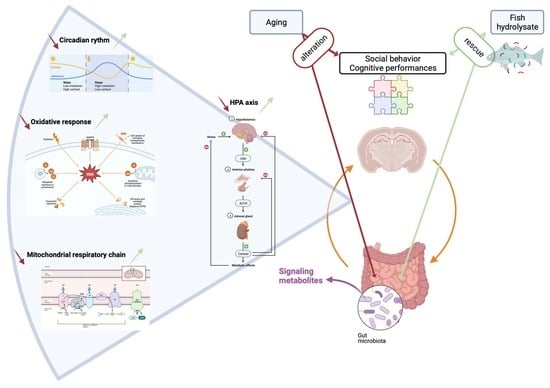
Dietary Marine Hydrolysate Improves Memory Performance and Social Behavior through Gut Microbiota Remodeling during Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet
2.3. Behavioral Test
2.3.1. Short-Term Spatial Recognition Memory Assessed in the Y-Maze
2.3.2. Spatial Memory Assessed in the Morris Water Maze Task
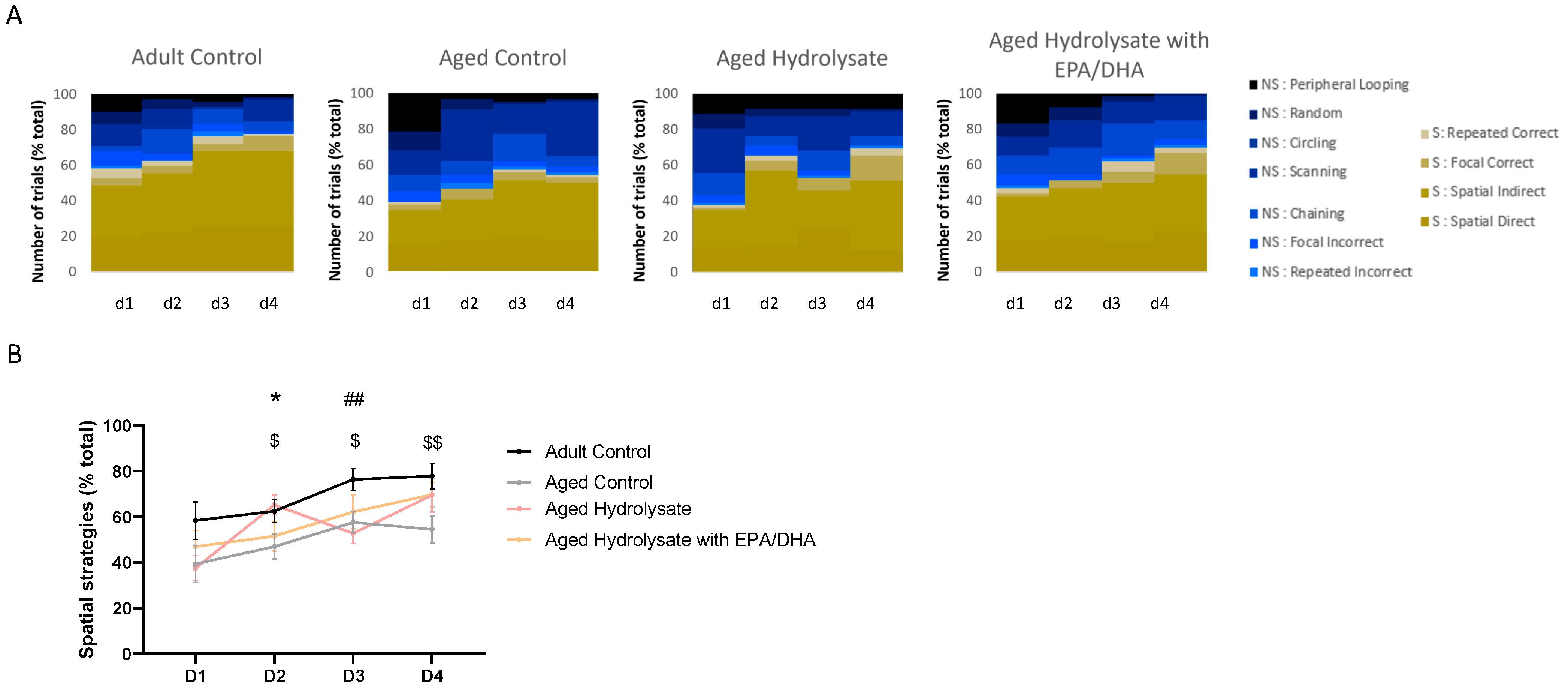
2.3.3. Examination of Navigation Strategies in the Morris Water Maze
2.3.4. Sociability and Interest in Social Novelty in the Three-Chamber Test
2.3.5. Restraint Test to Explore Stress Reactivity
2.4. Tissue Processing
2.5. Biochemical Measurements
2.5.1. Measurement of Corticosterone
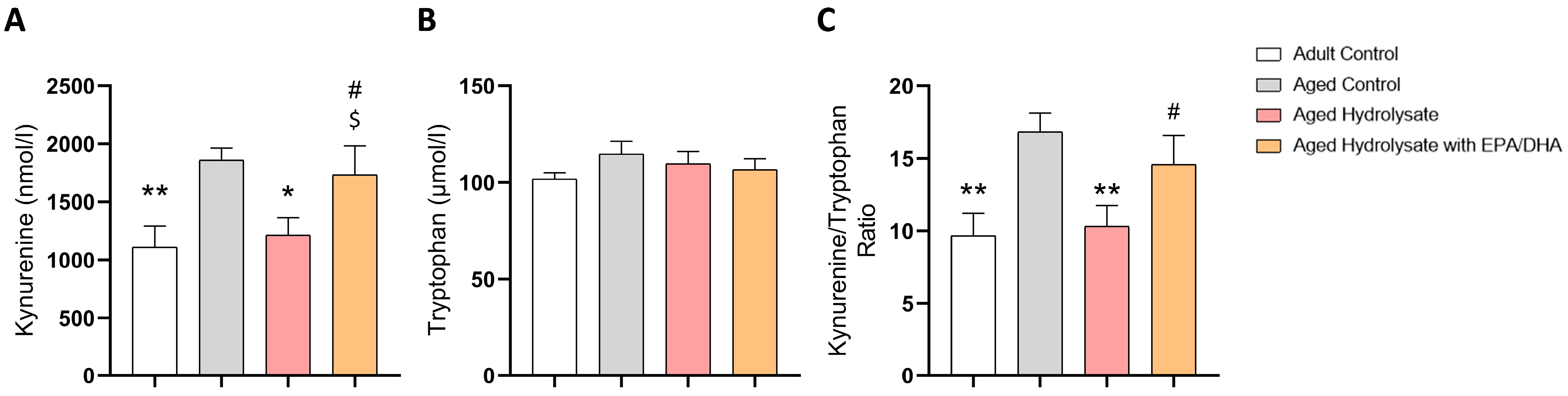
2.5.2. Tryptophan (TRP) and Kynurenine (KYN) Levels
