Reviewing the Source, Physiological Characteristics, and Aroma Production Mechanisms of Aroma-Producing Yeasts
Abstract
:1. Introduction
2. Source of Aroma-Producing Yeast
3. Biological Properties of Aroma-Producing Yeast
3.1. Pichia
3.2. Hansenula
3.3. Zygosaccharomyces
3.4. Candida
3.5. Other Yeasts
4. Physiological Characteristics of Aroma-Producing Yeast
4.1. Alcohol Resistance Characteristics
4.2. Acid-Resistant Characteristics
4.3. Salt Tolerance
5. Interaction between Aroma-Producing Yeast and Other Microorganisms
6. Mechanisms of Yeast Aroma
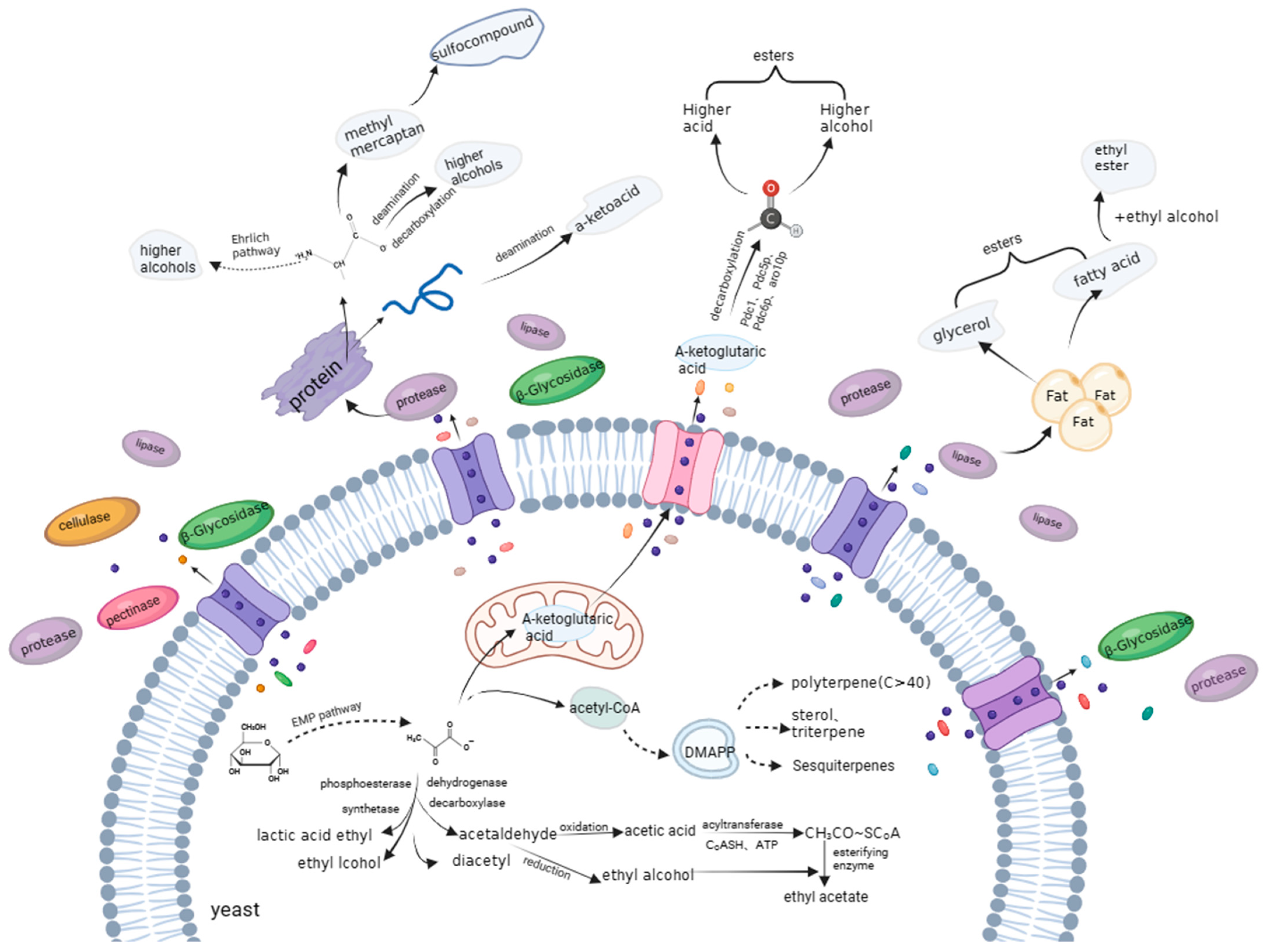
6.1. Formation of Esters
6.2. Formation of Terpenoids
6.3. Formation of Alcohols
7. Artificial Modification of Aroma-Producing Yeast Based on Synthetic Biology
8. Application of Aroma-Producing Yeast
8.1. The Application of Essence
8.2. Application in the Food Industry
8.3. Application in Cosmetics
9. Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non- Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, J.H.; Bai, D.H.; Ahn, B. Feasibility of brewing Makgeolli using Pichia anomala Y197-13, a non-Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2012, 22, 1749–1757. [Google Scholar] [PubMed]
- Cai, X.; Wu, L.; Chen, M.; Chen, J.; Wang, K.; Yang, L.; Luo, A. Screening and identification of aroma-producing yeast in distiller’s grains of Moutai-flavor Baijiu. China Brew. 2017, 36, 42–47. [Google Scholar]
- Wu, Y.; Chen, X.E.; Fang, X.; Ji, L.; Tian, F.; Yu, H.; Chen, Y. Isolation and Identification of Aroma-producing Yeast from Mackerel Fermentation Broth and Its Fermentation Characteristics. J. Aquat. Food Prod. Technol. 2021, 30, 1264–1280. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef]
- Feng, C.; Liu, Y.; Wang, Z.; Wang, C. Application and prospect of aroma-producing yeast. J. Green Sci. Technol. 2016, 190–191. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Chmielewska, J.; Nofer, J. Effect of Different Yeast Strains and Temperature of Fermentation on Basic Enological Parameters, Polyphenols and Volatile Compounds of Aurore White Wine. Foods 2019, 8, 599. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S.; van der Westhuizen, T.J.; Augustyn, O.P.H. Yeast Biodiversity in Vineyards and Wineries and Its Importance to the South African Wine Industry. A Review. S. Afr. J. Enol. Vitic. 1999, 20, 61–70. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, W.; Wang, X.; Kong, W.; Huang, W.; Zhan, J.; Xia, G.; You, Y. Indigenous yeast can increase the phenolic acid and volatile ester compounds in Petit Manseng wine. Front. Nutr. 2022, 9, 1031594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, J.; Wu, H.; Deng, M.; Gong, Y.; Zhong, Z.; Gong, J. Screening of yeast in citrus peels and their volatile aromatic components analysis. J. Food Saf. Qual. 2014, 5, 4050–4055. [Google Scholar]
- Lu, Y.; Liu, Z.; Zhang, S.; Niu, J.; Hu, R.; Tian, L.; Yang, Y. Screening, Identification and Fermentation Properties of Aroma Producing Yeast for Kiwi Fruit. J. Henan Agric. Sci. 2021, 50, 166–173. [Google Scholar]
- Tang, H.; Wang, H.; Wu, H.; Deng, J.; Liu, Y.; Wang, Y. Screening, Identification and Characterization of Aroma-Producing and Salt-Tolerant Yeast Strains from Pickles from South Sichuan, China. Food Sci. 2020, 41, 150–157. [Google Scholar]
