Abstract
Benincasa hispida peel, a type of postconsumer waste, is considered a source of beneficial phytochemicals. Therefore, it was subjected to investigation for biological activities in this study. B. hispida peel was extracted using 95% v/v, 50% v/v ethanol and water. The obtained extracts were B95, B50 and BW. B95 had a high flavonoid content (212.88 ± 4.73 mg QE/g extract) and phenolic content (131.52 ± 0.38 mg GAE/g extract) and possessed high antioxidant activities as confirmed by DPPH, ABTS and lipid peroxidation inhibition assays. Moreover, B95 showed inhibitory effects against collagenase and hyaluronidase with values of 41.68 ± 0.92% and 29.17 ± 0.66%, which related to anti-aging activities. Via the HPLC analysis, one of the potential compounds found in B95 was rutin. Molecular docking has provided an understanding of the molecular mechanisms underlying the interaction of extracts with collagenase and hyaluronidase. All extracts were not toxic to fibroblast cells and did not irritate the hen’s egg chorioallantoic membrane, which indicated its safe use. In conclusion, B. hispida peel extracts are promising potential candidates for further use as antioxidant and anti-aging agents in the food and cosmetic industries.
1. Introduction
Ultraviolet (UV) radiation is a potent initiator of reactive oxygen species (ROS) generation in the skin, leading to oxidative imbalance [1]. ROS can impair the skin barrier functions by altering squalene, cholesterol and unsaturated lipids, which are essential components of the skin’s structure. The effect of skin oxidative stress caused by excessive ROS leads to the initiation of several skin problems such as atypical pigmentation, skin inflammation, increasing sebum secretion and increased levels of oxidized lipids, including skin aging [2,3]. Collagen, elastin and hyaluronic acid (HA) are biological compounds that help promote healthy and youthful skin. Collagen and elastin, the proteins abundant in the dermal layer, play essential roles in maintaining skin flexibility, elasticity and integrity, whereas HA, a glucose-based polymer, in the dermis and the epidermis layers mainly promotes skin rejuvenation and moisture [4,5]. The over-accumulation of ROS leads to the activation of dermal enzymes including collagenase, elastase and hyaluronidase, which in turn degrade collagen, elastin and HA [6]. Thus, various antioxidants simplify skin protection against oxidative damage caused by ROS. Both chemical and natural antioxidants are investigated and incorporated into numerous skin care products to mitigate skin damage. Medicinal plants have been reported to provide potential antioxidation activity through various mechanisms. For example, Thunbergia laurifolia Lindl. leaf extracts have demonstrated antioxidation activities through radical scavenging and inhibiting peroxidation mechanisms [7], and Acacia concinna Linn. pod extracts exhibited scavenging activity against free radicals [8].
Benincasa hispida (Thunb.) Cogn, a winter melon also known as Fuk-Kiew in Thailand, is an edible plant in the family Cucurbitaceae, generally found in Asia, especially in northern Thailand. Scientific reports suggest that B. hispida is rich in nutrients, minerals, vitamins and phytochemical compounds [9]. The bioactive compounds in B. hispida extract include phenolics, triterpenoids, flavonoids, glycosides, carotenes and β-sitosterin [10,11]. Related studies have reported that B. hispida exhibits pharmacologic effects including antioxidation, anti-angiotensin-converting enzyme, anti-inflammation and antibacterial effects. [12,13]. In addition, the efficacy and phytochemical components of the pulp, fruit and seeds of B. hispida were determined [11]. Gallic acid, catechin and ascorbic acid, which are phenolic compounds found in the fruit of B. hispida, exhibit antioxidant activity by reducing free radicals [14,15,16].
The fruit of B. hispida is broadly cylindrical in shape, as shown in Figure 1A. Generally, the edible pulp of the fruit is used in cooking and the production of various food products such as tea and juice. Thus, the peel of the fruit then becomes a form of postconsumer waste, as shown in Figure 1B. Interestingly, B. hispida juice has gained popularity as a consumer product. This trend has resulted in an increased production of B. hispida peel, which is considered a waste product in the food industry. Notably, B. hispida fruit can be stored for several months, possibly because of the presence of a protective wax coating on the peel. Thus, investigating the constituents within the peel responsible for the ability to protect the pulp from external environmental influences as shown in Figure 1B would be intriguing. Therefore, the present study focuses on investigating the antioxidant and anti-aging properties of B. hispida peel, conducting simulations of the possible docking mechanisms of the potential compounds in the peel and performing safety tests. These research methods are designed to transform B. hispida peel, traditionally considered a waste product, into a high-value material for potential applications as an innovative cosmetic ingredient or another innovative material using its biological activities and the mechanisms of action determined through docking simulations.
Figure 1.
B. hispida fruit (A) and its peel (B) used in investigation.
2. Materials and Methods
2.1. Materials
The plant material used in this study was fresh B. hispida fruit, collected from Phrae Province, Thailand. The plant was identified, and its voucher specimen was stored in the Herbarium of the Faculty of Pharmacy, Chiang Mai University, Thailand.
The reagents and chemicals were of analytical grade. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS) were purchased from Fluka (Buchs, Switzerland). Folin–Ciocalteu reagent was purchased from Merck (Darmstadt, Germany). 6-Hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid (Trolox), gallic acid, ascorbic acid, kojic acid, epigallocatechin-3-gallate (EGCG), hyaluronic acid, linoleic acid, rutin, 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT) and 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) were purchased from Sigma (St. Louis, MO, USA). Dulbecco’s Modified Eagle medium (DMEM) and penicillin–streptomycin were acquired from Invitrogen (Grand Island, NY, USA). Fetal bovine serum (FBS) and bovine serum albumin (BSA) were obtained from Biochrom AG (Berlin, Germany). Ethanol and dimethyl sulfoxide (DMSO) were purchased from Labscan Asia Co., Ltd. (Bangkok, Thailand). HPLC-grade acetonitrile was purchased from Merck (Darmstadt, Germany). Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (Darmstadt, Germany), and sodium carbonate, aluminum chloride and sodium nitrite were purchased from United Chemical & Trading Co., Ltd. (Chiang Mai, Thailand). Other chemicals and solvents were of the highest grade available.
2.2. Plant Extraction
The procedure was performed following a related study with modification [17]. Briefly, the fresh peel of B. hispida fruit was dried in a hot air oven (Memmert, WI, USA) at 50 °C for 24 to 48 h and then ground to a fine powder. The obtained plant powder was extracted with various solvents using ultrasound-assisted extraction (UAE). For extraction, 95% v/v ethanol, 50% v/v ethanol and deionized (DI) water were used as solvents. In each solvent extraction, an ultrasonic bath (Elma Schmidbauer GmbH, Singen, Germany) was used to generate the ultrasound effect for 30 min at 65.0 ± 1.0 °C. Each UAE was performed in triplicate. The extracted mixture (plant powder mixed with solvent) was filtered through a Whatman No. 1 filter paper. The filtrates obtained from 95% v/v ethanol and 50% v/v ethanol were subjected to a rotary vacuum evaporator (M.A-3S, Eyela, Tokyo, Japan) to remove the solvent, whereas the filtrate obtained from DI water was subjected to a lyophilizer (Christ Beta 2-8 LD plus, Osterode am Harz, Germany). After the solvents were completely removed, the extracts from 95% v/v ethanol (B95), 50% v/v ethanol (B50) and DI water (BW) were obtained, and all extracts were stored at 4 °C until use. The %yield of each extract was calculated using the following equation:
where E represents the weight of the extract and P represents the weight of the plant powder used for extraction.