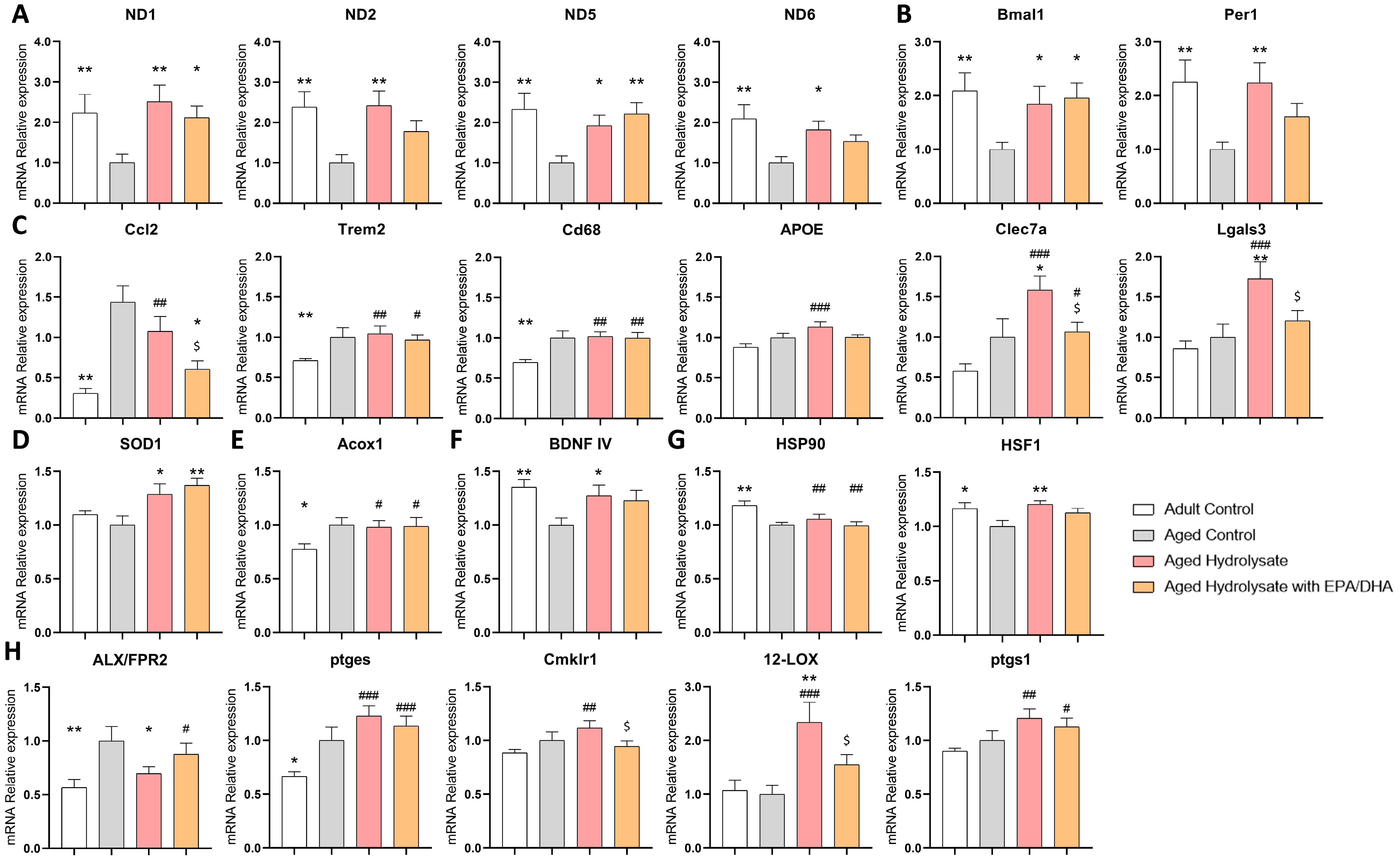
2.5.3. RNA Expression by Fluidigm Microfluidics Arrays
2.5.4. Analysis of the Microbiome in 16S
2.6. Statistical Analysis
3. Results
3.1. Both Fish Hydrolysate Supplementations Promote Social Novelty Preference in Aged Mice
3.2. Both Fish Hydrolysate Supplementations Prevent Short-Term and Long-Term Spatial Memory Deficits in Aged Mice
3.3. Fish Hydrolysate Supplementation Facilitates the Early Adoption of Spatial Strategies in the Process of Spatial Learning
3.4. Both Supplementations Decrease Basal Stress and Modulate Stress Response in Aged Mice
3.5. Fish Hydrolysate Supplementation Decreases the Levels of Plasmatic Kynurenine in Aged Mice
3.6. Both Supplementations Modulate Gene Expression Changes Associated with Aging