- Van Mullem, J.J.; Zhang, J.; Dias, D.R.; Schwan, R.F. Using wild yeasts to modulate the aroma profile of low-alcoholic meads. Braz. J. Microbiol. 2022, 53, 2173–2184. [Google Scholar] [CrossRef]
- Sottil, C.; Salor-Torregrosa, J.M.; Moreno-Garcia, J.; Peinado, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Using Torulaspora delbrueckii, Saccharomyces cerevisiae and Saccharomyces bayanus wine yeasts as starter cultures for fermentation and quality improvement of mead. Eur. Food Res. Technol. 2019, 245, 2705–2714. [Google Scholar] [CrossRef]
- Barry, J.P.; Metz, M.S.; Hughey, J.; Quirk, A.; Bochman, M.L. Two novel strains of Torulaspora delbrueckii isolated from the honey bee microbiome and their use in honey fermentation. Fermentation 2018, 4, 22. [Google Scholar] [CrossRef]
- Chen, K.; Liu, C.; Wang, Y.; Wang, Z.; Li, F.; Ma, L.; Li, J. Predominance of indigenous non-Saccharomyces yeasts in the traditional fermentation of greengage wine and their significant contribution to the evolution of terpenes and ethyl esters. Food Res. Int. 2021, 143, 110253. [Google Scholar] [CrossRef]
- Han, X.; Qing, X.; Yang, S.; Li, R.; Zhan, J.; You, Y.; Huang, W. Study on the diversity of non-Saccharomyces yeasts in Chinese wine regions and their potential in improving wine aroma by β-glucosidase activity analyses. Food Chem. 2021, 360, 129886. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The sensory quality improvement of citrus wine through co-fermentations with selected non-Saccharomyces yeast strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, M.; Xie, N.; Huang, M.; Feng, Y. Community structure of yeast in fermented soy sauce and screening of functional yeast with potential to enhance the soy sauce flavor. Int. J. Food Microbiol. 2022, 370, 109652. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Song, K.; Cui, M. Performance of non-Saccharomyces yeasts isolated from Jiaozi in dough fermentation and steamed bread making. LWT 2019, 111, 46–54. [Google Scholar] [CrossRef]
- Fu, Z.; Sun, B.; Li, X.; Fan, G.; Teng, C.; Alaa, A.; Jia, Y. Isolation and characterization of a high ethyl acetate-producing yeast from Laobaigan Daqu and its fermentation conditions for producing high-quality Baijiu. Biotechnol. Biotechnol. Equip. 2018, 32, 1218–1227. [Google Scholar] [CrossRef]
- Xu, A. Characterization of Fungal Community in Fuzhuan Brick-Tea and Its Intestinal Regulation Functions Investigation. Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2011. [Google Scholar]
- Li, R.-R.; Xu, M.; Zheng, J.; Liu, Y.-J.; Sun, C.-H.; Wang, H.; Guo, X.-W.; Xiao, D.-G.; Wu, X.-L.; Chen, Y.-F. Application potential of baijiu non-Saccharomyces yeast in winemaking through sequential fermentation with Saccharomyces cerevisiae. Front. Microbiol. 2022, 13, 902579. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, K.; Liu, C.; Wang, Y.; Chen, T.; Yan, G.; Li, J. Non-Saccharomyces yeasts highly contribute to characterisation of flavour profiles in greengage fermentation. Food Res. Int. 2022, 157, 111391. [Google Scholar] [CrossRef]