%yield = (E/P) × 100
2.3. Determination of Total Flavonoid Content
Total flavonoid content (TFC) was determined using aluminum chloride colorimetry following our related study [18]. All B. hispida extracts were prepared at a concentration of 1.0 mg/mL in DI water. The extract with 100 µL was mixed with 30 µL of 5% v/v sodium nitride. After 5 min, 50 µL of 2% v/v aluminum chloride was added. The solution mixture was left for 6 min, followed by adding 50 µL of 1N sodium hydroxide. The mixture was stored in the dark for 10 min at room temperature before analysis. The mixed solution was measured for absorbance at 510 nm using a UV spectrophotometer (UV-2600i, Shimadzu, Kyoto, Japan) against a blank without the extract. The standard curve for TFC determination was constructed using a quercetin (QE) solution (0.0 to 1.0 mg/mL) under the same procedure as the tested B. hispida extract. The TFC value was expressed as milligrams of QE equivalents per gram of extract (mg QE/g extract).
2.4. Determination of Total Phenolic Content
Total phenolic content (TPC) was analyzed using the Folin–Ciocalteu method [19]. First, 50 µL of each B. hispida extract (3 mg/mL in DI water) was mixed with 100 µL of 10% v/v Folin–Ciocalteu reagent. After 5 min, 50 µL of 10% w/v sodium carbonate was added. The mixture was vortexed and stored in the dark at ambient temperature for 2 h. Then, the absorbance of mixtures was measured using a UV spectrophotometer (UV-2600i, Shimadzu) at 765 nm against a blank without the extract. The TPC value was determined using a standard curve constructed with a gallic acid standard solution (0.0 to 1.0 mg/mL) and expressed as milligrams of gallic acid equivalents (GAEs) per gram of extract (mg GAE/g extract).
2.5. Antioxidant Activities
The B. hispida extracts were prepared to determine the antioxidant activities using various procedures. Trolox and ascorbic acid were used as standards in all assays.
2.5.1. DPPH Radical Scavenging Assay
This experiment was determined using the modified method [20]. In total, 20 µL of the extract solution (0.0 to 1.0 mg/mL) was mixed with 180 µL of DPPH reagent (167 µM) and then incubated in the dark at ambient temperature for 30 min. The absorbance of the extract–reagent mixture was investigated at 520 nm using a microplate reader (SpectraMax M3, Molecular Devices, San Jose, CA, USA). The efficacy against DPPH radicals was expressed as the percentage of inhibition (% inhibition), which was estimated using the following equation:
where Ac is the absorbance of positive control that used ethanol mixed with tested reagent, Aw is the absorbance of negative control (ethanol), Ae is the absorbance of tested extract, and Ab is the absorbance of blank that used extract solution mixed with ethanol.
% Inhibition = [(Ac − Aw) − (Ae − Ab)/(Ac − Ab)] × 100
2.5.2. ABTS Radical Scavenging Assay
The potential of B. hispida peel extracts was assessed in terms of scavenging activity using the ABTS assay following the method of Poomanee [21] with modifications. The ABTS reagent was prepared by mixing an ABTS solution in DI water (7 mM) with potassium persulfate solution in DI water (3 mM) in a ratio of 1:0.5 and incubated in a dark area for 18 h. The measurement was performed using a microplate reader (SpectraMax M3, Molecular Devices) at 734 nm. Before testing, the prepared ABTS reagent was diluted to provide an absorbance of 0.7 and was then used as a tested ABTS reagent. Altogether, 20 µL of the extract solution (0.0 to 1.0 mg/mL) and 180 µL of tested ABTS reagent were mixed and incubated in the dark at ambient temperature for 30 min. The absorbance of the extract–reagent mixture was measured at 520 nm using a microplate reader (SpectraMax M3, Molecular Devices). The % inhibition against ABTS radicals (% inhibition) was estimated using Equation (2) described above.
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
This experiment was modified from the related study [22]. The tested samples, which were 1.0 mg/mL of each of the B. hispida extracts, were used in this experiment. Briefly, 20 µL of the extract solution was added to the mixed solution, which was 0.3 M acetate buffer (pH 3.6) with 10 mM TPTZ in 40 mM of 37% w/v hydrochloric solution. The extract–reagent mixture was incubated under dark conditions at ambient temperature for 5 min and then analyzed using a microplate reader (SpectraMax M3, Molecular Devices) at 595 nm. FRAP values are expressed as mg ferrous sulfate (FeSO4) per gram extract and were calculated from a linear regression equation constructed from various concentrations of FeSO4 solutions (100 to 1000 µM).
2.5.4. Lipid Peroxidation Inhibition Assay
This investigation was based on the method in a related study [23]. The stock solution of B. hispida extracts was prepared at a concentration of 50.0 mg/mL in 50% DMSO. Then, 150 µL of extract solution was mixed with 100 µL of DI water, 350 µL of 20 mM phosphate buffer solution (PBS) (pH 7.0), 350 µL of 1.3% v/v linoleic acid in methanol and 50 µL of 25.14 mg/g 2,2′-azobis-(2-amidinopropane dihydrochloride) or (APPH) in 20 mM PBS (pH 7.0). The tested mixture was incubated at 50 °C for 4 h. Afterward, 5 µL of the mixture was added to a 96-well plate with 5 µL of 10% ammonium thiocyanate and 5 µL of 20 mM ferrous chloride, followed by adding 185 µL of 75% methanol. After 3 min, the absorbance of the mixed solution at 500 nm was determined using a Genios Pro microplate reader (Tecan, Crailsheim, Germany). The potential values expressed as % inhibition were calculated using Equation (2).
2.6. Anti-Aging Activities
2.6.1. Collagenase Inhibition Assay
The collagenase inhibition activity of B. hispida extracts was determined following the protocol of Thring [24] with some modification. The tested collagenase enzyme at a concentration of 2.0 mg/mL was prepared at 25 °C in PBS pH 7.5, containing 50 mM Tricine, 10 mM calcium chloride and 400 mM sodium chloride. As a substrate, 1.0 mM of N-[3- (2 furyl) acryloyl]-Leu-Gly-Pro-Ala, also known as FALGPA, in PBS pH 7.5 was used. B. hispida extracts (10 µL) at a concentration of 0.5 mg/mL were added to a 96-well plate, and then the tested collagen enzyme (10 µL) was added, followed by the substrate (10 µL). The reaction mixture was incubated for 30 min at 37 °C. After that, this mixture was analyzed using a microplate reader (SPECTROstar Nano, BMG Labtech, Aylesbury, Buckinghamshire, UK) at a wavelength of 340 nm. For comparison, 0.5 mg/mL of EGCG standard solution was used as a positive control. The results expressed as % collagenase enzyme inhibition were calculated according to the following equation:
where C is the control absorbance (DI water mixed with collagen enzyme and substrate), C0 is the blank control absorbance (DI water mixed with PBS pH 7.5 and collagen enzyme), Cs is the tested extract absorbance and Cb is the extract blank absorbance (extract mixed with PBS and substrate).
% Collagenase enzyme inhibition = [(C − C0) − (Cs − Cb)/(C − C0)] × 100
2.6.2. Hyaluronidase Inhibition Assay
This assay was performed to determine the potential of B. hispida extract against the hyaluronidase enzyme following the sigma protocol with slight modification [25]. The diluted hyaluronidase enzyme (2.0 mg/mL) was prepared at 25 °C in the enzymatic diluent, comprising a mixture of PBS pH 7.0, 77 mM sodium chloride and 0.01% BSA. Then, 0.03% hyaluronic acid mixed with PBS pH 5.3, namely PHS, was prepared and incubated at 80 °C. The B. hispida extract at a concentration of 0.5 mg/mL in DI water was used for analysis by mixing 50 µL of the B. hispida extract with 100 µL of diluted hyaluronidase enzyme solution and incubating the mixture at 37.5 °C for 10 min; then, 100 µL of 0.03% hyaluronic acid was added and continuously incubated at 37.5 °C for 45 min. After that, acetic albumin solution pH 3.75, containing 24 mM sodium acetate, 79 mM acetic acid and 0.1% BSA, was added and mixed at 25 °C. This mixture was subjected to analysis at 600 nm using a microplate reader (SPECTROstar Nano, BMG Labtech). Tannic acid, at the same concentration of the tested extract, was used as a positive control. The activity was shown as % hyaluronidase enzyme inhibition and calculated using the following equation:
where H is the control absorbance (tested mixture without the extract and using DI water as the tested sample), H0 is the blank control absorbance (DI water mixed with enzymatic diluent), Hs is the tested extract absorbance and Hb is the extract blank absorbance (extract mixed with PHS).