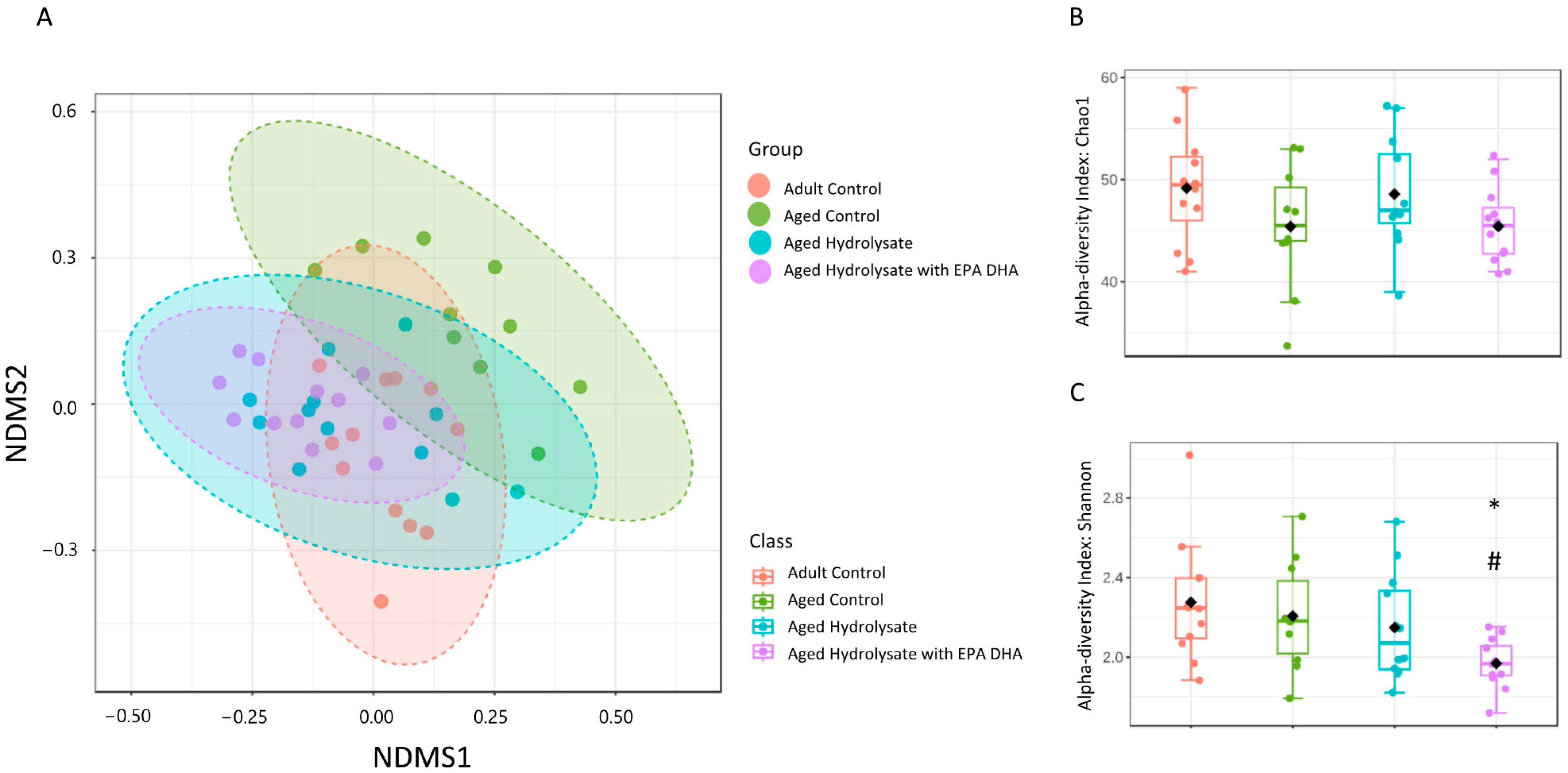
3.7. Age and Supplementations Have Distinct Microbiota Profiles
3.8. Both Supplementations Prevent Microbiota Impairments in Aged Mice
3.9. Preference for Social Novelty and Memory Performance Correlates with AUC of Corticosterone, Plasmatic Level of Kyn, Kyn/Trp Ratio, Gene Expression of Complex 1 of the Mitochondrial Respiratory Chain, and Gut Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Camfield, D.A.; Owen, L.; Scholey, A.B.; Pipingas, A.; Stough, C. Dairy Constituents and Neurocognitive Health in Ageing. Br. J. Nutr. 2011, 106, 159–174. [Google Scholar] [CrossRef]
- World Population Ageing, 2019 Highlights; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2020; ISBN 978-92-1-148325-3.
