Phosphorylated Fish Gelatin and the Quality of Jelly Gels: Gelling and Microbiomics Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
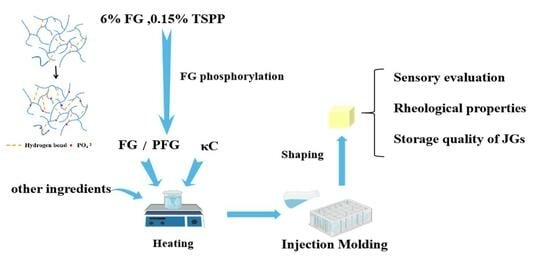
2.2. Preparation of Fish Gelatin Jelly Candy
2.3. Sensory Evaluation
2.4. Rheological Properties
2.5. Storage Quality of JGs
2.5.1. Textural Properties
2.5.2. Color Analysis
2.5.3. Deformation Resistance
2.5.4. Microbial Diversity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation
3.2. Analysis of Jelly Deformation and Fracture Properties
3.3. Storage Quality of JGs
3.3.1. Textural Properties
3.3.2. Color Analysis
3.3.3. Analysis of Deformation Resistance Rate
3.3.4. Statistical and Quality Analysis of High-Throughput Sequencing
3.3.5. Alpha Diversity Index Analysis of JGs
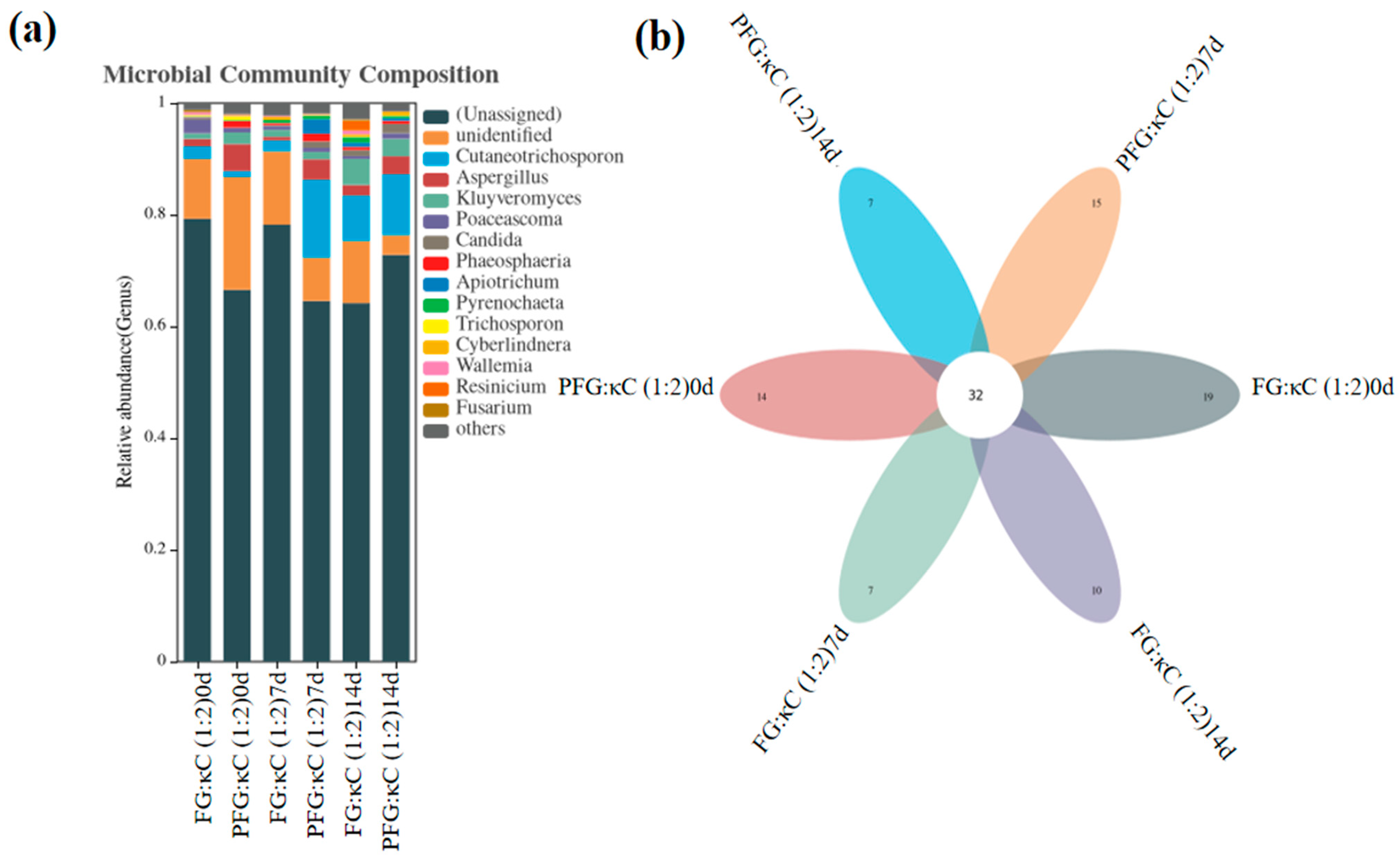
3.3.6. Analysis of Bacterial Flora in JGs at the Genus Level
3.3.7. Analysis of Fungal Flora in JGs at the Genus Level
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivero, R.; Archaina, D.; Sosa, N.; Leiva, G.; Baldi Coronel, B.; Schebor, C. Development of healthy gummy jellies containing honey and propolis. J. Sci. Food Agric. 2020, 100, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.M.; Sabuz, A.A.; Khan, M.H.H.; Chowdhury, M.G.F.; Miaruddin, M.; Alam, M.; Khatun, A. Effect of honey and lemon juice on the physicochemical, nutritional, microbial and antioxidant properties of guava–pineapple jelly during storage periods. Discov. Food 2022, 2, 31. [Google Scholar] [CrossRef]
- Riedel, R.; Böhme, B.; Rohm, H. Development of formulations for reduced-sugar and sugar-free agar-based fruit jellies. Int. J. Food Sci. Technol. 2015, 50, 1338–1344. [Google Scholar] [CrossRef]
- Konar, N.; Gunes, R.; Palabiyik, I.; Toker, O.S. Health conscious consumers and sugar confectionery: Present aspects and projections. Trends Food Sci. Technol. 2022, 123, 57–68. [Google Scholar] [CrossRef]