% Hyaluronidase enzyme inhibition = [(H − H0) − (Hs − Hb)/(H − Hb)] × 100
2.7. Chemical Marker Analysis by High-Performance Liquid Chromatography (HPLC)
Qualitative and quantitative analyses of the most promising B. hispida extract were performed using an HPLC system (Prominence LC2030C, Shimazu, Japan). A Knauer® vertex III reversed-phase HPLC column C18 (250 mm × 20 mm) (KNAUER Wissenschaftliche Geräte GmbH, Berlin, Germany) was used as the stationary phase. A gradient system was used as an analytical method with a detector at a wavelength of 360 nm. The mobile phase consisted of A (0.1% acetic acid in water) and B (acetonitrile) with a flow rate of 1.0 mL/min for 33 min. Gradient elution of the mobile phase was carried out as follows: 0.01 min, 95% A; 2 min, 95% A; 7 min, 80% A; 22 min, 70% A; 23 min, 95% A; and 33 min, 95% A. The analytical samples were dissolved in absolute ethanol and filtered through a 0.45 μm filter (Whatman, Marlborough, MA, USA) before analysis with an injection volume of 10 μL. B. hispida extract at 1.0 mg/mL was prepared for analysis. Rutin was used as a reference compound to analyze the active compounds in B. hispida extract. The retention time of rutin was approximately 17 min. The concentration of rutin in B. hispida extract was calculated from the peak area using a linear equation constructed from a standard curve of rutin (0.0 to 50 µg/mL).
2.8. Molecular Docking Simulation
The main ingredients of B. hispida peel, including ascorbic acid, quercetin and rutin, were submitted to molecular docking simulation. The 3D chemical structures of these compounds were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/ accessed on 12 June 2023). All structures were geometrically optimized using Gaussian09w [26] with the HF/6-31G(d,p) [27] basis set. The enzymes involved in the aging process such as collagenase (PDB ID: 1CGL) [28] and hyaluronidase (PDB ID: 2PE4) [29] were obtained from the RCSB Protein Data Bank (PDB) database (https://www.rcsb.org/ accessed on 12 June 2023). All co-crystallized ligands and water molecules were removed from the structures. Before molecular docking, the Gasteiger’s and Kollman potential charges were assigned to ligands and proteins, respectively, using AutoDockTools 1.5.7 [30]. Molecular docking was carried out using the AutoDock Vina [31] program. The grid center of each protein was set according to its active site coordinates. The compound with the highest binding affinity was chosen as the representative structure for further analysis. Hydrogen bonds and hydrophobic interactions were identified using LigandScout 4.4.8 [32] and Discovery Studio Visualizer 2021 [33]. The molecular visualization was performed using the UCSF ChimeraX [34] Program.
2.9. Cell Cytotoxicity Test
To investigate the skin toxicity of B. hispida extracts, human dermal fibroblast (HDF) cells, obtained from the American Type Culture Collection (ATCC), were employed in this experiment. The test was performed using the MTT assay that was described in a related study with modifications [35]. The HDF cells (1 × 104 cells/well) were cultured and treated in a 96-well plate with a medium culture containing 10% DMEM, 10% FBS and antibiotic solution (100 U/mL penicillin and 100 µg/mL streptomycin) and incubated at 37 °C in a 5% CO2 humidified atmosphere for 24 h. Then, the tested samples, B. hispida extracts at the concentration of 0.001 to 1.0 mg/mL, were added and continuously incubated in the same condition. After 24 h, the supernatant was removed, and 100 µL of 0.05 mg/mL MTT reagent was added to the well, followed by further incubation at 37 °C in a 5% CO2 humidified atmosphere for 6 h. The MTT reagent was then removed, and 100 µL of DMSO was added to dissolve the formazan crystals. After 15 min, the absorbance was measured at 560 nm using a microplate reader (Bio Tek Instruments, Winooski, VT, USA). The results were calculated and expressed as the percentage of cell viability using the following equation:
where A is the absorbance of the tested sample and A0 is the absorbance of the control, namely the tested well without B. hispida extract.
% Cell viability = (A/A0) × 100
2.10. In Vitro Irritation Test Using Hen’s Egg Chorioallantoic Membrane (HET-CAM) Assay
The irritation potential of B. hispida extracts was appraised using the HET-CAM assay according to a protocol described by Chaiyana [35]. This experiment serves as an alternative in vitro method for assessing skin irritation. For investigation using the HET-CAM assay, the placental membrane of a chicken embryo, providing a vascular network of capillaries, was used in the study. The fertilized hen eggs used in this study were obtained from the Faculty of Agriculture at Chiang Mai University, Thailand. Eggs aged between seven and nine days were employed. These eggs underwent incubation at a temperature of 37.5 ± 0.5 °C with a relative humidity of 62.5 ± 7.5%. The shell above the air cavitation of the egg was carefully removed using a rotating cutting blade attached to a Marathon champion 3 micromotor (Saeyang, Seoul, South Korea). A normal saline solution was then applied to an inner membrane that came into direct contact with the chorioallantoic membrane (CAM), followed by incubation under the same conditions as described above for 15 min. After this incubation period, the inner membrane was meticulously removed using forceps. Then, 30 µL of B. hispida extract at a concentration of 10 mg/mL was dropped onto the prepared CAM. In this study, the positive and negative controls were represented by 30 µL of 1% (w/v) sodium lauryl sulfate (SLS) and 30 µL of normal saline solution, respectively.
The assessment of irritation effects involved continuous observation using a stereomicroscope (Olympus, Tokyo, Japan) over a 5 min (300 s) period and expressed the short-term irritation effect. After 60 min of testing, observations were once again conducted to determine long-term irritation. The observed signs of irritation included vascular hemorrhage, vascular lysis and vascular irritation. The time of the first appearance of each of these irritating signs was recorded in seconds and was subsequently used to calculate an irritation score (IS) according to the following equation:
where Ht is the time of the initial appearance of vascular hemorrhage, Lt is the time of the initial appearance of vascular lysis and Ct is the time point of the initial appearance of vascular coagulation. Based on the calculated IS, IS = 0.0 to 0.9 was classified as no irritation, IS = 1.0 to 4.9 was classified as mild irritation, IS = 5.0 to 8.9 was classified as moderate irritation and IS = 9.0 to 21.0 was classified as severe irritation.
IS = [((301 − Ht)/300) × 5] + [((301 − Lt)/300) × 7] + [((301 − Ct)/300) × 9]
2.11. Statistical Analysis
All measurements were performed independently in triplicate. The results are expressed as mean ± S.D. Statistical analysis was conducted using SPSS Software, Version 17.0 for Windows, and differences between groups were determined using one-way analysis of variance (ANOVA) followed by Tukey’s test. The differences were considered significant at a p-value ≤ 0.05.
3. Results
3.1. Plant Extraction
From the extraction using various solvents, the obtained extracts from B. hispida peels and the percentage yields of each extract are shown in Table 1. The highest extract yield was found for BW, followed by B95 and B50. The extracts appeared viscous and light brown and had specific odors.

Table 1.
Percentage yields of B. hispida peel extracts from various solvents.