- Gallagher, M.; Rapp, P.R. The Use of Animal Models to Study the Effects of Aging on Cognition. Annu. Rev. Psychol. 1997, 48, 339–370. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, Neurodegeneration and Brain Rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Deary, I.J.; Corley, J.; Gow, A.J.; Harris, S.E.; Houlihan, L.M.; Marioni, R.E.; Penke, L.; Rafnsson, S.B.; Starr, J.M. Age-Associated Cognitive Decline. Br. Med. Bull. 2009, 92, 135–152. [Google Scholar] [CrossRef]
- Sadoun, A.; Rosito, M.; Fonta, C.; Girard, P. Key Periods of Cognitive Decline in a Nonhuman Primate Model of Cognitive Aging, the Common Marmoset (Callithrix Jacchus). Neurobiol. Aging 2019, 74, 1–14. [Google Scholar] [CrossRef]
- McQuail, J.A.; Frazier, C.J.; Bizon, J.L. Molecular Aspects of Age-Related Cognitive Decline: The Role of GABA Signaling. Trends Mol. Med. 2015, 21, 450–460. [Google Scholar] [CrossRef]
- Arioli, M.; Crespi, C.; Canessa, N. Social Cognition through the Lens of Cognitive and Clinical Neuroscience. BioMed Res. Int. 2018, 2018, 4283427. [Google Scholar] [CrossRef]
- Yang, Y.C.; Boen, C.; Gerken, K.; Li, T.; Schorpp, K.; Harris, K.M. Social Relationships and Physiological Determinants of Longevity across the Human Life Span. Proc. Natl. Acad. Sci. USA 2016, 113, 578–583. [Google Scholar] [CrossRef]
- Charles, S.T.; Carstensen, L.L. Social and Emotional Aging. Annu. Rev. Psychol. 2010, 61, 383–409. [Google Scholar] [CrossRef]
- Ratsika, A.; Cruz Pereira, J.S.; Lynch, C.M.K.; Clarke, G.; Cryan, J.F. Microbiota-Immune-Brain Interactions: A Lifespan Perspective. Curr. Opin. Neurobiol. 2023, 78, 102652. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the Social Brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut Microbiota and Aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Saraswati, S.; Sitaraman, R. Aging and the Human Gut Microbiota—From Correlation to Causality. Front. Microbiol. 2015, 5, 764. [Google Scholar] [CrossRef]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent Developments in Understanding the Role of the Gut Microbiota in Brain Health and Disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. Toward an Improved Definition of a Healthy Microbiome for Healthy Aging. Nat. Aging 2022, 2, 1054–1069. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary Polyphenols: Regulate the Advanced Glycation End Products-RAGE Axis and the Microbiota-Gut-Brain Axis to Prevent Neurodegenerative Diseases. Crit. Rev. Food Sci. Nutr. 2022, 1–27. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Boehme, M.; Guzzetta, K.E.; Bastiaanssen, T.F.S.; van de Wouw, M.; Moloney, G.M.; Gual-Grau, A.; Spichak, S.; Olavarría-Ramírez, L.; Fitzgerald, P.; Morillas, E.; et al. Microbiota from Young Mice Counteracts Selective Age-Associated Behavioral Deficits. Nat. Aging 2021, 1, 666–676. [Google Scholar] [CrossRef]
- Mossad, O.; Nent, E.; Woltemate, S.; Folschweiller, S.; Buescher, J.M.; Schnepf, D.; Erny, D.; Staeheli, P.; Bartos, M.; Szalay, A.; et al. Microbiota-Dependent Increase in δ-Valerobetaine Alters Neuronal Function and Is Responsible for Age-Related Cognitive Decline. Nat. Aging 2021, 1, 1127–1136. [Google Scholar] [CrossRef]
- Sylvia, K.E.; Demas, G.E. A Gut Feeling: Microbiome-Brain-Immune Interactions Modulate Social and Affective Behaviors. Horm. Behav. 2018, 99, 41–49. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Claesson, M.J. Gut Microbiota: Changes throughout the Lifespan from Infancy to Elderly. Int. Dairy J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
- Jawhara, S. Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection. Microorganisms 2023, 11, 1556. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Soy Bioactive Peptides and the Gut Microbiota Modulation. Appl. Microbiol. Biotechnol. 2020, 104, 9009–9017. [Google Scholar] [CrossRef]
- Tsafack, P.B.; Li, C.; Tsopmo, A. Food Peptides, Gut Microbiota Modulation, and Antihypertensive Effects. Molecules 2022, 27, 8806. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]
- Szklany, K.; Engen, P.A.; Naqib, A.; Green, S.J.; Keshavarzian, A.; Lopez Rincon, A.; Siebrand, C.J.; Diks, M.A.P.; van de Kaa, M.; Garssen, J.; et al. Dietary Supplementation throughout Life with Non-Digestible Oligosaccharides and/or n-3 Poly-Unsaturated Fatty Acids in Healthy Mice Modulates the Gut-Immune System-Brain Axis. Nutrients 2021, 14, 173. [Google Scholar] [CrossRef]
