Characterization and evaluation of umami taste: A review. Trends Anal. Chem. 2020, 127, 115876. [Google Scholar] [CrossRef]
- Duan, Z.; Dong, S.; Sun, Y.; Dong, Y.; Gao, Q. Response of Atlantic salmon (Salmo salar) flavor to environmental salinity while culturing between freshwater and seawater. Aquaculture 2021, 530, 735953. [Google Scholar] [CrossRef]
- Nishimura, T.; Kato, H. Taste of free amino acids and peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar] [CrossRef]
- Hoehl, K.; Schoenberger, G.; Busch-Stockfisch, M. Stimulus and recognition thresholds for the basic tastes in deionized water. Are the recommendations for citric acid too high. Ernahr. Umsch. 2014, 61, 130–136. [Google Scholar]
- Guennoun, Y.; Benajiba, N.; Elkari, K.; Bouziani, A.; Elammari, L.; Al-Jawaldeh, A.; Elhaloui, N.; Barkat, A.; Benkirane, H.; Aguenaou, H. The threshold of sweet taste recognition among a sample of Moroccan population. Food Sci. Nutr. 2022, 52, 45–60. [Google Scholar] [CrossRef]
- Ferrante, M.; Napoli, S.; Grasso, A.; Zuccarello, P.; Cristaldi, A.; Copat, C. Systematic review of arsenic in fresh seafood from the Mediterranean Sea and European Atlantic coasts: A health risk assessment. Food Chem. Toxicol. 2019, 126, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Dahl, L.; Molin, M.; Amlund, H.; Meltzer, H.M.; Julshamn, K.; Alexander, J.; Sloth, J.J. Stability of arsenic compounds in seafood samples during processing and storage by freezing. Food Chem. 2010, 123, 720–727. [Google Scholar] [CrossRef]
- FAO/WHO. Safety Evaluation of Certain Contaminants in Food: Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Rome, Italy, 2011; pp. 1–799. [Google Scholar]
- Park, G.-y.; Kang, D.-e.; Davaatseren, M.; Shin, C.; Kang, G.-J.; Chung, M.-S. Reduction of total, organic, and inorganic arsenic content in Hizikia fusiforme (Hijiki). Food Sci. Biotechnol. 2019, 28, 615–622. [Google Scholar] [CrossRef]
- Lin, S.-J.; Chen, L.-F.; Jia, Y.-B.; Xiao, H.-L.; Xue, Y.-P.; Zheng, Y.-G. Distribution and chemoenzymatic removal of heavy metals in sea cucumber Acaudina leucoprocta. Food Sci. Technol. Res. 2018, 24, 223–229. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant potential of sea cucumbers and their beneficial effects on human health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, M.; Bascón, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J. Appl. Phycol. 2013, 25, 1793–1803. [Google Scholar] [CrossRef]
- Korczek, K.R.; Tkaczewska, J.; Duda, I.; Migdał, W. Effect of Heat Treatment on the Antioxidant Activity as Well as In vitro Digestion Stability of Herring (Clupea harengus) Protein Hydrolysates. J. Aquat. Food Prod. 2021, 30, 806–825. [Google Scholar] [CrossRef]
- Senadheera, T.R.; Hossain, A.; Dave, D.; Shahidi, F. Antioxidant and ACE-Inhibitory Activity of Protein Hydrolysates Produced from Atlantic Sea Cucumber (Cucumaria frondosa). Molecules 2023, 28, 5263. [Google Scholar] [CrossRef]