3.2. Total Flavonoid and Total Phenolic Contents
The values of total flavonoid content (TFC) and total phenolic content (TPC) in B. hispida extracts are shown in Table 2. Flavonoid contents in different extracts were calculated from the regression equation of the quercetin standard curve (y = 0.3502x + 0.0565, r2 = 0.993) and expressed as mg QE/g extract. Phenolic contents in different extracts were calculated from the regression equation of the gallic acid standard curve (y = 8.2221x + 0.006, r2 = 0.998) and expressed as mg GAE/g extract.

Table 2.
Total phenolic and total flavonoid contents of B. hispida peel extracts.
The highest TFC was found in B95 with a value of 212.88 ± 4.73 mg QE/g extract and was followed by the TFCs of BW and B50, consecutively. However, the highest TPC was found in B50 with the value of 140.43 ± 0.77 mg GAE/g extract and was followed by the TPCs of B95 and BW, consecutively. These results might be related to the extracted solvents; some flavonoid and phenolic compounds are well extracted in semi-polar solvents such as 95% ethanol, which was presented in the study as having a high value of TFC and TPC in B95.
3.3. Antioxidant Activities
The antioxidative activities of B. hispida peel extracts were investigated using various methods to evaluate various mechanisms of action. The results of B. hispida extracts from these procedures are shown in Table 3.

Table 3.
Antioxidant activities with various assays.
DPPH and ABTS assays were performed to determine the radical scavenging activity of the extracts. From the DPPH assay, the results were expressed as the concentration of extract that could be active against radicals where the % inhibition is equal to 50 (IC50). The IC50 values of extracts in the DPPH assay were calculated from the regression equations of each extract, namely y = 0.0128x + 12.75, r2 = 0.986 for B95; y = 0.0308x + 2.6124, r2 = 0.9895 for B50; and y = 1.3828x + 16.589, r2 = 0.925 for BW. The standard compounds that were considered in comparison were ascorbic acid and Trolox. The IC50 values of these standard compounds were also calculated from the regression equations of each compound, namely y = 6.1021 + 20.349, r2 = 0.936 for ascorbic acid and y = 3.031 + 14.631, r2 = 0.989 for Trolox. The results demonstrated that B50 exhibited the significantly highest scavenging activity with an IC50 value of 1.73 ± 0.13 mg/mL, followed by B95 with an IC50 of 2.91 ± 0.07 mg/mL and BW with an IC50 value of 23.63 ± 0.60 mg/mL (p < 0.05).
The ABTS assay results were expressed as IC50 values, the same as the results obtained from the DPPH assay. IC50 values of the B. hispida extracts were calculated using the equations of linear regression of each extract. The obtained equations used to calculate IC50 values of B95, B50 and BW were y = 0.227x + 9.7036 (r2 = 0.9907), y = 0.2387x + 10.612 (r2 = 0.9708) and y = 0.1387x + 10.685 (r2 = 0.9279), respectively. Ascorbic acid and Trolox were used as the standard compounds, and IC50 values were also calculated from the regression equations. The calculated equations of ascorbic acid and Trolox were y = 7.2955x + 6.7331 (r2 = 0.9841) and y = 3.9231x + 2.1272 (r2 = 0.9803), respectively. The obtained IC50 values from ABTS assays showed that B95 and B50 provided the highest ABTS scavenging activity with IC50 values of 0.17± 3.13 mg/mL and 0.16 ± 3.95 mg/mL, followed by BW with an IC50 value of 0.27 ± 12.09 mg/mL (p < 0.05).
The FRAP assay was used to determine the ability of radical reduction. The results were expressed as the mg FeSO4/g extract which was calculated from the regression equation of FeSO4 (y = 6.724x + 0.1818, r2 = 0.995). A high FRAP value indicated a high radical reduction activity. B. hispida extracts (B95, B50 and BW) at a concentration of 1.0 mg/mL were used in this experiment. The BW showed the highest reducing property with a FRAP value of 17.33 ± 0.19 mg FeSO4/g extract. Nevertheless, B95 showed less activity in comparison with others (p < 0.05).
The lipid peroxidation inhibition assay was performed to determine the antioxidant ability against oxidative degradation of lipids by peroxide radicals. The results were expressed as % inhibition obtained from B95, B50 and BW extracts including the standard compounds (ascorbic acid and Trolox) at the same concentration of 0.1 mg/mL. B95 possessed high activity with the % inhibition of 53.42 ± 0.02, against peroxide radicals, followed by B50 and BW, consecutively.
Ascorbic acid and Trolox, the compounds that were used for comparing the antioxidant activity in all tested assays, showed the significantly strongest activities when compared with all B. hispida peel extracts (p < 0.05). Interestingly, the overall antioxidative assays illustrated that B95 obtained from B. hispida peel has potential antioxidant activity related to all antioxidant mechanisms.
3.4. Anti-Aging Activities
To determine anti-aging activities of B. hispida peel extracts, the ability to inhibit collagenase and hyaluronidase enzymes was investigated. The results were expressed as % collagenase enzyme inhibition and % hyaluronidase enzyme inhibition. The obtained inhibitory effects of B. hispida peel extracts are illustrated in Figure 2. Among the samples tested against the collagenase enzyme (Figure 2A), B95 showed an inhibition effect that does not significantly differ (p > 0.05) from EGCG, a standard compound, at the same concentration of 0.5 mg/mL. B95 showed an inhibition of the collagenase enzyme with the value of 41.68 ± 0.92%. Furthermore, the % collagenase inhibition exhibited by B50 and BW did not demonstrate statistically significant differences when compared with that of B95 (p > 0.05). Concerning the activity against the hyaluronidase enzyme, the obtained results are demonstrated in Figure 2B. B95, with a value of 29.17 ± 0.66%, showed obviously higher hyaluronidase inhibition activity than other extracts (p < 0.05). Nevertheless, B50 and BW demonstrated hyaluronidase inhibition of the same potential (p > 0.05). However, all B. hispida peel extracts possessed an inhibitory effect against the hyaluronidase enzyme that was less than that of tannic acid, the standard compound in this experiment (p < 0.05).
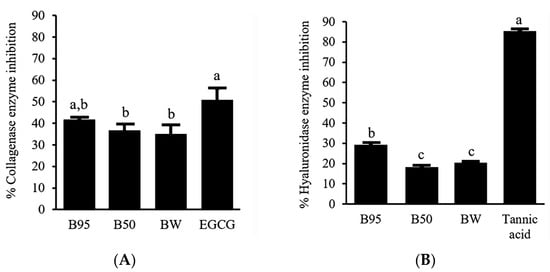
Figure 2.
Potential of B. hispida extracts against the collagenase enzyme compared with EGCG (A) and the hyaluronidase enzyme compared with tannic acid (B). Different letters indicate significance level at p < 0.05.
3.5. Chemical Marker Analysis by High-Performance Liquid Chromatography (HPLC)
The results obtained from antioxidant and anti-aging assays showed that B95 exhibited the most potent effects among all the tested extracts of B. hispida. Consequently, B95 was selected to determine its chemical marker using HPLC. Rutin, a reference flavonoid compound used to identify the active compound in B95, was also included in the analysis for comparison. The HPLC chromatograms of B95 and rutin are presented in Figure 3. Several components were detected in B95, as indicated by the observed HPLC chromatograms; one of these components was rutin, which exhibited a retention time of approximately 17 min. The quantification of rutin in B95 was calculated using the linear equation of rutin, namely y = 8914.9x + 793.63 (r2 = 0.9999). The results demonstrated that 1 mg of B95 contained 4.81 ± 0.03 µg of rutin.