- Portela, N.D.; Galván, C.; Sanmarco, L.M.; Bergero, G.; Aoki, M.P.; Cano, R.C.; Pesoa, S.A. Omega-3-Supplemented Fat Diet Drives Immune Metabolic Response in Visceral Adipose Tissue by Modulating Gut Microbiota in a Mouse Model of Obesity. Nutrients 2023, 15, 1404. [Google Scholar] [CrossRef]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health Benefits of Docosahexaenoic Acid and Its Bioavailability: A Review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef]
- Chataigner, M.; Mortessagne, P.; Lucas, C.; Pallet, V.; Layé, S.; Mehaignerie, A.; Bouvret, E.; Dinel, A.L.; Joffre, C. Dietary Fish Hydrolysate Supplementation Containing N-3 LC-PUFAs and Peptides Prevents Short-Term Memory and Stress Response Deficits in Aged Mice. Brain. Behav. Immun. 2020, 91, 716–730. [Google Scholar] [CrossRef]
- Dellu, F.; Mayo, W.; Cherkaoui, J.; Le Moal, M.; Simon, H. A Two-Trial Memory Task with Automated Recording: Study in Young and Aged Rats. Brain Res. 1992, 588, 132–139. [Google Scholar] [CrossRef]
- Bensalem, J.; Servant, L.; Alfos, S.; Gaudout, D.; Layé, S.; Pallet, V.; Lafenetre, P. Dietary Polyphenol Supplementation Prevents Alterations of Spatial Navigation in Middle-Aged Mice. Front. Behav. Neurosci. 2016, 10, 9. [Google Scholar] [CrossRef]
- Morris, R. Developments of a Water-Maze Procedure for Studying Spatial Learning in the Rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Boyer, F.; Jaouen, F.; Ibrahim, E.C.; Gascon, E. Deficits in Social Behavior Precede Cognitive Decline in Middle-Aged Mice. Front. Behav. Neurosci. 2019, 13, 55. [Google Scholar] [CrossRef]
- Dinel, A.-L.; André, C.; Aubert, A.; Ferreira, G.; Layé, S.; Castanon, N. Lipopolysaccharide-Induced Brain Activation of the Indoleamine 2,3-Dioxygenase and Depressive-like Behavior Are Impaired in a Mouse Model of Metabolic Syndrome. Psychoneuroendocrinology 2014, 40, 48–59. [Google Scholar] [CrossRef]
- Gohl, D.M.; Vangay, P.; Garbe, J.; MacLean, A.; Hauge, A.; Becker, A.; Gould, T.J.; Clayton, J.B.; Johnson, T.J.; Hunter, R.; et al. Systematic Improvement of Amplicon Marker Gene Methods for Increased Accuracy in Microbiome Studies. Nat. Biotechnol. 2016, 34, 942–949. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Machanda, Z.P.; Rosati, A.G. Shifting Sociality during Primate Ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190620. [Google Scholar] [CrossRef]
- Wrzus, C.; Hänel, M.; Wagner, J.; Neyer, F.J. Social Network Changes and Life Events across the Life Span: A Meta-Analysis. Psychol. Bull. 2013, 139, 53–80. [Google Scholar] [CrossRef]
- Shoji, H.; Miyakawa, T. Age-Related Behavioral Changes from Young to Old Age in Male Mice of a C57BL/6J Strain Maintained under a Genetic Stability Program. Neuropsychopharmacol. Rep. 2019, 39, 100–118. [Google Scholar] [CrossRef]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C.; et al. Revisiting Metchnikoff: Age-Related Alterations in Microbiota-Gut-Brain Axis in the Mouse. Brain. Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, Variability, and Temporal Stability of the Intestinal Microbiota of the Elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The Gut Microbiome as a Modulator of Healthy Ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut Microbiome Pattern Reflects Healthy Ageing and Predicts Survival in Humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Boehme, M.; Guzzetta, K.E.; Wasén, C.; Cox, L.M. The Gut Microbiota Is an Emerging Target for Improving Brain Health during Ageing. Gut Microbiome 2023, 4, E2. [Google Scholar] [CrossRef]
- Wang, Z.; Ying, X.; Gao, P.; Wang, C.; Wang, Y.; Yu, X.; Chen, J.; Wang, B.; Luo, H. Anti-Inflammatory Activity of a Peptide from Skipjack (Katsuwonus Pelamis). Mar. Drugs 2019, 17, 582. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Yang, Y.; Wang, Z.; Wang, B.; Zhang, B.; Yu, J.; Lu, W.; Pan, M.; Zhao, J.; et al. The Diversity of Gut Microbiota in Type 2 Diabetes with or without Cognitive Impairment. Aging Clin. Exp. Res. 2021, 33, 589–601. [Google Scholar] [CrossRef]