- Gao, J.; Xu, J.; Zuo, Y.; Ye, C.; Jiang, L.; Feng, L.; Huang, L.; Xu, Z.; Lian, J. Synthetic biology toolkit for marker-less integration of multigene pathways into Pichia pastoris via CRISPR/Cas9. ACS Synth. Biol. 2022, 11, 623–633. [Google Scholar] [CrossRef]
- Vogl, T.; Sturmberger, L.; Kickenweiz, T.; Wasmayer, R.; Schmid, C.; Hatzl, A.-M.; Gerstmann, M.A.; Pitzer, J.; Wagner, M.; Thallinger, G.G.; et al. A toolbox of diverse promoters related to methanol utilization: Functionally verified parts for heterologous pathway expression in Pichia pastoris. ACS Synth. Biol. 2016, 5, 172–186. [Google Scholar] [CrossRef]
- Türkanoğlu Özçelik, A.; Yılmaz, S.; Inan, M. Pichia pastoris promoters. Recomb. Protein Prod. Yeas 2019, 1923, 97–112. [Google Scholar]
- Wang, X.; Sun, W.; Zhang, Z.; Pang, W.; Shi, H.; Tan, J. Function Research Progress of P. pastoris. Mod. Food 2021, 1–5. [Google Scholar] [CrossRef]
- Kuang, Y.; Liao, L.; Shen, F.; Li, Q.; Zhang, L.; Xiao, B.; Luo, Q. Optimization of aroma-producing conditions and analysis of fermentation fluid volatile components of Pichiakluyveri S23. Food Sci. Technol. 2019, 44, 23–29. [Google Scholar]
- Liu, Y.; Wei, W.; Su, Y.; Wu, L.; Tong, L.; Zhang, W. Breeding and Characteristic Analysis of Aroma-Producing Yeast for Mulberry Wine. Food Sci. Technol. 2022, 47, 1–7. [Google Scholar]
- Wang, G.; Liu, L.; Zheng, Y.; Ma, G.; Zhang, Z.; Wang, Y.; Yang, X. Isolation of a yeast with high-yield for β-phenylethanol and its aroma characteristics. Food Ferment. Ind. 2022, 48, 68–74. [Google Scholar]
- Cheng, F.J.; Lu, S.F.; Hu, D.X. Advances in the Expression of Foreign genes in Hansenula polymorpha. Chin. J. Biotechnol. 2001, 17, 246–249. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, H.; Wang, T.; Feng, G.; Qian, W.; Cai, C.; Mao, P. Differentiating transcriptomic patterns and functional analysis of Hansenula anomala during cultivation. Food Ferment. Ind. 2019, 45, 1–6. [Google Scholar]
- Izquierdo Canas, P.M.; García Romero, E.; Huertas Nebreda, B.; Gómez Alonso, S.; Gómez-Alonso, S.; Collins, V.J.; Corona, G. Enhancement of flavour properties in wines using sequential inoculations of non. VITIS-J. Grapevine Res. 2011, 50, 177–182. [Google Scholar]
- Ai, F.; Hu, H.; Peng, L. Screening and growth characteristics of aroma-producing yeast in citrus juice. China Brew. 2010, 67–70. [Google Scholar] [CrossRef]
- Solieri, L.; Cassanelli, S.; Giudici, P. A new putative Zygosaccharomyces yeast species isolated from traditional balsamic vinegar. Yeast 2007, 24, 403–417. [Google Scholar] [CrossRef]
- Aidoo, K.E.; Rob Nout, M.J.; Sarkar, P.K. Occurrence and function of yeasts in Asian indigenous fermented foods. FEMS Yeast Res. 2006, 6, 30–39. [Google Scholar] [CrossRef]
- Fan, Z.; Li, H.; Zhang, Q.; Chen, G.; Li, J.; Deng, W.; Chen, X. Screening of Aroma-Producing Yeast from Pixian Broadbean Paste and Optimization of Its Growth Condition. J. Food Sci. Biotechnol. 2020, 39, 76–83. [Google Scholar]
- Zhao, L. Isolation of flavor producing yeast and analysis on aroma characteristics in the natural soy sauce mash. Food Mach. 2012, 28, 11–14. [Google Scholar]