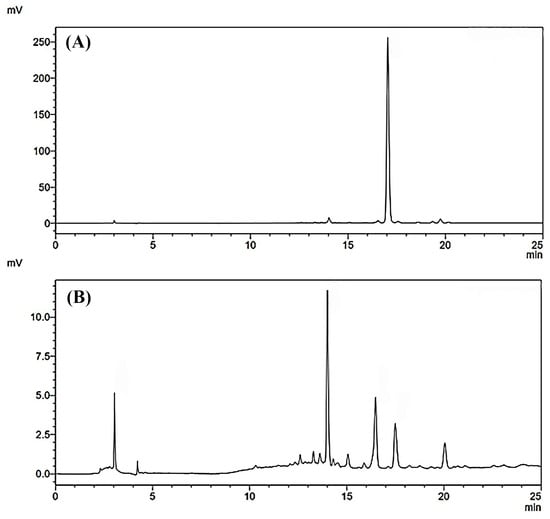
Figure 3.
HPLC chromatograms of rutin at the concentration of 50 µg/mL (A) and the most promising B. hispida extract, B95 (B) at a concentration of 1.0 mg/mL, analysis at 360 nm.
3.6. Molecular Docking Simulation
The main ingredients of B. hispida peel extract, including ascorbic acid, quercetin and rutin, were submitted to molecular docking simulation. The molecular docking results indicated that quercetin exhibited the highest binding affinity against the collagenase enzyme (z8.9 kcal/mol), followed by rutin (−7.9 kcal/mol) and ascorbic acid (−6.3 kcal/mol), consecutively. The molecular recognition of these compounds against collagenase is depicted in Figure 4. All compounds fit well in the binding pocket of collagenase, as shown in Figure 4A. Ascorbic acid interacts with key amino acids, including Ala182, Arg214, His218, Glu219 and Pro238, via hydrogen bonding and also forms a metal interaction with Zn301 (Figure 4B). Quercetin forms hydrogen bonds with Arg214, Glu219 and Tyr237 and interacts with Leu181 and Val215 via hydrophobic interactions (Figure 4C). Rutin exhibits interactions with amino acids such as Asn180, Arg214, Tyr237 and Tyr240 through hydrogen bonds and forms hydrophobic interactions with Val215 (Figure 4D).
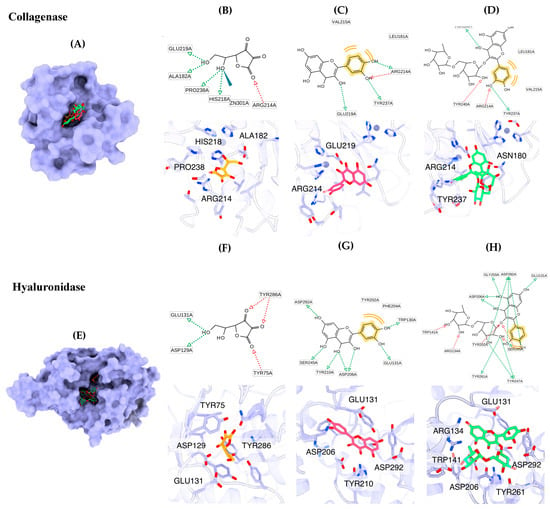
Figure 4.
Binding modes of representative phytochemicals binding to collagenase (A) and hyaluronidase (E). The 2D and 3D interactions of ascorbic acid (B), quercetin (C) and rutin (D) complexed with collagenase, along with the 2D and 3D binding modes of ascorbic acid (F), quercetin (G) and rutin (H) in the binding site of hyaluronidase. The pharmacophore features include hydrogen bond acceptor (red arrow), hydrogen bond donor (green arrow) and hydrophobic property (yellow).
The molecular docking results for the hyaluronidase enzyme showed that rutin possessed the highest docking affinity (−9.8 kcal/mol), followed by quercetin (−8.1 kcal/mol) and ascorbic acid (−5.6 kcal/mol). The graphical illustration indicates that the molecular binding patterns of the compounds differ in terms of their positions within the binding pocket (Figure 4E). Ascorbic acid fits well in the narrow pocket to form hydrogen bonds with residues including Tyr75, Asp129, Glu131 and Tyr286 (Figure 4F). Quercetin interacts with Trp130, Glu131, Asp206, Tyr210, Ser245 and Asp292 via hydrogen bonds and forms hydrophobic interactions with Tyr202 and Phe204 (Figure 4G). Rutin is located on the same site as quercetin. The core structure of rutin forms hydrogen bonds with Glu131, Gly203, Tyr202, Asp206, Ser245 and Asp292, and the glycoside substituent forms hydrogen bonds with Arg134, Trp141, Tyr247 and Tyr261 (Figure 3).
3.7. Cell Cytotoxicity Test
A cytotoxicity test of B. hispida extracts (B95, B50 and BW) on HAF cells was performed using the MTT assay. The tested samples showing cell viability of above 80% were regarded as nontoxic to the cells. The obtained results after 24 h of exposure of the tested samples to the HAF cells are shown in Figure 5. All B. hispida extracts were not toxic to HAF cells at concentrations of 0.001 to 0.01 mg/mL, with cell viability of more than 90%. In addition, B50 and BW at a concentration of 1.0 mg/mL also exhibited nontoxicity to HAF cells with a % cell viability of 99.74 ± 8.63, and 92.35 ± 17.30, respectively. However, B95 at a concentration of 1.0 mg/mL showed slight toxicity to HAF cells with a % cell viability of 77.58 ± 2.82%.
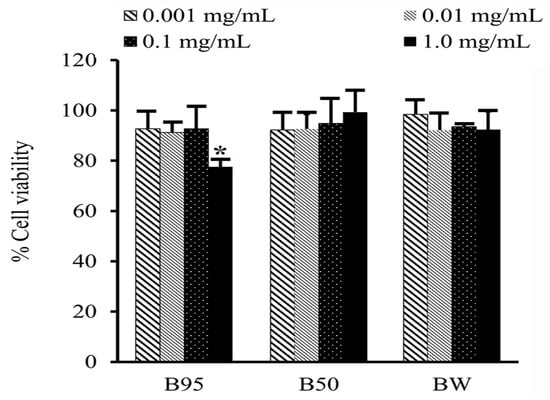
Figure 5.
Percentage cell viability of each B. hispida extract at concentrations from 0.001 to 1.0 mg/mL. The values are presented mean ± S.D. (n = 3). Asterisk (*) represents a different value at p < 0.05.
3.8. In Vitro Irritation Test Using the HET-CAM Assay
To further investigate the effective activities of B. hispida peel extracts in vivo and in a clinical study, an in vitro irritation test using the HET-CAM assay was then employed. The irritation induced by B. hispida peel extracts (B95, B50 and BW) was evaluated and compared with that of the negative control, 0.9% w/v NaCl, and the positive control, 1% (w/v) SLS. The obtained results as irritation scores are shown in Table 4, and the observations of the alterations under a stereomicroscope are shown in Figure 6. None of the B. hispida peel extracts at a concentration of 10 mg/mL induced considerable alterations, namely vascular hemorrhage, vascular lysis and vascular irritation, in short-term testing (5 min) and long-term testing (60 min), similar to the result obtained from 0.9% w/v NaCl. However, 1% (w/v) SLS, the positive control, exhibited signs of severe irritation in 5 min of testing with vascular hemorrhages and vascular lysis.

Table 4.
Irritation score of B. hispida peel extracts, 0.9% w/v NaCl and 1% w/v SLS.
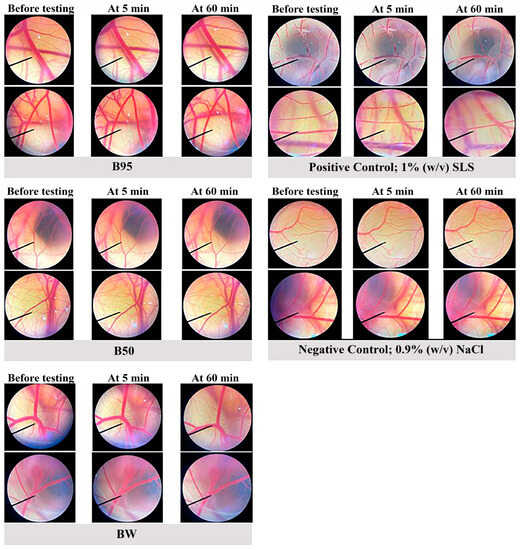
Figure 6.