- Cumby, N.; Zhong, Y.; Naczk, M.; Shahidi, F. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem. 2008, 109, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Husni, A.; Shin, I.-S.; You, S.; Chung, D. Antioxidant properties of water and aqueous ethanol extracts and their crude saponin fractions from a far-eastern sea cucumber, Stichopus japonicus. Food Sci. Biotechnol. 2009, 18, 419–424. [Google Scholar]
- John, J.A.; Shahidi, F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J. Funct. Foods 2010, 2, 196–209. [Google Scholar] [CrossRef]
- Zeng, H.; Qin, L.; Liu, X.; Miao, S. Increases of lipophilic antioxidants and anticancer activity of coix seed fermented by Monascus purpureus. Foods 2021, 10, 566. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical composition and biological activities of the nord-west romanian wild bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.) leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chen, G.; Liu, H. Antioxidative categorization of twenty amino acids based on experimental evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, T.R.; Hossain, A.; Dave, D.; Shahidi, F. In silico analysis of bioactive peptides produced from underutilized sea cucumber by-products—A bioinformatics approach. Mar. Drugs 2022, 20, 610. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Savino, R.J.; Kempisty, B.; Mozdziak, P. The potential of a protein model synthesized absent of methionine. Molecules 2022, 27, 3679. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; McClements, D.J.; Decker, E.A. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase β-lactoglobulin in oil-in-water emulsions. J. Agric. Food Chem. 2005, 53, 10248–10253. [Google Scholar] [CrossRef] [PubMed]
- Wallner, S.; Hermetter, A.; Mayer, B.; Wascher, T. The alpha-amino group of l-arginine mediates its antioxidant effect. Eur. J. Clin. Investig. 2001, 31, 98–102. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of L-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Borrajo, P.; Pateiro, M.; Gagaoua, M.; Franco, D.; Zhang, W.; Lorenzo, J.M. Evaluation of the antioxidant and antimicrobial activities of porcine liver protein hydrolysates obtained using alcalase, bromelain, and papain. Appl. Sci. 2020, 10, 2290. [Google Scholar] [CrossRef]
- O’Sullivan, S.M.; Lafarga, T.; Hayes, M.; O’Brien, N.M. Bioactivity of bovine lung hydrolysates prepared using papain, pepsin, and Alcalase. J. Food Biochem. 2017, 41, e12406. [Google Scholar] [CrossRef]
- Bah, C.S.; Carne, A.; McConnell, M.A.; Mros, S.; Bekhit, A.E.-D.A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016, 202, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Phanturat, P.; Benjakul, S.; Visessanguan, W.; Roytrakul, S. Use of pyloric caeca extract from bigeye snapper (Priacanthus macracanthus) for the production of gelatin hydrolysate with antioxidative activity. LWT-Food Sci. Technol. 2010, 43, 86–97. [Google Scholar] [CrossRef]
- Je, J.-Y.; Lee, K.-H.; Lee, M.H.; Ahn, C.-B. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res. Int. 2009, 42, 1266–1272. [Google Scholar] [CrossRef]