- Jiang, X.; Peng, D.; Zhang, W.; Duan, M.; Ruan, Z.; Huang, S.; Zhou, S.; Fang, Q. Effect of aroma-producing yeasts in high-salt liquid-state fermentation soy sauce and the biosynthesis pathways of the dominant esters. Food Chem. 2021, 344, 128681. [Google Scholar] [CrossRef]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Characterization of different non-Saccharomyces yeasts via mono-fermentation to produce polyphenol-enriched and fragrant kiwi wine. Food Microbiol. 2022, 103, 103867. [Google Scholar] [CrossRef]
- Zuehlke, J.M.; Petrova, B.; Edwards, C.G. Advances in the control of wine spoilage by Zygosaccharomyces and Dekkera/Brettanomyces. Annu. Rev. Food Sci. Technol. 2013, 4, 57–78. [Google Scholar] [CrossRef]
- Escott, C.; Manuel del Fresno, J.; Loira, I.; Morata, A.; Antonio Suarez-Lepe, J. Zygosaccharomyces rouxii: Control Strategies and Applications in Food and Winemaking. Fermentation 2018, 4, 69. [Google Scholar] [CrossRef]
- Solieri, L. The revenge of Zygosaccharomyces yeasts in food biotechnology and applied microbiology. World J. Microbiol. Biotechnol. 2021, 37, 96. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Torchio, F.; Rolle, L.; Gerbi, V.; Cocolin, L. Exploitation of the non-Saccharomyces yeast Starmerella bacillaris (synonym Candida zemplinina) in wine fermentation: Physiological and molecular characterizations. Int. J. Food Microbiol. 2015, 199, 33–40. [Google Scholar] [CrossRef]
- Tofalo, R.; Patrignani, F.; Lanciotti, R.; Perpetuini, G.; Schirone, M.; Di Gianvito, P.; Pizzoni, D.; Arfelli, G.; Suzzi, G. Aroma profile of Montepulciano d’Abruzzo wine fermented by single and co-culture starters of autochthonous saccharomyces and non-Saccharomyces yeasts. Front. Microbiol. 2016, 7, 610. [Google Scholar] [CrossRef]
- Nisiotou, A.; Sgouros, G.; Mallouchos, A.; Nisiotis, C.-S.; Michaelidis, C.; Tassou, C.; Banilas, G. The use of indigenous Saccharomyces cerevisiae and Starmerella bacillaris strains as a tool to create chemical complexity in local wines. Food Res. Int. 2018, 111, 498–508. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, H. Study on the Effects of Aroma-producing Yeast on the Flavor of Yellow Rice Wine by Biological Polypeptide Fermentation. Liquor-Mak. Sci. 2014, 53–55. [Google Scholar] [CrossRef]
- Peña, R.; Ganga, M.A. Novel antimicrobial peptides produced by Candida intermedia LAMAP1790 active against the wine-spoilage yeast Brettanomyces bruxellensis. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2019, 112, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Yuzuki, M.; Ito, K.; Shiga, K.; Bamba, T.; Fukusaki, E. Influence of yeast and lactic acid bacterium on the constituent profile of soy sauce during fermentation. J. Biosci. Bioeng. 2017, 123, 203–208. [Google Scholar] [CrossRef]
- van der Sluis, C.; Tramper, J.; Wijffels, R.H. Enhancing and accelerating flavour formation by salt-tolerant yeasts in Japanese soy-sauce processes. Trends Food Sci. Technol. 2001, 12, 322–327. [Google Scholar] [CrossRef]
- Chen, B.; Lu, F.; Wang, F.; Yin, L. Effect of salt-tolerant yeast on the flavor of soy sauce and its research progress. China Brew. 2010, 1–3. [Google Scholar] [CrossRef]
- Li, Y.-C.; Rao, J.-W.; Meng, F.-B.; Wang, Z.-W.; Liu, D.-Y.; Yu, H. Combination of mutagenesis and adaptive evolution to engineer salt-tolerant and aroma-producing yeast for soy sauce fermentation. J. Sci. Food Agric. 2021, 101, 4288–4297. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, H.; Liu, Z. Screening of an aroma-producing yeast and application in fermented Mantou. China Brew. 2020, 39, 69–73. [Google Scholar]