The stereomicroscope images of CAM vasculature before testing, at 5 min after treatment and at 60 min after treatment with 10 mg/mL of each extract (B95, B50 and BW), 0.9% w/v NaCl and 1% w/v SLS.
4. Discussion
It has been realized that the potential of antioxidants against free radical damage to the skin is a significant property that can decelerate skin aging and skin damage related to transduction pathways and epigenetic changes caused by oxidative stress [36]. Moreover, countless studies have explored the antioxidant activity from novel natural sources, including plants, animals, insects, algae and waste products, to protect the skin from damage caused by free radicals [37,38]. Phenolic compounds are powerful antioxidant agents with wide mechanisms of action including the capacity to scavenge free radicals such as hydroxyl radical (•OH) and superoxide anion (O2−•) and chelate metal ions (Fe2+, Fe3+, Cu2+ and Cu+) which react to hydrogen peroxide (H2O2). Highly reactive ▪OH is formed by the dismutation of O2−• by SOD, enhancing the activity or expression of intracellular antioxidant enzymes [39]. Flavonoids are a group of natural substances with variable phenolic structures. Flavones and catechins have been reported to be the most powerful flavonoids for protecting the body against ROS [40].
B. hispida is a potentially edible plant that has been used for remedies and protection against many diseases related to the phytochemicals in the used part [11]. The obtained extracts of B. hispida in this study showed a high yield in BW and B95, which could indicate that the chemical constituents in B. hispida peel are well solubilized in water and 95% ethanol. Related studies have reported the presence of phenols, alkaloids, saponins, steroids, carbohydrates and flavonoids in chloroform, alcoholic and aqueous extracts obtained from B. hispida peel [10,13]. This was consistent with the results of this study that found flavonoids in B. hispida peel extracts, especially in B95 (95% v/v ethanolic extract). Moreover, phenolic compounds were found in all extracts. The phytochemical profile of phenolic compounds in B. hispida has been reported by Islam et al., and the results revealed the presence of many bioactive chemicals, such as quercetin, rutin, astilbin, catechin, naringenin and hispidulin [11]. The antioxidant activities of B. hispida extracts were investigated with standard procedures to evaluate the different mechanisms of action. DPPH and ABTS methods have commonly been used to evaluate the antioxidant activity of compounds that act as scavengers of O2−• and •OH radicals [41]. The FRAP assay is a method that possesses the power of antioxidation by reducing ferric iron (Fe3+) to ferrous iron (Fe2+) [42]. In this study, B95 obviously possessed antioxidant activities with free radical scavenging mechanisms via DPPH and ABTS assays. Moreover, it could inhibit lipid peroxidation by stopping lipid chain reactions. Further, BW possessed good potential to reduce Fe3+ via the FRAP assay. These results are consistent with related studies reporting that the methanolic extracts of B. hispida peel provided antioxidant activity against free radicals when determined using DPPH and FRAP methods [10,43]. In addition, the high flavonoid and phenolic contents found in B95 possessed good potential antioxidant activities in all assays, referring to the synergistic action of both compounds [44].
Collagenase is a key enzyme that acts by degrading collagen in the extracellular matrix, promoting premature skin aging. Hyaluronidase is also a key enzyme that degrades hyaluronic acid, a critical component of the extracellular matrix in the skin for maintaining the normal hydration of the skin [45,46]. The inhibition of both enzymes provides the potential to prevent skin aging and its signs. Our study found that the strongest anticollagenase activity was provided by B95 (72.70 ± 1.24%), and the strongest antihyaluronidase activity was also exhibited by B95 (74.88 ± 4.84%). The results presumably recommend that flavonoid compounds that were found to possess the highest B95 levels may be the active compounds for these activities. In a related study, rutin and quercetin, which belong to the group of flavonoid compounds, were detected in the fruit of B. hispida, indicating good anti-aging activities [16,47]. In addition, the literature extensively supports the efficacy of flavonols as inhibitors of collagenase [48]. Quercetin and kaempferol exhibited a more significant inhibitory effect compared with flavones, isoflavones and flavanones, with the latter demonstrating a very negligible impact. The arrangement of hydroxyl groups in the B-ring of the flavonoid structure may play a crucial role in determining the inhibitory effect on enzyme activity [49]. Similarly, the antioxidative and anti-enzymatic activities of Thai plants were found to be influenced by their phenolic and flavonoid contents [6]. Thus, both compounds were investigated in a molecular docking simulation against collagenase and hyaluronidase enzymes.
Various phytochemicals have been identified in B. hispida extracts, primarily in the fruit portion [16]. However, limited information is available regarding the composition of phytochemicals obtained from B. hispida peel. In this study, the most promising B. hispida extract, namely B95, was used to determine the phytochemical active compounds through HPLC analysis. Rutin, a flavonoid that has been detected in various parts of B. hispida, was employed as a reference compound for the HPLC analysis of B95. This analysis revealed the presence of numerous compounds, which manifested as multiple HPLC peaks observed in the chromatogram. Rutin is one of the compounds identified in B95. This result aligns with related studies that have also detected rutin in other parts of B. hispida [50,51]. However, it is necessary to identify and characterize the other compounds in B95. This will provide a comprehensive understanding of B95′s composition and support the feasibility of its mechanism of action and efficacy in various applications. Conducting a broader analysis to identify and understand the various compounds in B95 can significantly enhance the ability to assess its potential benefits and applications. Molecular docking contributes to a better understanding of the molecular basis underlying the interaction between promising compounds from B. hispida and the collagenase and hyaluronidase enzymes, which are the key enzymes related to anti-aging properties. Ascorbic acid, quercetin and rutin were identified as the main bioactive compounds in the B. hispida peel extract and were further investigated for their molecular recognition within the binding sites of these enzymes. All phytochemicals are capable of binding to the collagenase active site cleft through hydrogen and hydrophobic interactions. The strength of the binding of these phytochemicals correlates to the number of hydrogen bonds and steric effects. Quercetin and rutin form interactions with Arg214 at the bottom of the S1′ pocket, which is suitable in size for an aromatic ring [52]. Moreover, the hydrogen bond with Glu219 also supports the binding affinity of quercetin within the binding pocket [28]. In hyaluronidase, rutin and quercetin form hydrogen bonds with Asp129 and Glu131, which are essential amino acids for the catalytic site of hyaluronidase [53,54]. The interaction of quercetin and rutin with Tyr202 has been observed, further supporting the binding strength [55]. This interaction information can provide insights into the potential use of the phytochemicals from B. hispida peel extract as anti-aging agents that inhibit the activity of collagenase and hyaluronidase, which are enzymes associated with the skin aging processes of collagen degradation and hyaluronic acid breakdown.
Regarding safety, the cytotoxicity and the irritation of B. hispida peel extracts need to be investigated before application and further development for food, cosmetics or cosmeceutical products. The cytotoxicity of all B. hispida peel extracts on human dermal fibroblast cells was evaluated using the MTT assay, which is a standard protocol for assessing in vitro cytotoxicity. This assay is based on the reduction of the yellow tetrazolium salt of MTT to purple formazan crystals by metabolically active cells. Thus, the high detection of purple formazan crystals in the experiment indicates a high density of living cells. A test sample showing cell viability above 80% is considered as safe or possessing non-cytotoxicity [56,57]. In the present study, B. hispida peel extracts (B95, B50 and BW) demonstrated cell viability of more than 80% after being exposed to the cells for 24 h. These results were consistent with one related study reporting that B. hispida extract is not toxic to normal cells [58]. Therefore, it could be concluded that B. hispida peel extracts are not harmful to dermal cells and can be safely applied on the skin.
