A Comparison of the Meat Quality, Nutritional Composition, Carcass Traits, and Fiber Characteristics of Different Muscular Tissues between Aged Indigenous Chickens and Commercial Laying Hens
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Sample Collection
2.2. pH and Meat Color
2.3. Drip Loss, Cooking Loss, and Shear Force
2.4. Chemical Composition
2.5. Amino Acid Profile
2.6. Myofiber Characteristics
2.7. Statistical Analysis
3. Results
3.1. Carcass Traits
3.2. pH and Meat Color
3.3. Drip Loss, Cooking Loss, and Shear Force
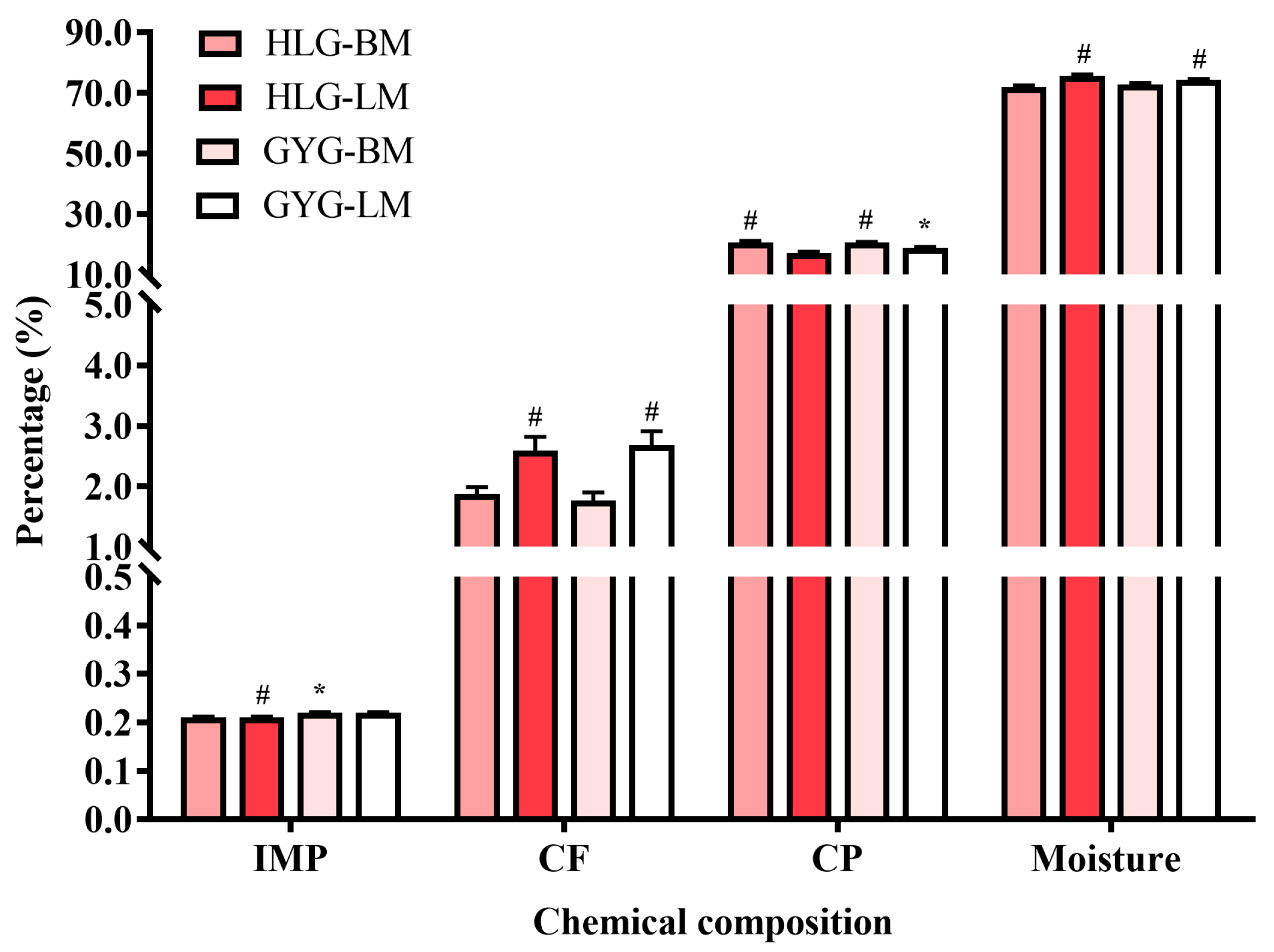
3.4. Meat Chemical Analysis
3.5. Amino Acid Profile
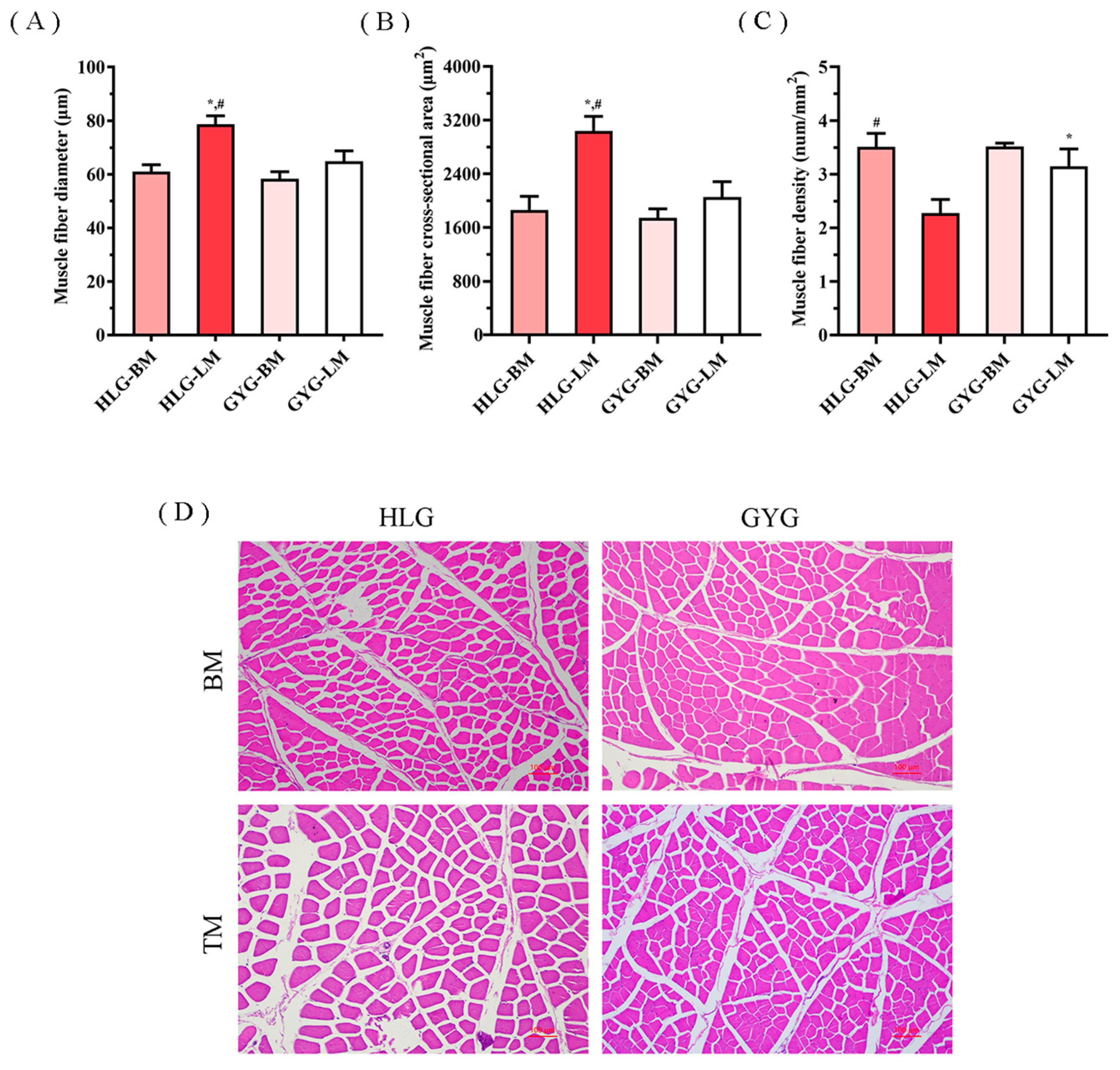
3.6. Myofiber Characteristics
3.7. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.G.; Naveena, B.M.; Jo, C.; Sakata, R.; Zhou, G.H.; Banerjee, R.; Nishiumi, T. Technological demands of meat processing-An Asian perspective. Meat Sci. 2017, 132, 35–44. [Google Scholar] [CrossRef]
- Bromfield, J.I.; Hoffman, L.C.; Horyanto, D.; Soumeh, E.A. Enhancing Growth Performance, Organ Development, Meat Quality, and Bone Mineralisation of Broiler Chickens through Multi-Enzyme Super-Dosing in Reduced Energy Diets. Animal 2021, 11, 2791. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.; Marrugo Padilla, A.; Mendez Cuadro, D.; Rodriguez Cavallo, E. Residues of tetracyclines and beta-lactams antibiotics induce carbonylation of chickenbreast. F1000Research 2021, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.B.; Wu, J.P. Conventional use and sustainable valorization of spent egg-laying hens as functional foods and biomaterials: A review. Bioresour. Bioprocess. 2022, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.C.; Webster, A.B.; Lewis, N.J.; Van Arnam, C. Management of spent hens. J. Appl. Anim. Welf. Sci. JAAWS 1999, 2, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Alders, R.G.; Dumas, S.E.; Rukambile, E.; Magoke, G.; Maulaga, W.; Jong, J.; Costa, R. Family poultry: Multiple roles, systems, challenges, and options for sustainable contributions to household nutrition security through a planetary health lens. Matern. Child Nutr. 2018, 14, e12668. [Google Scholar] [CrossRef]
- Yang, Z.; Rose, S.P.; Yang, H.M.; Pirgozliev, V.; Wang, Z.Y. Egg production in China. Worlds Poult. Sci. J. 2018, 74, 417–426. [Google Scholar] [CrossRef]