- Šližytė, R.; Mozuraitytė, R.; Martínez-Alvarez, O.; Falch, E.; Fouchereau-Peron, M.; Rustad, T. Functional, bioactive and antioxidative properties of hydrolysates obtained from cod (Gadus morhua) backbones. Process Biochem. 2009, 44, 668–677. [Google Scholar] [CrossRef]
- Jamdar, S.; Rajalakshmi, V.; Sharma, A. Antioxidant and ace inhibitory properties of poultry viscera protein hydrolysate and its peptide fractions. J. Food Biochem. 2012, 36, 494–501. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Liang, Q.; Ahmed, F.; Zhang, M.; Sperou, N.; Franco, C.M.; Feng, Q.; Zhang, W. In Vivo and Clinical Studies of Sea Cucumber-Derived Bioactives for Human Health and Nutrition From 2012–2021. Front. Mar. Sci. 2022, 9, 917857. [Google Scholar] [CrossRef]


| Sample | Water | Ash | Protein | Lipid |
|---|---|---|---|---|
| C. frondosa [%] | 86.2 ± 1.5 | 0.6 ± 0.1 | 4.7 ± 0.4 | 0.5 ± 0.5 |
| Total Amino Acids | Free Amino Acids | |||
|---|---|---|---|---|
| Amino Acids | (mg/g Wet Weight) | (mg/g Dry Weight) | (mg/g Wet Weight) | (mg/g Dry Weight) |
| Alanine | 3.5 ± 0.6 | 25.3 ± 4.3 | 0.16 ± 0.09 | 1.14 ± 0.66 |
| Asparagine | 0.1 ± 0.0 | 0.5 ± 0.1 | 0.00 ± 0.00 | 0.01 ± 0.00 |
| Aspartic acid | 5.5 ± 0.7 | 39.7 ± 4.9 | 0.01 ± 0.01 | 0.09 ± 0.04 |
| Glutamic acid | 6.8 ± 0.9 | 49.0 ± 6.7 | 0.33 ± 0.06 | 2.37 ± 0.44 |
| Glutamine | 0.1 ± 0.0 | 0.5 ± 0.1 | 0.07 ± 0.01 | 0.48 ± 0.08 |
| Glycine/Arginine | 5.7 ± 1.3 | 41.4 ± 9.4 | 0.32 ± 0.28 | 2.31 ± 2.03 |
| Histidine | 1.0 ± 0.0 | 7.0 ± 0.3 | 0.00 ± 0.00 | 0.01 ± 0.00 |
| Isoleucine | 2.2 ± 0.2 | 15.6 ± 1.1 | 0.00 ± 0.00 | 0.03 ± 0.01 |
| Leucine | 2.1 ± 0.2 | 15.4 ± 1.2 | 0.01 ± 0.00 | 0.07 ± 0.02 |
| Lysine | 3.8 ± 0.3 | 27.3 ± 2.1 | 0.08 ± 0.03 | 0.57 ± 0.25 |
| Methionine | 0.2 ± 0.0 | 1.6 ± 0.3 | 0.00 ± 0.00 | 0.02 ± 0.02 |
| Phenylalanine | 2.5 ± 0.2 | 18.2 ± 1.5 | 0.01 ± 0.00 | 0.05 ± 0.02 |
| Serine | 3.0 ± 0.4 | 21.5 ± 2.8 | 0.01 ± 0.00 | 0.10 ± 0.02 |
| Threonine | 2.9 ± 0.2 | 21.0 ± 1.7 | 0.01 ± 0.01 | 0.10 ± 0.05 |
| Tyrosine | 1.9 ± 0.2 | 13.4 ± 1.2 | 0.01 ± 0.00 | 0.04 ± 0.01 |
| Valine | 1.0 ± 0.1 | 7.4 ± 0.5 | 0.01 ± 0.00 | 0.05 ± 0.01 |
| ∑Total amino acids | 42.2 ± 5.3 | 304.7 ± 38.3 | ||
| ∑Free amino acids | 1.0 ± 0.5 | 7.4 ± 3.7 | ||
| Sample | Hydrolysate without Enzyme | Bromelain + Papain | Alcalase |
|---|---|---|---|
| Protein content [mg/g] | 133.8 ± 35.3 a | 321.4 ± 52.0 b | 182.5 ± 43.8 a |
| Protein yield [%] | 8.3 ± 2.6 a | 36.8 ± 6.6 b | 11.8 ± 2.9 a |
| Total Amino Acids | Free Amino Acids | |||||
|---|---|---|---|---|---|---|
| Amino Acids | Without Enzyme | Bromelain + Papain | Alcalase | Without Enzyme | Bromelain + Papain | Alcalase |
| Alanine | 10.4 ± 2.1 a | 27.8 ± 5.8 b | 16.5 ± 4.5 a | 2.54 ± 1.73 a | 2.53 ± 1.11 a | 2.90 ± 1.57 a |
| Asparagine | 0.2 ± 0.1 ab | 0.3 ± 0.1 b | 0.1 ± 0.1 a | 0.01 ± 0.00 a | 0.07 ± 0.03 b | 0.02 ± 0.01 a |
| Aspartic acid | 15.8 ± 4.3 a | 39.4 ± 5.2 b | 21.9 ± 4.5 a | 0.17 ± 0.06 a | 0.13 ± 0.06 a | 0.36 ± 0.13 b |