- Gallego, M.; Arnal, M.; Talens, P.; Toldra, F.; Mora, L. Effect of Gelatin Coating Enriched with Antioxidant Tomato By-Products on the Quality of Pork Meat. Polymers 2020, 12, 1032. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Gunes, R.; Palabiyik, I.; Konar, N.; Toker, O.S. Soft confectionery products: Quality parameters, interactions with processing and ingredients. Food Chem. 2022, 385, 132735. [Google Scholar] [CrossRef]
- Cen, S.; Yu, W.; Yang, W.; Lou, Q.; Huang, T. Reversibility of the gel, rheological, and structural properties of alcohol pretreated fish gelatin: Effect of alcohol types. J. Texture Stud. 2022, 53, 266–276. [Google Scholar] [CrossRef]
- Wangtueai, S.; Noomhorm, A.; Regenstein, J.M. Effect of Microbial Transglutaminase on Gel Properties and Film Characteristics of Gelatin from Lizardfish (Saurida spp.) Scales. J. Food Sci. 2010, 75, C731–C739. [Google Scholar] [CrossRef]
- Kaewruang, P.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Nalinanon, S. Impact of divalent salts and bovine gelatin on gel properties of phosphorylated gelatin from the skin of unicorn leatherjacket. LWT 2014, 55, 477–482. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, M.; Ma, M. Emulsifying properties of ovalbumin: Improvement and mechanism by phosphorylation in the presence of sodium tripolyphosphate. Food Hydrocoll. 2016, 60, 29–37. [Google Scholar] [CrossRef]
- Miedzianka, J.; Peksa, A. Effect of pH on phosphorylation of potato protein isolate. Food Chem. 2013, 138, 2321–2326. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Cen, S.; Zhang, L.; Liu, L.; Lou, Q.; Wang, C.; Huang, T. Phosphorylation modification on functional and structural properties of fish gelatin: The effects of phosphate contents. Food Chem. 2022, 380, 132209. [Google Scholar] [CrossRef]
- Announcement of Newly Approved National Standards of P. R. China 2005 No.11. China Stand. 2006, 1, 21–28.