- Huang, C.B.; Xiao, L.; Xing, S.C.; Chen, J.Y.; Yang, Y.W.; Zhou, Y.; Chen, W.; Liang, J.B.; Mi, J.D.; Wang, Y.; et al. The microbiota structure in the cecum of laying hens contributes to dissimilar H2S production. BMC Genom. 2019, 20, 770. [Google Scholar] [CrossRef]
- Deng, S.L.; Xing, T.; Li, C.B.; Xu, X.L.; Zhou, G.H. The Effect of Breed and Age on the Growth Performance, Carcass Traits and Metabolic Profile in Breast Muscle of Chinese Indigenous Chickens. Foods 2022, 11, 483. [Google Scholar] [CrossRef]
- Yin, L.Q.; Xu, M.X.; Huang, Q.K.; Zhang, D.H.; Lin, Z.Z.; Wang, Y.; Liu, Y.P. Nutrition and Flavor Evaluation of Amino Acids in Guangyuan Grey Chicken of Different Ages, Genders and Meat Cuts. Animal 2023, 13, 1235. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Pathak, V.; Fayaz, H. Effect of refrigerated storage on the quality characteristics of microwave cooked chicken seekh kababs extended with different non-meat proteins. J. Food Sci. Technol. 2013, 50, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Li, Z.Q.; Ran, J.S.; Yang, C.W.; Lin, Z.Z.; Liu, Y.P. LC/MS-based lipidomics to characterize breed-specific and tissue-specific lipid composition of chicken meat and abdominal fat. LWT-Food Sci. Technol. 2022, 163, 113611. [Google Scholar] [CrossRef]
- Bett, H.K.; Peters, K.J.; Nwankwo, U.M.; Bokelmann, W. Estimating consumer preferences and willingness to pay for the underutilised indigenous chicken products. Food Policy 2013, 41, 218–225. [Google Scholar] [CrossRef]
- Sheng, Z.Y.; Pettersson, M.E.; Hu, X.X.; Luo, C.L.; Qu, H.; Shu, D.M.; Shen, X.; Carlborg, O.; Li, N. Genetic dissection of growth traits in a Chinese indigenous x commercial broiler chicken cross. BMC Genom. 2013, 14, 151. [Google Scholar] [CrossRef]
- Yuan, C.Y.; Jiang, Y.; Wang, Z.X.; Chen, G.H.; Bai, H.; Chang, G.B. Indigenous, Yellow-Feathered Chickens Body Measurements, Carcass Traits, and Meat Quality Depending on Marketable Age. Animal 2022, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Mao, H.G.; Dong, X.Y.; Cao, H.Y.; Liu, K.; Yin, Z.Z. Expression of MSTN gene and its correlation with pectoralis muscle fiber traits in the domestic pigeons (Columba livia). Poult. Sci. 2019, 98, 5265–5271. [Google Scholar] [CrossRef] [PubMed]