- Ma, M.; Liu, Z.L. Mechanisms of ethanol tolerance in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 87, 829–845. [Google Scholar] [CrossRef]
- Javier De la Torre-Gonzalez, F.; Alberto Narvaez-Zapata, J.; Eric Lopez-y-Lopez, V.; Larralde-Corona, C.P. Ethanol tolerance is decreased by fructose in Saccharomyces and non-Saccharomyces yeasts. LWT-Food Sci. Technol. 2016, 67, 1–7. [Google Scholar] [CrossRef]
- Archana, K.M.; Ravi, R.; Anu-Appaiah, K.A. Correlation between ethanol stress and cellular fatty acid composition of alcohol producing non-Saccharomyces in comparison with Saccharomyces cerevisiae by multivariate techniques. J. Food Sci. Technol.-Mysore 2015, 52, 6770–6776. [Google Scholar] [CrossRef]
- Yamaoka, C.; Kurita, O.; Kubo, T. Improved ethanol tolerance of Saccharomyces cerevisiae in mixed cultures with Kluyveromyces lactis on high-sugar fermentation. Microbiol. Res. 2014, 169, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [PubMed]
- Mira, N.P.; Becker, J.D.; Sa-Correia, I. Genomic expression program involving the Haa1p-Regulon in Saccharomyces cerevisiae response to acetic acid. OMICS J. Integr. Biol. 2010, 14, 587–601. [Google Scholar] [CrossRef]
- Palma, M.; Dias, P.J.; Roque, F.D.C.; Luzia, L.; Guerreiro, J.F.; Sa-Correia, I. The Zygosaccharomyces bailii transcription factor Haa1 is required for acetic acid and copper stress responses suggesting subfunctionalization of the ancestral bifunctional protein Haa1/Cup2. BMC Genomics 2017, 18, 75. [Google Scholar] [CrossRef]
- Vazquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Beltran, G.; Jesus Torija, M. The role of the membrane lipid composition in the oxidative stress tolerance of different wine yeasts. Food Microbiol. 2019, 78, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Brewster, J.L.; de Valoir, T.; Dwyer, N.D.; Winter, E.; Gustin, M.C. An osmosensing signal transduction pathway in yeast. Science 1993, 259, 1760–1763. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.H.; Chudek, J.A.; Foster, R.; Gadd, G.M. Osmotic significance of glycerol accumulation in exponentially growing yeasts. Appl. Environ. Microbiol. 1987, 53, 2119–2123. [Google Scholar] [CrossRef]
- Iwaki, T.; Kurono, S.; Yokose, Y.; Kubota, K.; Tamai, Y.; Watanabe, Y. Cloning of glycerol-3-phosphate dehydrogenase genes (ZrGPD1 and ZrGPD2) and glycerol dehydrogenase genes (ZrGCY1 and ZrGCY2) from the salt-tolerant yeast Zygosaccharomyces rouxii. Yeast Chichester Engl. 2001, 18, 737–744. [Google Scholar] [CrossRef]
- Wang, D.; Hao, Z.; Zhao, J.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Comparative physiological and transcriptomic analyses reveal salt tolerance mechanisms of Zygosaccharomyces rouxii. Process Biochem. 2019, 82, 59–67. [Google Scholar] [CrossRef]
- Vejarano, R.; Gil-Calderón, A. Commercially Available Non-Saccharomyces Yeasts for Winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility, and Safety. Fermentation 2021, 7, 171. [Google Scholar] [CrossRef]
- Jin, H.; Wang, F.; Lu, Y.; Zhang, S.; Niu, J.; Tang, C.; Zuo, W.; Yang, Y. Optimization of fermentation conditions for mixed bacteria of ki-wifruit wine and its antioxidant activity. Food Ferment. Ind. 2022, 48, 177–185. [Google Scholar]