- Yang, D.; Yang, H. Effects of ethanol on gelation of iota-carrageenan. LWT 2020, 126, 109281. [Google Scholar] [CrossRef]
- Seremet, D.; Mandura, A.; Cebin, A.V.; Martinic, A.; Galic, K.; Komes, D. Challenges in confectionery industry: Development and storage stability of innovative white tea-based candies. J. Food Sci. 2020, 85, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.; Long, J.; Seyfoddin, A.; Guerrero, P.; de la Caba, K.; Etxabide, A. Characterization of ribose-induced crosslinking extension in gelatin films. Food Hydrocoll. 2020, 99, 105324. [Google Scholar] [CrossRef]
- Pulatsu, E.; Su, J.-W.; Kenderes, S.M.; Lin, J.; Vardhanabhuti, B.; Lin, M. Restructuring cookie dough with 3D printing: Relationships between the mechanical properties, baking conditions, and structural changes. J. Food Eng. 2022, 319, 110911. [Google Scholar] [CrossRef]
- Sow, L.C.; Nicole Chong, J.M.; Liao, Q.X.; Yang, H. Effects of κ-carrageenan on the structure and rheological properties of fish gelatin. J. Food Eng. 2018, 239, 92–103. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, B.; Qiao, D.; Yan, X.; Zhao, S.; Jia, C.; Niu, M.; Xu, Y. Addition of κ-carrageenan increases the strength and chewiness of gelatin-based composite gel. Food Hydrocoll. 2022, 128, 107565. [Google Scholar] [CrossRef]
- Sow, L.C.; Yang, H. Effects of salt and sugar addition on the physicochemical properties and nanostructure of fish gelatin. Food Hydrocoll. 2015, 45, 72–82. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Hemar, Y. Physical and sensory properties of gelatin from seabass (Lates calcarifer) as affected by agar and κ-carrageenan. J. Texture Stud. 2018, 49, 47–55. [Google Scholar] [CrossRef]
- Deng, R.; Mars, M.; Van der Sman, R.G.M.; Smeets, P.A.M.; Janssen, A.E.M. The importance of swelling for in vitro gastric digestion of whey protein gels. Food Chem. 2020, 330, 127182. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Shangguan, X.; Wang, H.; Sha, X.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef]
- Xia, Z.; Yao, X.; Liu, W.; Li, L.; Kang, D.; Yan, C. Effects of conjugates of epsilon-polylysine-dextran created through Maillard reaction on quality and storage stability of the chicken gel. Food Res Int. 2023, 164, 112360. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Nhi, T.T.Y.; Vu, N.D.; Quyen, N.N.; Thinh, P.V.; Tho, N.T.M.; Truc, T.T. The effect of malt, pectin, and gelatin concentrations on elasticity, color and sensory evaluation of soursop (Annona muricata L.) jelly candy. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012013. [Google Scholar] [CrossRef]
- McCann, T.H.; Homer, S.H.; Oiseth, S.K.; Day, L.; Newberry, M.; Regina, A.; Lundin, L. High amylose wheat starch increases the resistance to deformation of wheat flour dough. J. Cereal Sci. 2018, 79, 440–448. [Google Scholar] [CrossRef]