- Haunshi, S.; Devatkal, S.; Prince, L.L.L.; Ullengala, R.; Ramasamy, K.; Chatterjee, R. Carcass Characteristics, Meat Quality and Nutritional Composition of Kadaknath, a Native Chicken Breed of India. Foods 2022, 11, 3603. [Google Scholar] [CrossRef]
- Baeza, E.; Le Bihan-Duval, E. Chicken lines divergent for low or high abdominal fat deposition: A relevant model to study the regulation of energy metabolism. Animal 2013, 7, 965–973. [Google Scholar] [CrossRef]
- Fouad, A.M.; El-Senousey, H.K. Nutritional Factors Affecting Abdominal Fat Deposition in Poultry: A Review. Asian Austral. J. Anim. 2014, 27, 1057–1068. [Google Scholar] [CrossRef]
- Yang, B.W.; Huang, S.M.; Zhao, G.X.; Ma, Q.G. Dietary supplementation of porcine bile acids improves laying performance, serum lipid metabolism and cecal microbiota in late-phase laying hens. Anim. Nutr. 2022, 11, 283–292. [Google Scholar] [CrossRef]
- Xing, J.Y.; Kang, L.; Hu, Y.; Xu, Q.Y.; Zhang, N.B.; Jiang, Y.L. Effect of Dietary Betaine Supplementation on mRNA Expression and Promoter CpG Methylation of Lipoprotein Lipase Gene in Laying Hens. J. Poult. Sci. 2009, 46, 224–228. [Google Scholar] [CrossRef]
- Kirmizibayrak, T.; Onk, K.; Ekiz, B.; Yalcintan, H.; Yilmaz, A.; Yazici, K.; Altinel, A. Effects of Age and Sex on Meat Quality of Turkish Native Geese Raised Under A Free-Range System. Kafkas Univ. Vet. Fak. Derg. 2011, 17, 817–823. [Google Scholar]
- Smiecinska, K.; Stepien, A.; Kubiak, D. Effect of Variety and Sex on the Carcass and Meat Quality Traits of Guinea Fowl (Numida meleagris L.). Animal 2022, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lin, H.; Song, Z.G.; Jiao, H.C. Corticosterone alters meat quality by changing preand postslaughter muscle metabolism. Poult. Sci. 2008, 87, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.C.; Kim, B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005, 71, 351–357. [Google Scholar] [CrossRef]
- Henckel, P.; Karlsson, A.; Jensen, M.T.; Oksbjerg, N.; Petersen, J.S. Metabolic conditions in Porcine longissimus muscle immediately pre-slaughter and its influence on peri- and post mortem energy metabolism. Meat Sci. 2002, 62, 145–155. [Google Scholar] [CrossRef]