- Manderino, L.; Carroll, I.; Azcarate-Peril, M.A.; Rochette, A.; Heinberg, L.; Peat, C.; Steffen, K.; Mitchell, J.; Gunstad, J. Preliminary Evidence for an Association Between the Composition of the Gut Microbiome and Cognitive Function in Neurologically Healthy Older Adults. J. Int. Neuropsychol. Soc. JINS 2017, 23, 700–705. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Wang, C.; Wang, L.; He, T.; Hu, H.; Song, J.; Cui, C.; Qiao, J.; Qing, L.; et al. Enterotype Bacteroides Is Associated with a High Risk in Patients with Diabetes: A Pilot Study. J. Diabetes Res. 2020, 2020, 6047145. [Google Scholar] [CrossRef]
- Higarza, S.G.; Arboleya, S.; Arias, J.L.; Gueimonde, M.; Arias, N. Akkermansia Muciniphila and Environmental Enrichment Reverse Cognitive Impairment Associated with High-Fat High-Cholesterol Consumption in Rats. Gut Microbes 2021, 13, 1880240. [Google Scholar] [CrossRef]
- Anderson, J.R.; Carroll, I.; Azcarate-Peril, M.A.; Rochette, A.D.; Heinberg, L.J.; Peat, C.; Steffen, K.; Manderino, L.M.; Mitchell, J.; Gunstad, J. A Preliminary Examination of Gut Microbiota, Sleep, and Cognitive Flexibility in Healthy Older Adults. Sleep Med. 2017, 38, 104–107. [Google Scholar] [CrossRef]
- Verdi, S.; Jackson, M.A.; Beaumont, M.; Bowyer, R.C.E.; Bell, J.T.; Spector, T.D.; Steves, C.J. An Investigation into Physical Frailty as a Link Between the Gut Microbiome and Cognitive Health. Front. Aging Neurosci. 2018, 10, 398. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Yu, S.; Wang, L.; Jing, X.; Wang, Y.; An, C. Features of Gut Microbiota and Short-Chain Fatty Acids in Patients with First-Episode Depression and Their Relationship with the Clinical Symptoms. Front. Psychol. 2023, 14, 1088268. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Yuan, Y.; Liu, L.; Zhao, Y.; Wang, X. Gut Microbiota Alteration Is Associated With Cognitive Deficits in Genetically Diabetic (Db/Db) Mice During Aging. Front. Aging Neurosci. 2022, 13, 815562. [Google Scholar] [CrossRef]
- Li, T.; Lin, L.; Li, C.; Zheng, J.; Chen, B.; Shen, Y.; Ren, D. Amelioration of Walnut-Derived Novel Peptides against D-Galactose-Induced Cognitive Impairment by Modulating the Gut Microbiota Composition. Food Funct. 2023, 14, 4228–4241. [Google Scholar] [CrossRef]
- Bondar, N.P.; Lepeshko, A.A.; Reshetnikov, V.V. Effects of Early-Life Stress on Social and Anxiety-Like Behaviors in Adult Mice: Sex-Specific Effects. Behav. Neurol. 2018, 2018, 1538931. [Google Scholar] [CrossRef]
- Jacobson, L. Hypothalamic-Pituitary-Adrenocortical Axis: Neuropsychiatric Aspects. Compr. Physiol. 2014, 4, 715–738. [Google Scholar] [CrossRef]
- Du, X.; Pang, T.Y. Is Dysregulation of the HPA-Axis a Core Pathophysiology Mediating Co-Morbid Depression in Neurodegenerative Diseases? Front. Psychiatry 2015, 6, 32. [Google Scholar] [CrossRef]
- Sriram, K.; Rodriguez-Fernandez, M.; Doyle, F.J. Modeling Cortisol Dynamics in the Neuro-Endocrine Axis Distinguishes Normal, Depression, and Post-Traumatic Stress Disorder (PTSD) in Humans. PLoS Comput. Biol. 2012, 8, e1002379. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the Gut-Brain Axis: Regulation by the Microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Mackos, A.R.; Maltz, R.; Bailey, M.T. The Role of the Commensal Microbiota in Adaptive and Maladaptive Stressor-Induced Immunomodulation. Horm. Behav. 2017, 88, 70–78. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Tronche, C.; Piérard, C.; Coutan, M.; Chauveau, F.; Liscia, P.; Béracochéa, D. Increased Stress-Induced Intra-Hippocampus Corticosterone Rise Associated with Memory Impairments in Middle-Aged Mice. Neurobiol. Learn. Mem. 2010, 93, 343–351. [Google Scholar] [CrossRef]
- Knight, B.G.; Durbin, K. Aging and the Effects of Emotion on Cognition: Implications for Psychological Interventions for Depression and Anxiety. PsyCh J. 2015, 4, 11–19. [Google Scholar] [CrossRef]
- Puigoriol-Illamola, D.; Griñán-Ferré, C.; Vasilopoulou, F.; Leiva, R.; Vázquez, S.; Pallàs, M. 11β-HSD1 Inhibition by RL-118 Promotes Autophagy and Correlates with Reduced Oxidative Stress and Inflammation, Enhancing Cognitive Performance in SAMP8 Mouse Model. Mol. Neurobiol. 2018, 55, 8904–8915. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of Circadian Rhythms in Health and Disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Versace, S.; Pellitteri, G.; Sperotto, R.; Tartaglia, S.; Da Porto, A.; Catena, C.; Gigli, G.L.; Cavarape, A.; Valente, M. A State-of-Art Review of the Vicious Circle of Sleep Disorders, Diabetes and Neurodegeneration Involving Metabolism and Microbiota Alterations. Int. J. Mol. Sci. 2023, 24, 10615. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef]