| Glutamic acid | 26.1 ± 2.8 a | 54.3 ± 9.8 b | 37.7 ± 5.2 ab | 5.16 ± 1.71 a | 3.93 ± 1.36 a | 4.91 ± 1.24 a |
| Glutamine | 0.2 ± 0.1 a | 0.4 ± 0.1 a | 0.2 ± 0.1 a | 1.18 ± 0.11 a | 1.18 ± 0.30 a | 1.01 ± 0.16 a |
| Glycine/Arginine | 17.5 ± 6.8 a | 52.2 ± 15.2 b | 34.3 ± 12.9 ab | 4.71 ± 3.55 a | 4.29 ± 2.72 a | 4.61 ± 3.19 a |
| Histidine | 2.2 ± 0.9 a | 5.5 ± 0.6 b | 2.5 ± 0.4 a | 0.07 ± 0.01 a | 0.15 ± 0.05 b | 0.10 ± 0.03 a |
| Isoleucine | 5.7 ± 2.0 a | 12.0 ± 1.1 b | 6.0 ± 1.6 a | 0.13 ± 0.02 a | 0.33 ± 0.09 b | 0.21 ± 0.06 a |
| Leucine | 9.7 ± 3.0 a | 20.3 ± 2.9 b | 10.3 ± 3.1 a | 0.26 ± 0.07 a | 1.95 ± 0.47 b | 0.59 ± 0.20 a |
| Lysine | 10.0 ± 2.2 a | 21.8 ± 2.9 b | 10.1 ± 1.7 a | 1.18 ± 0.47 a | 1.28 ± 0.58 a | 1.30 ± 0.54 a |
| Methionine | 2.0 ± 0.7 a | 6.0 ± 0.5 b | 2.3 ± 0.6 a | 0.14 ± 0.03 a | 0.71 ± 0.16 b | 0.24 ± 0.09 a |
| Phenylalanine | 5.9 ± 2.2 a | 11.8 ± 1.4 b | 5.6 ± 1.6 a | 0.22 ± 0.05 a | 0.93 ± 0.21 b | 0.36 ± 0.11 a |
| Serine | 8.1 ± 2.0 a | 22.2 ± 3.5 b | 11.5 ± 2.4 a | 0.37 ± 0.06 a | 0.55 ± 0.15 a | 0.48 ± 0.13 a |
| Threonine | 8.6 ± 2.3 a | 19.7 ± 1.1 b | 10.4 ± 2.2 a | 0.37 ± 0.05 a | 0.62 ± 0.16 b | 0.50 ± 0.08 ab |
| Tyrosine | 4.6 ± 1.8 a | 10.7 ± 0.8 b | 4.8 ± 1.2 a | 0.17 ± 0.03 a | 0.82 ± 0.19 b | 0.32 ± 0.08 a |
| Valine | 6.7 ± 2.0 a | 17.0 ± 1.0 b | 8.2 ± 1.7 a | 0.20 ± 0.04 a | 0.62 ± 0.17 b | 0.30 ± 0.09 a |
| ∑Total amino acids | 133.8 ± 35.3 | 321.4 ± 52.0 | 182.5 ± 43.8 | |||
| ∑Free amino acids | 16.9 ± 8.0 | 20.1 ± 7.8 | 18.2 ± 7.7 | |||
| Unhydrolyzed Raw Material | Hydrolyzed Raw Material | ||||
|---|---|---|---|---|---|
| Without Enzyme | Bromelain + Papain | Alcalase | |||
| Sample | Wet Weight | Dry Weight | Dry Weight | Dry Weight | Dry Weight |
| C. frondosa | 4.2 ± 0.5 | 30.5 ± 0.4 | 13.4 ± 0.4 | 32.1 ± 0.5 | 18.3 ± 4.4 |
| Conventional seafood products | |||||
| Protein content (g/100 g wet weight) | Tuna 19.5–26.5 | Salmon 9.6–14.7 | Caviar 10.0–22.3 | Calamari 6.1–11.0 | Shrimps 14.5–17.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, D.T.; Falch, E.; Elvevoll, E.O.; Jensen, I.-J. Enzymatic Hydrolysis of Orange-Footed Sea Cucumber (Cucumaria frondosa)—Effect of Different Enzymes on Protein Yield and Bioactivity. Foods 2023, 12, 3685. https://doi.org/10.3390/foods12193685
Vu DT, Falch E, Elvevoll EO, Jensen I-J. Enzymatic Hydrolysis of Orange-Footed Sea Cucumber (Cucumaria frondosa)—Effect of Different Enzymes on Protein Yield and Bioactivity. Foods. 2023; 12(19):3685. https://doi.org/10.3390/foods12193685
Chicago/Turabian StyleVu, Dat Trong, Eva Falch, Edel O. Elvevoll, and Ida-Johanne Jensen. 2023. "Enzymatic Hydrolysis of Orange-Footed Sea Cucumber (Cucumaria frondosa)—Effect of Different Enzymes on Protein Yield and Bioactivity" Foods 12, no. 19: 3685. https://doi.org/10.3390/foods12193685
APA StyleVu, D. T., Falch, E., Elvevoll, E. O., & Jensen, I.-J. (2023). Enzymatic Hydrolysis of Orange-Footed Sea Cucumber (Cucumaria frondosa)—Effect of Different Enzymes on Protein Yield and Bioactivity. Foods, 12(19), 3685. https://doi.org/10.3390/foods12193685