- Zanetti, E.; De Marchi, M.; Dalvit, C.; Molette, C.; Remignon, H.; Cassandro, M. Carcase characteristics and qualitative meat traits of three Italian local chicken breeds. Brit. Poult. Sci. 2010, 51, 629–634. [Google Scholar] [CrossRef][Green Version]
- Xie, X.X.; Meng, Q.X.; Cui, Z.L.; Ren, L.P. Effect of Cattle Breed on Meat Quality, Muscle Fiber Characteristics, Lipid Oxidation and Fatty Acids in China. Asian Austral. J. Anim. 2012, 25, 824–831. [Google Scholar] [CrossRef]
- Tomasevic, I.; Djekic, I.; Font-i-Furnols, M.; Terjung, N.; Lorenzo, J.M. Recent advances in meat color research. Curr. Opin. Food Sci. 2021, 41, 81–87. [Google Scholar] [CrossRef]
- Ruedt, C.; Gibis, M.; Weiss, J. Meat color and iridescence: Origin, analysis, and approaches to modulation. Compr. Rev. Food. Sci. Food Saf. 2023, 22, 3366–3394. [Google Scholar] [CrossRef]
- Suman, S.P.; Wang, Y.F.; Gagaoua, M.; Kiyimba, F.; Ramanathan, R. Proteomic approaches to characterize biochemistry of fresh beef color. J. Proteom. 2023, 281, 10. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mussa, N.J.; Kibonde, S.F.; Boonkum, W.; Chankitisakul, V. The Comparison between Tanzanian Indigenous (Ufipa Breed) and Commercial Broiler (Ross Chicken) Meat on the Physicochemical Characteristics, Collagen and Nucleic Acid Contents. Food Sci. Anim. Resour. 2022, 42, 833–848. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Mostert, A.C.; Kidd, M.; Laubscher, L.L. Meat quality of kudu (Tragelaphus strepsiceros) and impala (Aepyceros melampus): Carcass yield, physical quality and chemical composition of kudu and impala Longissimus dorsi muscle as affected by gender and age. Meat Sci. 2009, 83, 788–795. [Google Scholar] [CrossRef]
- Kathuria, D.; Dhiman, A.K.K.; Attri, S. Sous vide, a culinary technique for improving quality of food products: A review. Trends Food Sci. Technol. 2022, 119, 57–68. [Google Scholar] [CrossRef]
- van der Sman, R.G.M. Model for electrical conductivity of muscle meat during Ohmic heating. J. Food Eng. 2017, 208, 37–47. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.Y. Comparison of Quality and Sensory Characteristics of Spent Hen and Broiler in South Korea. Animal 2021, 11, 2565. [Google Scholar] [CrossRef]