- Alfaro, A.d.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, W.; Guo, R.; Yao, W. Effect of dietary copper level on the gut microbiota and its correlation with serum inflammatory cytokines in Sprague-Dawley rats. J. Microbiol. 2017, 55, 694–702. [Google Scholar] [CrossRef] [PubMed]



| Sample Name | Critical Strain (Cr) | Modulus of Intersection | Cohesive Energy (Ec, J/m3) |
|---|---|---|---|
| FG:κC (2:1) | 28.97 ± 4.82 b | 63.70 ± 6.45 b | 25,128.60 ± 435.27 b |
| PFG:κC (2:1) | 29.93 ± 0.61 b | 79.8 ± 2.57 a | 42,822.21 ± 754.03 a |
| FG:κC (1:1) | 52.29 ± 15.64 a | 16.55 ± 5.86 c | 24,458.40 ± 535.48 c |
| PFG:κC (1:1) | 49.84 ± 7.41 a | 17.63 ± 5.40 c | 22,306.51 ± 545.66 d |
| FG:κC (1:2) | 22.86 ± 6.90 b | 91.02 ± 11.63 a | 17,076.40 ± 492.93 e |
| PFG:κC (1:2) | 20.21 ± 4.20 b | 64.18 ± 12.12 b | 16,122.56 ± 582.25 f |
| Storage Time/Day | Sample Name | Hardness (N) | Elasticity | Chewiness (N) | Resilience |
|---|---|---|---|---|---|
| 0 | FG:κC (2:1) | 11.08 ± 0.63 d | 0.84 ± 0.02 cd | 5.22 ± 0.39 e | 0.27 ± 0.02 ab |
| PFG:κC (2:1) | 11.64 ± 0.62 d | 0.86 ± 0.02 bc | 5.75 ± 0.27 de | 0.27 ± 0.01 abc | |
| FG:κC (1:1) | 2.89 ± 0.87 f | 0.71 ± 0.04 f | 0.45 ± 0.03 g | 0.11 ± 0.02 d | |
| PFG:κC (1:1) | 5.92 ± 0.57 e | 0.92 ± 0.00 a | 2.36 ± 0.43 f | 0.25 ± 0.02 c | |
| FG:κC (1:2) | 11.87 ± 0.56 d | 0.81 ± 0.01 de | 6.02 ± 0.35 d | 0.28 ± 0.00 ab | |
| PFG:κC (1:2) | 13.25 ± 0.78 c | 0.84 ± 0.02 cd | 6.84 ± 0.45 c | 0.27 ± 0.01 abc | |
| 7 | FG:κC (1:2) | 15.06 ± 1.16 b | 0.81 ± 0.04 de | 7.66 ± 0.69 b | 0.28 ± 0.02 a |
| PFG:κC (1:2) | 17.48 ± 1.03 a | 0.87 ± 0.01 b | 9.3 ± 0.58 a | 0.26 ± 0.01 abc | |
| 14 | FG:κC (1:2) | 12.21 ± 0.32 cd | 0.79 ± 0.01 e | 6.02 ± 0.22 d | 0.26 ± 0.00 bc |
| PFG:κC (1:2) | 15.11 ± 0.88 b | 0.86 ± 0.02 bc | 8.02 ± 0.81 b | 0.25 ± 0.01 bc |
| Storage Time/Day | Sample Name | L* | a* | b* | W |
|---|---|---|---|---|---|
| 0 | FG:κC (2:1) | 55.47 ± 0.35 c | −3.76 ± 0.04 g | −4.10 ± 0.13 c | 55.13 ± 0.35 ab |
| PFG:κC (2:1) | 54.33 ± 0.11 d | −3.44 ± 0.04 f | −3.69 ± 0.09 b | 54.05 ± 0.12 ab | |
| FG:κC (1:1) | 59.89 ± 0.22 a | −4.14 ± 0.03 h | −3.30 ± 0.02 a | 59.54 ± 0.22 abc | |
| PFG:κC (1:1) | 57.36 ± 0.15 b | −3.68 ± 0.03 g | −3.10 ± 0.08 a | 57.09 ± 0.15 a | |
| FG:κC (1:2) | 51.71 ± 0.49 e | −3.24 ± 0.08 e | −4.50 ± 0.12 d | 51.39 ± 0.48 abc | |
| PFG:κC (1:2) | 54.01 ± 0.18 d | −3.77 ± 0.03 g | −4.59 ± 0.07 d | 53.63 ± 0.19 ab | |
| 7 | FG:κC (1:2) | 40.32 ± 0.66 i | −1.31 ± 0.15 a | −5.02 ± 0.14 g | 40.10 ± 0.67 c |
| PFG:κC (1:2) | 44.56 ± 0.42 g | −2.03 ± 0.12 c | −6.25 ± 0.38 i | 44.24 ± 0.36 bc | |
| 14 | FG:κC (1:2) | 41.21 ± 0.40 h | −1.65 ± 0.06 b | −4.73 ± 0.17 d | 40.99 ± 0.40 c |
| PFG:κC (1:2) | 47.26 ± 0.22 f | −2.35 ± 0.12 d | −5.93 ± 0.21 h | 46.88 ± 0.23 bc |
| Storage Time/Day | Sample Name | Deformation Resistance | Sample Name | Deformation Resistance |