- Wang, L.; Xu, Y. The Influence of Mixed Culture on Volatiles in Apple Wine and Fermentation Efficiency. Food Sci. 2005, 151–155. [Google Scholar] [CrossRef]
- Tan, F.; Wang, B.; Hu, P.; Liu, C.; Zhang, M. Application and challenge of non-Saccharomyces cerevisiae yeast in the mixed fermentation of fruit wine. Food Ferment. Ind. 2020, 46, 282–286. [Google Scholar]
- Zhang, W.; Weng, P.; Wu, Z. Fermentation Efficiency and Flavor Characteristics of Bayberry Wine with Mixed Starter Culture of Issatchenkio orientalis and Saccharomyces cerevisiae. Food Sci. 2019, 40, 144–151. [Google Scholar]
- Li, W.; Fan, G.; Fu, Z.; Wang, W.; Xu, Y.; Teng, C.; Zhang, C.; Yang, R.; Sun, B.; Li, X. Effects of fortification of Daqu with various yeasts on microbial community structure and flavor metabolism. Food Res. Int. Ott. Ont 2020, 129, 108837. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Genoves, S.; Valles, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef]
- Curiel, J.A.; Morales, P.; Gonzalez, R.; Tronchoni, J. Different non-Saccharomyces yeast species stimulate nutrient consumption in S. cerevisiae mixed cultures. Front. Microbiol. 2017, 8, 2121. [Google Scholar] [CrossRef]
- Lin, M.M.-H.; Boss, P.K.; Walker, M.E.; Sumby, K.M.; Grbin, P.R.; Jiranek, V. Evaluation of indigenous non-Saccharomyces yeasts isolated from a South Australian vineyard for their potential as wine starter cultures. Int. J. Food Microbiol. 2020, 312, 108373. [Google Scholar] [CrossRef]
- Xin, C.; Xu, L.; Dong, Q.; Cai, P.; Yu, W.; Yu, P.; Zhao, J. Effect of Brewing Microorganisms on Liquor Quality and Style. Liquor Mak. 2016, 43, 38–47. [Google Scholar]
- Escudero, A.; Campo, E.; Farina, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
- Boscaino, F.; Ionata, E.; La Cara, F.; Guerriero, S.; Marcolongo, L.; Sorrentino, A. Impact of Saccharomyces cerevisiae and Metschnikowia fructicola autochthonous mixed starter on Aglianico wine volatile compounds. J. Food Sci. Technol.-Mysore 2019, 56, 4982–4991. [Google Scholar] [CrossRef]
- Luzzini, G.; Slaghenaufi, D.; Ugliano, M. Volatile compounds in monovarietal wines of two amarone della valpolicella terroirs: Chemical and sensory impact of grape variety and origin, yeast strain and spontaneous fermentation. Foods 2021, 10, 2474. [Google Scholar] [CrossRef]
- Fernandez, M.; Ubeda, J.F.; Briones, A.I. Typing of non-Saccharomyces yeasts with enzymatic activities of interest in wine-making. Int. J. Food Microbiol. 2000, 59, 29–36. [Google Scholar] [CrossRef]
- Wang, R.; Wang, B. Research into the Regularity of Ester Formation in Producing Heavy Fragrant Type Liquor. J. Shandong Inst. Light Ind. 1994, 8, 57–61. [Google Scholar]
- Ma, L.; Ding, P.; Yang, G.; He, G. Advances on the plant terpenoid isoprenoid biosynthetic pathway and its key enzymes. Biotechnol. Bull. 2006, 1, 22–30. [Google Scholar]
- Bisotto, A.; Julien, A.; Rigou, P.; Schneider, R.; Salmon, J.M. Evaluation of the inherent capacity of commercial yeast strains to release glycosidic aroma precursors from Muscat grape must. Aust. J. Grape Wine Res. 2015, 21, 194–199. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Q. Advances in Understanding the Formation Mechanism of Terpenoids during Winemaking and Factors Influencing It. Food Sci. 2021, 42, 249–258. [Google Scholar]