- Cai, C.X.; Zhang, L.J.; Liu, X.X.; Li, J.Z.; Ma, Y.C.; Jiang, R.R.; Li, Z.J.; Li, G.X.; Tian, Y.D.; Kang, X.T.; et al. Carcass composition, meat quality, leg muscle status, and its mRNA expression profile in broilers affected by valgus-varus deformity. Poult. Sci. 2023, 102, 102682. [Google Scholar] [CrossRef]
- Ou, Z.M.; Shi, Y.Y.; Li, Q.Q.; Wu, Y.; Chen, F.F. Effects of Sex on the Muscle Development and Meat Composition in Wuliangshan Black-Bone Chickens. Animal 2022, 12, 2565. [Google Scholar] [CrossRef]
- Weng, K.Q.; Huo, W.R.; Li, Y.; Zhang, Y.; Zhang, Y.; Chen, G.H.; Xu, Q. Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers. Poult. Sci. 2022, 101, 101537. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, G.D.; Jeong, J.Y.; Hur, S.J.; Joo, S.T. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010, 86, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.Q.; Huo, W.R.; Gu, T.T.; Bao, Q.; Hou, L.E.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G.H. Effects of marketable ages on meat quality through fiber characteristics in the goose. Poult. Sci. 2021, 100, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.F.; Fang, C.K.; Ma, Y.J.; He, S.P.; Ajuwon, K.M.; He, J.H. Dietary resveratrol supplement improves carcass traits and meat quality of Pekin ducks. Poult. Sci. 2021, 100, 100802. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Fletcher, D.L.; Smith, D.P.; Northcutt, J.K. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001, 80, 676–680. [Google Scholar] [CrossRef]
- Karunanayaka, D.S.; Jayasena, D.D.; Jo, C. Prevalence of pale, soft, and exudative (PSE) condition in chicken meat used for commercial meat processing and its effect on roasted chicken breast. J. Anim. Sci. Technol. 2016, 58, 27. [Google Scholar] [CrossRef]
- Qiao, M.; Fletcher, D.L.; Smith, D.P.; Northcutt, J.K. Effects of raw broiler breast meat color variation on marination and cooked meat quality. Poult. Sci. 2002, 81, 276–280. [Google Scholar] [CrossRef]
- Li, J.J.; Yang, C.W.; Peng, H.; Yin, H.D.; Wang, Y.; Hu, Y.D.; Yu, C.L.; Jiang, X.S.; Du, H.R.; Li, Q.Y.; et al. Effects of Slaughter Age on Muscle Characteristics and Meat Quality Traits of Da-Heng Meat Type Birds. Animal 2020, 10, 69. [Google Scholar] [CrossRef]
- Kaewthong, P.; Waiyagan, K.; Wattanachant, S. Imaging Analysis by Digital Camera for Separating Broiler Breast Meat with Low Water-Holding Capacity. J. Poult. Sci. 2017, 54, 253–261. [Google Scholar] [CrossRef]