- Froy, O. The Circadian Clock and Metabolism. Clin. Sci. Lond. Engl. 1979 2011, 120, 65–72. [Google Scholar] [CrossRef]
- Duncan, M.J.; Prochot, J.R.; Cook, D.H.; Tyler Smith, J.; Franklin, K.M. Influence of Aging on Bmal1 and Per2 Expression in Extra-SCN Oscillators in Hamster Brain. Brain Res. 2013, 1491, 44–53. [Google Scholar] [CrossRef]
- Price, K.H.; Dziema, H.; Aten, S.; Loeser, J.; Norona, F.E.; Hoyt, K.; Obrietan, K. Modulation of Learning and Memory by the Targeted Deletion of the Circadian Clock Gene Bmal1 in Forebrain Circuits. Behav. Brain Res. 2016, 308, 222–235. [Google Scholar] [CrossRef]
- Kwapis, J.L.; Alaghband, Y.; Kramár, E.A.; López, A.J.; Vogel Ciernia, A.; White, A.O.; Shu, G.; Rhee, D.; Michael, C.M.; Montellier, E.; et al. Epigenetic Regulation of the Circadian Gene Per1 Contributes to Age-Related Changes in Hippocampal Memory. Nat. Commun. 2018, 9, 3323. [Google Scholar] [CrossRef]
- Al-Rawashdeh, S.Y.; Lennie, T.A.; Chung, M.L. The Association of Sleep Disturbances with Quality of Life in Heart Failure Patient-Caregiver Dyads. West. J. Nurs. Res. 2017, 39, 492–506. [Google Scholar] [CrossRef]
- Borbolis, F.; Mytilinaiou, E.; Palikaras, K. The Crosstalk between Microbiome and Mitochondrial Homeostasis in Neurodegeneration. Cells 2023, 12, 429. [Google Scholar] [CrossRef]
- Fehér, J.; Élő, Á.; István, L.; Nagy, Z.Z.; Radák, Z.; Scuderi, G.; Artico, M.; Kovács, I. Microbiota Mitochondria Disorders as Hubs for Early Age-Related Macular Degeneration. GeroScience 2022, 44, 2623–2653. [Google Scholar] [CrossRef]
- Navarro, A.; Gomez, C.; López-Cepero, J.M.; Boveris, A. Beneficial Effects of Moderate Exercise on Mice Aging: Survival, Behavior, Oxidative Stress, and Mitochondrial Electron Transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R505–R511. [Google Scholar] [CrossRef]
- Navarro, A.; Gómez, C.; Sánchez-Pino, M.-J.; González, H.; Bández, M.J.; Boveris, A.D.; Boveris, A. Vitamin E at High Doses Improves Survival, Neurological Performance, and Brain Mitochondrial Function in Aging Male Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1392–R1399. [Google Scholar] [CrossRef]
- Papa, S.; De Rasmo, D. Complex I Deficiencies in Neurological Disorders. Trends Mol. Med. 2013, 19, 61–69. [Google Scholar] [CrossRef]
- Corral-Debrinski, M.; Horton, T.; Lott, M.T.; Shoffner, J.M.; Beal, M.F.; Wallace, D.C. Mitochondrial DNA Deletions in Human Brain: Regional Variability and Increase with Advanced Age. Nat. Genet. 1992, 2, 324–329. [Google Scholar] [CrossRef]
- Lin, M.T.; Simon, D.K.; Ahn, C.H.; Kim, L.M.; Beal, M.F. High Aggregate Burden of Somatic MtDNA Point Mutations in Aging and Alzheimer’s Disease Brain. Hum. Mol. Genet. 2002, 11, 133–145. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; El Aidy, S.; Crispie, F.; O’Sullivan, O.; Cotter, P.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. N-3 Polyunsaturated Fatty Acids (PUFAs) Reverse the Impact of Early-Life Stress on the Gut Microbiota. PLoS ONE 2015, 10, e0139721. [Google Scholar] [CrossRef]
- Roussel, C.; Anunciação Braga Guebara, S.; Plante, P.-L.; Desjardins, Y.; Di Marzo, V.; Silvestri, C. Short-Term Supplementation with ω-3 Polyunsaturated Fatty Acids Modulates Primarily Mucolytic Species from the Gut Luminal Mucin Niche in a Human Fermentation System. Gut Microbes 2022, 14, 2120344. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Bernet, F.; Montel, V.; Noël, B.; Dupouy, J.P. Diazepam-like Effects of a Fish Protein Hydrolysate (Gabolysat PC60) on Stress Responsiveness of the Rat Pituitary-Adrenal System and Sympathoadrenal Activity. Psychopharmacology 2000, 149, 34–40. [Google Scholar] [CrossRef]
- Kuijer, E.J.; Steenbergen, L. The Microbiota-Gut-Brain Axis in Hippocampus-Dependent Learning and Memory: Current State and Future Challenges. Neurosci. Biobehav. Rev. 2023, 152, 105296. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096. [Google Scholar] [CrossRef]







| Components | Percent (%) | ||
|---|---|---|---|
| Control Diet | Hydrolysate-Enriched Diet | Hydrolysate-Enriched Diet with EPA/DHA | |