- Mas, A.; Manuel Guillamon, J.; Jesus Torija, M.; Beltran, G.; Cerezo, A.B.; Troncoso, A.M.; Carmen Garcia-Parrilla, M. Bioactive compounds derived from the yeast metabolism of aromatic amino acids during alcoholic fermentation. Biomed Res. Int. 2014, 2014, 898045. [Google Scholar] [CrossRef]
- Höhne, M.; Kabisch, J. Brewing painkillers: A yeast cell factory for the production of opioids from sugar. Angew. Chem. Int. Ed. 2016, 55, 1248–1250. [Google Scholar] [CrossRef]
- Galanie, S.; Thodey, K.; Trenchard, I.J.; Interrante, M.F.; Smolke, C.D. Complete biosynthesis of opioids in yeast. Science 2015, 349, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, S. Bioproduction of monoterpene indole alkaloids in a single cell factory. Eng. Microbiol. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Madhavan, A.; Jose, A.A.; Binod, P.; Sindhu, R.; Sukumaran, R.K.; Pandey, A.; Castro, G.E. Synthetic biology and metabolic engineering approaches and its impact on non-conventional yeast and biofuel production. Front. Energy Res. 2017, 5, 8. [Google Scholar] [CrossRef]
- Awan, A.R.; Blount, B.A.; Bell, D.J.; Shaw, W.M.; Ho, J.C.H.; McKiernan, R.M.; Ellis, T. Biosynthesis of the antibiotic nonribosomal peptide penicillin in baker’s yeast. Nat. Commun. 2017, 8, 15202. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.P.; Etschmann, M.M.W.; Schrader, J.; de Billerbeck, G.M. Cell factory applications of the yeast Kluyveromyces marxianus for the biotechnological production of natural flavour and fragrance molecules. Yeast 2015, 32, 3–16. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Kildegaard, K.R.; Cernuda Pastor, M.; Jayachandran, S.; Kristensen, M.; Borodina, I. Yarrowia lipolytica Strains Engineered for the Production of Terpenoids. Front. Bioeng. Biotechnol. 2020, 8, 945. [Google Scholar] [CrossRef]
- Agrawal, A.; Yang, Z.; Blenner, M. Engineering Y. lipolytica for the biosynthesis of geraniol. bioRxiv 2023. [Google Scholar] [CrossRef]
- Promdonkoy, P.; Sornlek, W.; Preechakul, T.; Tanapongpipat, S.; Runguphan, W. Metabolic Engineering of Saccharomyces cerevisiae for Production of Fragrant Terpenoids from Agarwood and Sandalwood. Fermentation 2022, 8, 429. [Google Scholar] [CrossRef]
- Li, R.; Wang, K.; Wang, D.; Xu, L.; Shi, Y.; Dai, Z.; Zhang, X. Production of plant volatile terpenoids (rose oil) by yeast cell factories. Green Chem. 2021, 23, 5088–5096. [Google Scholar] [CrossRef]
- Schempp, F.M.; Drummond, L.; Buchhaupt, M.; Schrader, J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J. Agric. Food Chem. 2018, 66, 2247–2258. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Cho, B.-R.; Hahn, J.-S. Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnol. Bioeng. 2014, 111, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Beekwilder, J.; van Rossum, H.M.; Koopman, F.; Sonntag, F.; Buchhaupt, M.; Schrader, J.; Hall, R.D.; Bosch, D.; Pronk, J.T.; van Maris, A.J.A.; et al. Polycistronic expression of a β-carotene biosynthetic pathway in Saccharomyces cerevisiae coupled to β-ionone production. J. Biotechnol. 