- Huo, W.R.; Weng, K.Q.; Gu, T.T.; Zhang, Y.; Zhang, Y.; Chen, G.H.; Xu, Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021, 100, 101264. [Google Scholar] [CrossRef]

| Traits | HLG | GYG | p-Value |
|---|---|---|---|
| Carcass weight (g) | 1583.00 ± 62.31 | 1345.00 ± 60.80 | * |
| Eviscerated weight (g) | 1174.00 ± 52.39 | 1069.00 ± 31.64 | NS |
| Semi-eviscerated weight (g) | 1039.40 ± 45.90 | 920.00 ± 39.75 | NS |
| Breast muscle weight (g) | 175.58 ± 8.21 | 135.55 ± 9.88 | * |
| Breast muscle yield (%) | 16.95 ± 0.50 | 14.80 ± 0.95 | NS |
| Thigh muscle weight (g) | 229.26 ± 11.29 | 221.67 ± 7.97 | NS |
| Thigh muscle yield (%) | 22.03 ± 0.38 | 24.31 ± 0.88 | * |
| Abdominal fat weight (g) | 51.26 ± 6.49 | 21.88 ± 3.17 | * |
| Abdominal fat yield (%) | 4.54 ± 0.43 | 2.37 ± 0.36 | * |
| Traits | HLG-BM | HLG-TM | GYG-BM | GYG-TM |
|---|---|---|---|---|
| pH | 6.00 ± 0.04 * | 6.16 ± 0.02 # | 5.74 ± 0.01 | 6.13 ± 0.02 # |
| L* | 40.16 ± 0.53 # | 35.50 ± 0.85 | 41.44 ± 0.50 # | 36.23 ± 0.82 |
| a* | 2.28 ± 0.10 | 9.92 ± 0.60 # | 2.60 ± 0.16 | 11.14 ± 0.70 # |
| b* | 8.02 ± 0.25 | 9.58 ± 0.50 # | 8.73 ± 0.26 | 10.88 ± 0.45 *,# |
| Traits | HLG-BM | HLG-TM | GYG-BM | GYG-TM |
|---|---|---|---|---|
| Cooking loss (%) | 25.82 ± 1.03 | 35.10 ± 0.80 # | 26.72 ± 0.92 | 35.99 ± 0.74 # |
| Drip loss (%) | 2.70 ± 0.23 | 2.58 ± 0.17 | 2.79 ± 0.20 | 2.64 ± 0.19 |
| Shear force (kg) | 3.45 ± 0.23 | 5.86 ± 0.14 *,# | 3.48 ± 0.21 | 4.75 ± 0.17 # |
| Amino Acid | HLG-BM | HLG-TM | GYG-BM | GYG-TM | |
|---|---|---|---|---|---|
| Thr ▲ | 3.73 ± 0.01 | 3.74 ± 0.12 | 3.62 ± 0.09 | 3.94 ± 0.02 | |
| Val △ | 3.83 ± 0.08 | 3.65 ± 0.22 | 3.72 ± 0.10 | 3.83 ± 0.05 | |
| Met △ | 2.40 ± 0.06 | 2.45 ± 0.15 | 2.32 ± 0.06 | 2.58 ± 0.04 | |
| Ile △ | 3.79 ± 0.09 | 3.66 ± 0.21 | 3.64 ± 0.12 | 3.85 ± 0.04 | |
| Essential | Leu △ | 6.60 ± 0.16 | 6.29 ± 0.30 | 6.44 ± 0.15 | 6.63 ± 0.07 |
| Phe △ | 3.26 ± 0.08 | 3.11 ± 0.15 | 3.21 ± 0.06 | 3.29 ± 0.03 | |
| Lys | 7.41 ± 0.17 | 7.30 ± 0.45 | 7.17 ± 0.21 | 7.66 ± 0.10 | |
| His | 3.53 ± 0.06 *,# | 2.44 ± 0.23 | 2.93 ± 0.18 # | 2.33 ± 0.04 | |
| Arg △ | 5.37 ± 0.09 | 5.40 ± 0.27 | 5.26 ± 0.11 | 5.68 ± 0.08 | |