|---|---|---|---|---|
| 0 day | FG:κC (2:1) | 0.93 ± 0.02 a | PFG:κC (2:1) | 0.94 ± 0.00 a |
| FG:κC (1:1) | 0.78 ± 0.08 bc | PFG:κC (1:1) | 0.84 ± 0.02 b | |
| FG:κC (1:2) | 0.92 ± 0.02 a | PFG:κC (1:2) | 0.95 ± 0.02 a | |
| 7 day | FG:κC (1:2) | 0.67 ± 0.06 cd | PFG:κC (1:2) | 0.69 ± 0.05 d |
| 14 day | FG:κC (1:2) | 0.45 ± 0.07 e | PFG:κC (1:2) | 0.44 ± 0.05 e |
| Correlation Index | Species | Sample Name | |||||
|---|---|---|---|---|---|---|---|
| FG:κC (1:2) 0 d | PFG:κC (1:2) 0 d | FG:κC (1:2) 7 d | PFG:κC (1:2) 7 d | FG:κC (1:2) 14 d | PFG:κC (1:2) 14 d | ||
| Original sequence number | Bacteria | 131,359 | 120,967 | 131,101 | 127,243 | 120,750 | 125,948 |
| Fungus | 90,878 | 84,545 | 85,608 | 86,426 | 88,285 | 87,826 | |
| OUT/pc | Bacteria | 387 | 308 | 329 | 124 | 126 | 137 |
| Fungus | 132 | 104 | 92 | 110 | 121 | 97 | |
| Kingdoms | Bacteria | 1 | 1 | 1 | 1 | 1 | 1 |
| Fungus | 3 | 3 | 3 | 4 | 4 | 3 | |
| Phyla | Bacteria | 19 | 14 | 13 | 16 | 11 | 10 |
| Fungus | 7 | 7 | 8 | 8 | 8 | 6 | |
| Classes | Bacteria | 26 | 21 | 17 | 22 | 14 | 15 |
| Fungus | 18 | 17 | 20 | 17 | 18 | 14 | |
| Orders | Bacteria | 57 | 46 | 33 | 49 | 30 | 30 |
| Fungus | 23 | 23 | 29 | 26 | 23 | 25 | |
| Families | Bacteria | 104 | 89 | 56 | 91 | 50 | 54 |
| Fungus | 30 | 30 | 30 | 31 | 33 | 32 | |
| Genera | Bacteria | 162 | 145 | 69 | 134 | 60 | 75 |
| Fungus | 25 | 24 | 25 | 24 | 28 | 27 | |
| Correlation Index | Species | Sample Name | |||||
|---|---|---|---|---|---|---|---|
| FG:κC (1:2) 0 d | PFG:κC (1:2) 0 d | FG:κC (1:2) 7 d | PFG:κC (1:2) 7 d | FG:κC (1:2) 14 d | PFG:κC (1:2) 14 d | ||
| Richness | Bacteria | 387 | 308 | 329 | 124 | 126 | 137 |
| Fungus | 132 | 104 | 92 | 110 | 121 | 97 | |
| Chao1 | Bacteria | 387.1 | 308.1 | 329.2 | 124.4 | 126.8 | 138.6 |
| Fungus | 132.9 | 104.9 | 92.7 | 112.9 | 121.5 | 98.1 | |
| Reads | Bacteria | 89,108 | 89,615 | 84,265 | 101,127 | 84,087 | 99,476 |
| Fungus | 4394 | 3329 | 2169 | 3172 | 2204 | 4516 | |
| Simpson | Bacteria | 0.179 | 0.36 | 0.166 | 0.497 | 0.387 | 0.522 |
| Fungus | 0.276 | 0.0725 | 0.246 | 0.189 | 0.0869 | 0.293 | |
| Coverage | Bacteria | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Fungus | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |
| Bacteria | Sample Name | |||||
|---|---|---|---|---|---|---|
| Genus | FG:κC (1:2) 0 d | PFG:κC (1:2) 0 d | FG:κC (1:2) 7 d | PFG:κC (1:2) 7 d | FG:κC (1:2) 14 d | PFG:κC (1:2) 14 d |
| Pseudomonas | 4.01% | 4.74% | 13.35% | 94.72% | 90.87% | 92.25% |
| Shewanella | 34.03% | 57.85% | 32.21% | 2.77% | 4.51% | 3.63% |
| Geobacillus | 20.91% | 13.42% | 18.66% | 0.84% | 1.58% | 1.27% |
| Anoxybacillus | 8.79% | 5.33% | 7.84% | 0.42% | 0.70% | 0.63% |
| Lactococcus | 9.10% | 4.26% | 7.40% | 0.25% | 0.56% | 0.63% |
| Acinetobacter | 8.69% | 4.48% | 6.38% | 0.20% | 0.32% | 0.28% |