| Hydrochloric casein | 18 | 18 | 18 |
| Corn starch | 45.9 | 45.69 | 45.69 |
| Sucrose | 24 | 24 | 24 |
| Cellulose | 2 | 2 | 2 |
| Peanut Oil | 5 | 5 | 4.91 |
| Mineral Mix | 4 | 4 | 4 |
| Vitamin Mix | 1 | 1 | 1 |
| +DL methionine | 0.1 | 0.1 | 0.1 |
| +Vitamin A 5 UI/g | 5 UI/g | 5 UI/g | 5 UI/g |
| Hydrolysate | 0 | 0.21 | 0.21 |
| EPA/DHA (4:1) | 0 | 0 | 0.09 |
| Symbol | Name | Sequence (5′-3′) | Pathway |
|---|---|---|---|
| ND1-F | NADH dehydrogenase 1, mitochondrial | GAGTTCCCCTACCAATACCACACC | Mitochondrial respiratory chain |
| ND1-R | CGGCTCGTAAAGCTCCGAATAG | ||
| ND2-F | NADH dehydrogenase 2, mitochondrial | TTCATAGGGGCATGAGGAGGAC | |
| ND2-R | GTGAGGGATGGGTTGTAAGGAAG | ||
| ND5-F | NADH dehydrogenase 5, mitochondrial | GGAAGCATCTTTGCAGGATTTG | |
| ND5-R | CATGGTATTGTGAGGACTGGAATG | ||
| ND6-F | NADH dehydrogenase 6, mitochondrial | TGGTTTGGGAGATTGGTTGATG | |
| ND6-R | TATTGCCGCTACCCCAATCC | ||
| Bmal1-F | Basic helix-loop-helix ARNT like 1 | CGCTACGAAGTCGATGGTTCAG | Circadian clock |
| Bmal1-R | TGTTAGCTGCGGGAAGGTTG | ||
| Per1-F | Period circadian clock 1 | TGTCCTGCTGCGTTGCAAAC | |
| Per1-R | TTGAGACCTGAACCTGCAGAGG | ||
| SOD1-F | Superoxide dismutase 1 | TTGGCCGTACAATGGTGGTC | Antioxidant defenses |
| SOD1-R | GCAATCCCAATCACTCCACAG | ||
| APOE-F | Apolipoprotein E | TGCGAAGATGAAGGCTCTGTG | Microglial neurodegenerative phenotype |
| APOE-R | GGTTGGTTGCTTTGCCACTC | ||
| mTrem2-F | Triggering receptor expressed on myeloid cells 2 | TGCTGGCAAAGGAAAGGTGC | |
| mTrem2-R | ACATGACACCCTCAAGGACTGG | ||
| mCd68-F | Cluster of Differentiation 68 | TTCGGGCCATGTTTCTCTTG | |
| mCd68-R | ATTGTCGTCTGCGGGTGATG | ||
| mClec7a-F | C-Type Lectin Domain Containing 7A | TGGGTGCCCTAGCATTTTGG | |
| mClec7a-R | TGATTCTGTGGGCTTGTGGTTC | ||
| mLgals3-F | Galectin 3 | TATCCTGCTGCTGGCCCTTATG | |
| mLgals3-R | TGCGTTGGGTTTCACTGTGC | ||
| mCcl2-F | C-C Motif Chemokine Ligand 2 | ACCAGCCAACTCTCACTGAAGC | |
| mCcl2-R | TGGGGCGTTAACTGCATCTG | ||
| Acox1-F | Peroxisomal acyl-CoA oxidase | TCACTCGAAGCCAGCGTTAC | b-oxidation |
| Acox1-R | TTGAGGCCAACAGGTTCCAC | ||
| BDNFIV-F | Brain Derived Neurotrophic Factor | CAGAGCAGCTGCCTTGATGTT | Neuroprotection |
| BDNFIV-R | GCCTTGTCCGTGGACGTTTA | ||
| HSP90-F | Heat shock protein 90 | TTGGTGGACACAGGCATTGG | Aging |
| HSP90-R | AATCCGACACCAAACTGCCC | ||
| HSF1-F | Heat shock factor 1 | TGGCCATGAAGCACGAGAAC | |
| HSF1-R | TTTGCTGCTGGGCATGCTTC | ||
| Cmklr1-F | Chemerin Chemokine-Like Receptor 1 | AGTCACGCGCAGTAACAGAC | Synthesis of oxylipins |
| Cmklr1-R | TCGTTGTAAGCGTCGTACTCC | ||
| ALXFPR2-F | Formyl peptide receptor 2 | CATGAAGTCTGTGATAAGGGATGG | |
| ALXFPR2-R | CATAAGGTAAGGAAGGCAGAAGTG | ||
| 12LOX-F | 12-lipoxygenase | GCTGTTGCCACCATGAGATG | |
| 12LOX-R | AACGGATGTGTGGAACGAGG | ||
| Ptges-F | Prostaglandin E Synthase | ATCAAGATGTACGCGGTGGC | |
| Ptges-R | ATCCTCGGGGTTGGCAAAAG | ||
| Ptgs1-F | Prostaglandin-Endoperoxide Synthase 1 | ATCACCTGCGGCTCTTCAAG | |
| Ptgs1-R | ATCAACACGGACGCCTGTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mougin, C.; Chataigner, M.; Lucas, C.; Leyrolle, Q.; Pallet, V.; Layé, S.; Bouvret, E.; Dinel, A.-L.; Joffre, C. Dietary Marine Hydrolysate Improves Memory Performance and Social Behavior through Gut Microbiota Remodeling during Aging. Foods 2023, 12, 4199. https://doi.org/10.3390/foods12234199
Mougin C, Chataigner M, Lucas C, Leyrolle Q, Pallet V, Layé S, Bouvret E, Dinel A-L, Joffre C. Dietary Marine Hydrolysate Improves Memory Performance and Social Behavior through Gut Microbiota Remodeling during Aging. Foods. 2023; 12(23):4199. https://doi.org/10.3390/foods12234199
Chicago/Turabian StyleMougin, Camille, Mathilde Chataigner, Céline Lucas, Quentin Leyrolle, Véronique Pallet, Sophie Layé, Elodie Bouvret, Anne-Laure Dinel, and Corinne Joffre. 2023. "Dietary Marine Hydrolysate Improves Memory Performance and Social Behavior through Gut Microbiota Remodeling during Aging" Foods 12, no. 23: 4199. https://doi.org/10.3390/foods12234199
APA StyleMougin, C., Chataigner, M., Lucas, C., Leyrolle, Q., Pallet, V., Layé, S., Bouvret, E., Dinel, A.-L., & Joffre, C. (2023). Dietary Marine Hydrolysate Improves Memory Performance and Social Behavior through Gut Microbiota Remodeling during Aging. Foods, 12(23), 4199. https://doi.org/10.3390/foods12234199