As the irritation test, the HET-CAM assay was used to assess the irritation properties of B. hispida peel extracts. This protocol was developed by Lüepke [59] and is employed as an alternative to the Draize test for screening the potential of irritation and anti-irritation effect of cosmetic formulations and ingredients [60]. The HET-CAM test was compared with results obtained from the irritation test with human skin, and the results led to the conclusion that significant comparable efficacy patterns were found [61]. Thus, the HET-CAM assay has been used as a preliminary assessment of irritation before clinical trials. The results obtained in our study revealed that none of the B. hispida peel extracts showed any signs of irritation on the chorioallantoic membrane. The signs of irritation include hemorrhage, coagulation and vascular lysis. These results revealed that B. hispida peel extracts are suitable for further developing topical products or skin care products with safety similar to that of other extracts that have been reported to have no irritation potential after being tested using the HET-CAM assay [7,31,62]
5. Conclusions
In the present study, we have demonstrated that B. hispida peel extracts received from postconsumer waste, containing flavonoid and phenolic compounds, constitute a potential natural source. All the extracts possessed significant antioxidant and anti-aging activities, especially activities against enzymes that cause skin aging. The extract obtained from 95% ethanol as the extraction solvent showed the most promising antioxidant activities involving the mechanisms of radical scavenging of DPPH and ABTS radicals, reduced free radicals in the FRAP assay and inhibited peroxide radicals. Regarding the investigation of anti-aging activities, B. hispida peel extract, obtained from 95% ethanol, possesses good ability to inhibit the collagenase enzyme at the same potential as epigallocatechin gallate (standard control). Additionally, this extract also exhibits an inhibitory effect on the hyaluronidase enzyme. Based on the molecular docking results, the interaction of B. hispida peel extracts with collagenase and hyaluronidase suggests that they have the potential to be used as anti-aging agents. The in vitro toxicity against human dermal fibroblast cells and the in vitro irritation assay using hen’s egg chorioallantoic membrane demonstrate that all B. hispida peel extracts possess no toxicity and produce no irritating effect. In conclusion, these valuable results provide scientific evidence to support further studies concerning the physiochemical properties of B. hispida extracts and their active compounds. This research serves as a preliminary study via in vitro investigation; the mechanisms of in vivo antioxidant and in vivo anti-aging effects could be clarified in further investigations. Additionally, developing products containing B. hispida extracts for use in food, cosmetics or cosmeceuticals, as well as clinical studies, could also be investigated. Expanding upon this research can open opportunities for a wide range of practical applications.
Author Contributions
Conceptualization, K.K. and M.S.; Method, K.K., M.S., W.C. and S.C.; Software, S.C.; Formal Analysis, P.P., S.C., W.C. and K.K.; Resources, W.C. and K.K.; Data Curation, K.K., S.P., W.P. (Weeraya Preedalikit), W.P. (Worrapan Poomanee), P.P., W.C. and S.C.; Writing—Original Draft Preparation, P.P., K.K. and S.C.; Writing—Review and Editing, K.K., P.P., W.P. (Weeraya Preedalikit), W.P. (Worrapan Poomanee), M.S. and W.C.; Supervision, K.K.; Project Administration, K.K.; Funding Acquisition, W.C., K.K., M.S. and W.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was supported by the Fundamental Fund 2023, Chiang Mai University (funding number: 66A104000013).
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Chiang Mai University for supporting funding and are grateful to the Faculty of Pharmacy, Chiang Mai University, for providing us with the necessary resources. The authors would like to thank Thomas McManamon, Faculty of Pharmacy, Chiang Mai University, for English editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of Antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.T.; Jang, Y.; Myung, S.W.; Lee, J.M.; Kim, H.S.; Ko, S.C.; Lee, S.H. In Vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- Baier Leach, J.; Bivens, K.A.; Patrick, C.W.J.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef]
- Chatatikun, M.; Chiabchalard, A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement. Altern. Med. 2017, 17, 487. [Google Scholar] [CrossRef]
- Chaiyana, W.; Chansakaow, S.; Intasai, N.; Kiattisin, K.; Lee, K.-H.; Lin, W.-C.; Lue, S.-C.; Leelapornpisid, P. Chemical constituents, antioxidant, anti-MMPs, and anti-hyaluronidase activities of Thunbergia laurifolia Lindl. leaf extracts for skin aging and skin damage prevention. Molecules 2020, 25, 1923. [Google Scholar] [CrossRef]
- Poomanee, W.; Chaiyana, W.; Intasai, N. Leelapornpisid, Biological activities and charaterization of the pod extracts from Sompoi (Acacia concinna Linn.) grown in northern Thailand. Int. J. Pharm. Pharm. Sci. 2015, 7, 237–241. [Google Scholar]
- Rayees, B.; Dorcus, M.; Chitra, S. Nutritional composition and oil fatty acids of Indian winter melon Benincasa hispida (Thunb.) seeds. Int. Food Res. J. 2013, 20, 1151–1155. [Google Scholar]
- Rana, S.; Suttee, A. Phytochemical investigation and evaluation of free radical scavenging potential of Benincasa hispida peel extracts. Int. J. Curr. Pharm. Rev. Res. 2012, 3, 43–46. [Google Scholar]
- Islam, M.T.; Quispe, C.; El-Kersh, D.M.; Shill, M.C.; Bhardwaj, K.; Bhardwaj, P.; Sharifi-Rad, J.; Martorell, M.; Hossain, R.; Al-Harrasi, A.; et al. A Literature-based update on Benincasa hispida (Thunb.) Cogn.: Traditional uses, nutraceutical, and phytopharmacological profiles. Oxid. Med. Cell. Longev. 2021, 2021, 6349041. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Huang, J.-J.; Tso, T.; Ying Chieh, T.; Chang, C.-K. Antioxidant and angiotension-converting enzyme inhibition capacities of various parts of Benincasa hispida (Wax Gourd). Nahrung 2004, 48, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Doharey, V.; Kumar, M.; Kumar Upadhyay, S.; Singh, R.; Kumari, B. Pharmacognostical, physicochemical and pharmaceutical paradigm of ash gourd, Benincasa hispida (Thunb.) Fruit. Plant. Arch. 2021, 21, 249–252. [Google Scholar] [CrossRef]
- Fatariah, Z.; Zulkhairuazha, T.Y.T.; Rosli, W.W.I. Quantitative HPLC analysis of gallic acid in Benincasa hispida prepared with different extraction techniques. Sains Malays. 2014, 43, 1181–1187. [Google Scholar]
- Fatariah, Z.; Zulkhairuazha, T.Y.T.; Rosli, W.W.I. Ascorbic acid quantification in Benincasa hispida fruit extracted using different solvents. Int. Food Res. J. 2015, 22, 208–212. [Google Scholar]
- Busuioc, A.C.; Botezatu, A.V.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R.M. Comparative study of the chemical compositions and antioxidant activities of fresh juices from Romanian Cucurbitaceae varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef]
- Phumat, P.; Khongkhunthian, S.; Wanachantararak, P.; Okonogi, S. Comparative inhibitory effects of 4-allylpyrocatechol isolated from Piper betle on Streptococcus intermedius, Streptococcus mutans, and Candida albicans. Arch. Oral Biol. 2020, 113, 104690. [Google Scholar] [CrossRef]