| EAA | 39.91 ± 0.86 | 38.04 ± 2.16 | 38.32 ± 1.07 | 39.79 ± 0.45 | |
| Asp ▲ | 7.71 ± 0.17 | 7.59 ± 0.40 | 7.53 ± 0.18 | 8.01 ± 0.09 | |
| Ser ▲ | 3.23 ± 0.09 | 3.44 ± 0.15 | 3.18 ± 0.06 | 3.67 ± 0.02 # | |
| Glu ▲ | 12.37 ± 0.29 | 13.13 ± 0.61 | 12.00 ± 0.31 | 13.77 ± 0.19 # | |
| Gly ▲ | 3.31 ± 0.09 | 3.47 ± 0.17 | 3.43 ± 0.10 | 3.73 ± 0.10 | |
| Ala ▲ | 4.66 ± 0.11 | 4.51 ± 0.25 | 4.58 ± 0.08 | 4.77 ± 0.04 | |
| Non-essential | Cys | 0.40 ± 0.02 | 0.51 ± 0.03 # | 0.39 ± 0.01 | 0.52 ± 0.00 # |
| Tyr | 2.90 ± 0.05 | 2.89 ± 0.10 | 2.85 ± 0.06 | 3.04 ± 0.03 | |
| Pro △ | 2.82 ± 0.06 | 2.88 ± 0.13 | 2.86 ± 0.06 | 3.05 ± 0.05 | |
| NEAA | 37.40 ± 0.84 | 38.41 ± 1.75 | 36.82 ± 0.69 | 40.55 ± 0.48 | |
| FAA | 28.07 ± 0.61 | 27.44 ± 1.42 | 27.46 ± 0.61 | 28.90 ± 0.33 | |
| TAA | 35.01 ± 0.83 | 35.88 ± 1.76 | 34.35 ± 0.69 | 37.88 ± 0.43 | |
| EAA/NEAA | 1.07 ± 0.00 # | 0.99 ± 0.01 | 1.04 ± 0.013 # | 0.98 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Chen, Q.; Yin, L.; Tang, Y.; Lin, Z.; Zhang, D.; Liu, Y. A Comparison of the Meat Quality, Nutritional Composition, Carcass Traits, and Fiber Characteristics of Different Muscular Tissues between Aged Indigenous Chickens and Commercial Laying Hens. Foods 2023, 12, 3680. https://doi.org/10.3390/foods12193680
Liu L, Chen Q, Yin L, Tang Y, Lin Z, Zhang D, Liu Y. A Comparison of the Meat Quality, Nutritional Composition, Carcass Traits, and Fiber Characteristics of Different Muscular Tissues between Aged Indigenous Chickens and Commercial Laying Hens. Foods. 2023; 12(19):3680. https://doi.org/10.3390/foods12193680
Chicago/Turabian StyleLiu, Li, Qian Chen, Lingqian Yin, Yuan Tang, Zhongzhen Lin, Donghao Zhang, and Yiping Liu. 2023. "A Comparison of the Meat Quality, Nutritional Composition, Carcass Traits, and Fiber Characteristics of Different Muscular Tissues between Aged Indigenous Chickens and Commercial Laying Hens" Foods 12, no. 19: 3680. https://doi.org/10.3390/foods12193680
APA StyleLiu, L., Chen, Q., Yin, L., Tang, Y., Lin, Z., Zhang, D., & Liu, Y. (2023). A Comparison of the Meat Quality, Nutritional Composition, Carcass Traits, and Fiber Characteristics of Different Muscular Tissues between Aged Indigenous Chickens and Commercial Laying Hens. Foods, 12(19), 3680. https://doi.org/10.3390/foods12193680