| Thermus | 4.30% | 3.79% | 5.60% | 0.28% | 0.65% | 0.50% |
| Streptococcus | 3.88% | 1.77% | 3.21% | 0.12% | 0.23% | 0.26% |
| (Unassigned) | 0.63% | 0.55% | 0.52% | 0.16% | 0.25% | 0.18% |
| Bacillus | 0.70% | 0.55% | 0.58% | 0.03% | 0.03% | 0.04% |
| Chryseobacterium | 0.66% | 0.46% | 0.56% | 0.01% | 0.02% | 0.04% |
| Enhydrobacter | 0.40% | 0.23% | 0.32% | 0.01% | 0.02% | 0.02% |
| Carnobacterium | 0.35% | 0.24% | 0.27% | 0.01% | 0.02% | 0.03% |
| Aeromonas | 0.33% | 0.18% | 0.28% | 0.01% | 0.01% | 0.02% |
| Fusobacterium | 0.21% | 0.14% | 0.23% | 0.01% | 0.01% | 0.02% |
| Others | 3.00% | 2.00% | 2.58% | 0.14% | 0.20% | 0.21% |
| Fungus | Sample Name | |||||
|---|---|---|---|---|---|---|
| Genus | FG:κC (1:2) 0 d | PFG:κC (1:2) 0 d | FG:κC (1:2) 7 d | PFG:κC (1:2) 7 d | FG:κC (1:2) 14 d | PFG:κC (1:2) 14 d |
| (Unassigned) | 79.29% | 66.57% | 78.24% | 64.56% | 64.20% | 72.83% |
| Unidentified | 10.70% | 20.19% | 13.14% | 7.76% | 11.12% | 3.52% |
| Cutaneotrichosporon | 2.28% | 1.08% | 1.94% | 13.97% | 8.12% | 10.92% |
| Aspergillus | 1.34% | 4.84% | 0.65% | 3.66% | 1.91% | 3.21% |
| Kluyveromyces | 1.00% | 2.07% | 1.24% | 1.32% | 4.67% | 3.14% |
| Poaceascoma | 2.41% | 0.81% | 0.69% | 0.73% | 0.50% | 1.00% |
| Candida | 0.30% | 0.06% | 0.14% | 1.17% | 1.09% | 1.66% |
| Phaeosphaeria | 0.09% | 1.14% | 0.37% | 1.36% | 0.54% | 0.55% |
| Apiotrichum | 0.11% | 0.00% | 0.00% | 2.62% | 0.77% | 0.47% |
| Pyrenochaeta | 0.09% | 0.18% | 0.60% | 0.60% | 1.00% | 0.40% |
| Trichosporon | 0.27% | 0.72% | 0.00% | 0.19% | 0.14% | 0.11% |
| Cyberlindnera | 0.02% | 0.06% | 0.51% | 0.00% | 0.36% | 0.62% |
| Wallemia | 0.48% | 0.15% | 0.18% | 0.09% | 0.68% | 0.00% |
| Resinicium | 0.00% | 0.03% | 0.00% | 0.03% | 1.77% | 0.04% |
| Fusarium | 0.41% | 0.18% | 0.09% | 0.06% | 0.27% | 0.07% |
| Others | 1.20% | 1.91% | 2.20% | 1.88% | 2.85% | 1.45% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Sun, W.; Yang, Y.; Jia, R.; Zhan, S.; Ou, C.; Huang, T. Phosphorylated Fish Gelatin and the Quality of Jelly Gels: Gelling and Microbiomics Analysis. Foods 2023, 12, 3682. https://doi.org/10.3390/foods12193682
Wu S, Sun W, Yang Y, Jia R, Zhan S, Ou C, Huang T. Phosphorylated Fish Gelatin and the Quality of Jelly Gels: Gelling and Microbiomics Analysis. Foods. 2023; 12(19):3682. https://doi.org/10.3390/foods12193682
Chicago/Turabian StyleWu, Shiyu, Wanyi Sun, Yihui Yang, Ru Jia, Shengnan Zhan, Changrong Ou, and Tao Huang. 2023. "Phosphorylated Fish Gelatin and the Quality of Jelly Gels: Gelling and Microbiomics Analysis" Foods 12, no. 19: 3682. https://doi.org/10.3390/foods12193682
APA StyleWu, S., Sun, W., Yang, Y., Jia, R., Zhan, S., Ou, C., & Huang, T. (2023). Phosphorylated Fish Gelatin and the Quality of Jelly Gels: Gelling and Microbiomics Analysis. Foods, 12(19), 3682. https://doi.org/10.3390/foods12193682