- Phosri, S.; Kiattisin, K.; Intharuksa, A.; Janon, R.; Na Nongkhai, T.; Theansungnoen, T. Anti-aging, anti-acne, and cytotoxic activities of Houttuynia cordata extracts and phytochemicals analysis by LC-MS/MS. Cosmetics 2022, 9, 136. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Brem, B.; Seger, C.; Pacher, T.; Hartl, M.; Hadacek, F.; Hofer, O.; Vajrodaya, S.; Greger, H. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry 2004, 65, 2719–2729. [Google Scholar] [CrossRef]
- Poomanee, W.; Wattananapakasem, I.; Panjan, W.; Kiattisin, K. Optimizing anthocyanins extraction and the effect of cold plasma treatment on the anti-aging potential of purple glutinous rice (Oryza sativa L.). Cereal. Chem. 2021, 98, 571–582. [Google Scholar] [CrossRef]
- Payne, A.C.; Mazzer, A.; Clarkson, G.J.J.; Taylor, G. Antioxidant assays—Consistent findings from FRAP and ORAC reveal a negative impact of organic cultivation on antioxidant potential in Spinach but not watercress or rocket leaves. Food Sci. Nutr. 2013, 1, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Kiattisin, K.; Nitthikan, N.; Poomanee, W.; Leelapornpisid, P.; Viernstein, H.; Mueller, M. Anti-Inflammatory, Anti-inflammatory, antioxidant activities and safety of Coffea arabica leaf extract for alternative cosmetic ingredient. Chiang Mai J. Sci. 2019, 46, 284–294. [Google Scholar]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Rani, A.; Hamzah, R.; Arumwardana, S.; Afifah, E.; Kusuma, H.; Rihibiha, D.; Nufus, H.; Amalia, A. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. Nat. Prod. Sci. 2017, 23, 192–200. [Google Scholar] [CrossRef]
- Frisch, A. Gaussian 09W Reference; Gaussian, Inc.: Wallingford, CT, USA, 2009; 25p. [Google Scholar]
- Hoher, W.; Ditchfield, R.; Stewart, R.F.; Pople, J.A. Self-consistent molecular orbital methods. Iv. use of Gaussian expansions of slater-type orbitals. Extension to second-row molecules. J. Chem. Phys. 1970, 52, 2769–2773. [Google Scholar]
- Lovejoy, B.; Cleasby, A.; Hassell, A.M.; Longley, K.; Luther, M.A.; Weigl, D.; McGeehan, G.; McElroy, A.B.; Drewry, D.; Lambert, M.H.; et al. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science 1994, 263, 375–377. [Google Scholar] [CrossRef]
- Chao, K.L.; Muthukumar, L.; Herzberg, O. Structure of Human Hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry 2007, 46, 6911–6920. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Wolber, G.; Langer, T. LigandScout: 3-D Pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA Dassault Systèmes. Discovery Studio Visualizer 2021; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Jiamphun, S.; Bezuidenhout, S.; Krueathanasing, N.; Thammasorn, P.; Jittasai, P.; Tanakitvanicharoen, S.; Tima, S.; Anuchapreeda, S. Enhanced cosmeceutical potentials of the oil from Gryllus bimaculatus de Geer by Nanoemulsions. Int. J. Nanomed. 2023, 18, 2955–2972. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A Systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef] [PubMed]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant compounds from microalgae: A review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under In Vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Tabrizi, L.; Dao, D.Q.; Vu, T.A. Experimental and theoretical evaluation on the antioxidant activity of a copper(Ii) complex based on lidocaine and ibuprofen amide-phenanthroline agents. RSC Adv. 2019, 9, 3320–3335. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for Testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Abdullah, N.; Wan Saidatul, S.W.K.; Samicho, Z.; Zulkifli, K.S.; Aziman, N. Study on antioxidant capacity and phenolic content of various parts of wax gourd (Benincasa hispida). World Appl. Sci. J. 2012, 19, 1051–1056. [Google Scholar]
- Hubner, A.; Sobreira, F.; Vetore Neto, A.; Pinto, C.A.S.d.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The synergistic behavior of antioxidant phenolic compounds obtained from Winemaking Waste’s valorization, increased the efficacy of a sunscreen system. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Doshi, G.; Nalawade, V.; Mukadam, A.; Chaskar, P.; Zine, S.; Somani, R.; Une, H. Elucidation of flavonoids from Carissa congesta, Polyalthia longifolia, and Benincasa hispida plant extracts by hyphenated technique of liquid chromatography-mass spectroscopy. Pharmacogn. Res. 2016, 8, 281–286. [Google Scholar] [CrossRef]
- Bo, Y.S.; Hyun, P.K. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharm. Res. 2005, 28, 1152–1155. [Google Scholar]
- Sim, G.-S.; Lee, B.-C.; Cho, H.S.; Lee, J.W.; Kim, J.-H.; Lee, D.-H.; Kim, J.-H.; Pyo, H.-B.; Moon, D.C.; Oh, K.-W.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef]
- Shakya, A.; Gogoi, N.; Chaudhary, S.K.; Bhat, H.R.; Ghosh, S.K. Development and validation of a high-performance thin-layer chromatography method for the quantification of rutin in the fruit pulp of Benincasa hispida (Thunb.) Cogniaux. JPC—J. Planar. Chromat. 2019, 32, 371–377. [Google Scholar] [CrossRef]
- Doshi, G.M.; Une, H.D. Quantification of quercetin and rutin from Benincasa hispida seeds and Carissa congesta roots by high-performance thin layer chromatography and high-performance liquid chromatography. Pharmacogn. Res. 2016, 8, 37–42. [Google Scholar] [CrossRef]
- Sung, E.; Kim, S.; Shin, W. Binary image representation of a ligand binding site: Its application to efficient sampling of a conformational ensemble. BMC Bioinform. 2010, 11, 256. [Google Scholar] [CrossRef]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Arming, S.; Strobl, B.; Wechselberger, C.; Kreil, G. In Vitro Mutagenesis of PH-20 hyaluronidase from human sperm. Eur. J. Biochem. 1997, 247, 810–814. [Google Scholar] [CrossRef]
- Zhang, L.; Bharadwaj, A.G.; Casper, A.; Barkley, J.; Barycki, J.J.; Simpson, M.A. Hyaluronidase activity of human hyal1 requires active site acidic and tyrosine residues. J. Biol. Chem. 2009, 284, 9433–9442. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S. In Vitro cytotoxicity and cell viability assays: Principles, advantages, and disadvantages. In Genotoxicity; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell vability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Han, X.; Men, X.; Lee, S.J.; Oh, G.; Choi, Y.E.; Yang, J.M.; Cho, J.H.; Lee, O.H. Benincasa hispida extract prevents ovariectomy-induced osteoporosis in female ICR mice. Appl. Sci. 2023, 13, 832. [Google Scholar] [CrossRef]
- Budai, P.; Lehel, J.; Tavaszi, J.; Kormos, É. HET-CAM test for determining the possible eye irritancy of pesticides. Acta. Vet. Hung. 2010, 58, 369–377. [Google Scholar] [CrossRef]
- Rivero, M.N.; Lenze, M.; Izaguirre, M.; Pérez Damonte, S.H.; Aguilar, A.; Wikinski, S.; Gutiérrez, M.L. Comparison between HET-CAM protocols and a product use clinical study for eye irritation evaluation of personal care products including cosmetics according to their surfactant composition. Food. Chem. Toxicol. 2021, 153, 112229. [Google Scholar] [CrossRef]
- Wilson, T.D.; Steck, W.F. A Modified HET–CAM assay approach to the assessment of anti-irritant properties of plant extracts. Food Chem. Toxicol. 2000, 38, 867–872. [Google Scholar] [CrossRef]
- Hong-in, P.; Chaiyana, W. Potential cosmeceutical lamellar liquid crystals containing black longan (Dimocarpus longan Lour.) seed extract for MMP-1 and hyaluronidase inhibition. Sci. Rep. 2022, 12, 7683. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).