2014, 192, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; An, T.; Li, T.; Zhu, J.; Gao, K.; Sun, Z.; Xu, W.; Lin, P.; Zi, J. Reconstruction of the biosynthetic pathway of santalols under control of the Gal regulatory system in yeast. ACS Synth. Biol. 2020, 9, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.H.; Møller, B.L.; Kock, G.R.; Bünner, C.M.; Kristensen, C.; Jensen, O.R.; Okkels, F.T.; Olsen, C.E.; Motawia, M.S.; Hansen, J. De Novo Biosynthesis of Vanillin in Fission Yeast (Schizosaccharomyces pombe) and Baker’s Yeast (Saccharomyces cerevisiae). Appl. Environ. Microbiol. 2009, 75, 2765–2774. [Google Scholar] [CrossRef]
- Wriessnegger, T.; Augustin, P.; Engleder, M.; Leitner, E.; Müller, M.; Kaluzna, I.; Schürmann, M.; Mink, D.; Zellnig, G.; Schwab, H.; et al. Production of the sesquiterpenoid (+)-nootkatone by metabolic engineering of Pichia pastoris. Metab. Eng. 2014, 24, 18–29. [Google Scholar] [CrossRef]
- Chen, H.; Chen, W.; Mei, J. Producing of nature flavors and fragrances by bioconversion. Sci. Technol. Food Ind. 2011, 32, 317–320. [Google Scholar]
- Caputi, L.; Aprea, E. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat. Food Nutr. Agric. 2011, 3, 9–16. [Google Scholar] [CrossRef]
- Behrendorff, J.B.; Vickers, C.E.; Chrysanthopoulos, P.; Nielsen, L.K. 2,2-Diphenyl-1-picrylhydrazyl as a screening tool for recombinant monoterpene biosynthesis. Microb. Cell Factories 2013, 12, 76. [Google Scholar] [CrossRef]
- Berger, R.G. Biotechnology of flavours—The next generation. Biotechnol. Lett. 2009, 31, 1651–1659. [Google Scholar] [CrossRef]
- Hua, D.; Xu, P. Recent advances in biotechnological production of 2-phenylethanol. Biotechnol. Adv. 2011, 29, 654–660. [Google Scholar] [CrossRef]
- Etschmann, M.M.W.; Sell, D.; Schrader, J. Production of 2-phenylethanol and 2-phenylethylacetate from L-phenylalanine by coupling whole-cell biocatalysis with organophilic pervaporation. Biotechnol. Bioeng. 2005, 92, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Guerreiro, C.; Belo, I. Generation of flavors and fragrances through biotransformation and de novo synthesis. Food Bioprocess Technol. 2018, 11, 2217–2228. [Google Scholar] [CrossRef]
- Li, X.; Luo, C. Industrialized Production of Beer Yeast Extractive. Liquor Mak. Sci. Technol. 2002, 72–73. [Google Scholar]
- Cano-García, L.; Belloch, C.; Flores, M. Impact of Debaryomyces hansenii strains inoculation on the quality of slow dry-cured fermented sausages. Meat Sci. 2014, 96, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Mi, R.; Chen, X.; Xiong, S.; Qi, B.; Li, J.; Qiao, X.; Chen, W.; Qu, C.; Wang, S. Predominant yeasts in Chinese Dong fermented pork (Nanx Wudl) and their aroma-producing properties in fermented sausage condition. Food Sci. Hum. Wellness 2021, 10, 231–240. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Alvarez, J.P.; Neto, D.P.d.C.; Soccol, V.T.; Tanobe, V.O.A.; Rogez, H.; Góes-Neto, A.; Soccol, C.R. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT 2017, 84, 290–297. [Google Scholar] [CrossRef]
- Yan, S.; Xiangsong, C.; Xiang, X. Improvement of the aroma of lily rice wine by using aroma-producing yeast strain Wickerhamomyces anomalus HN006. AMB Express 2019, 9, 89. [Google Scholar] [CrossRef]
- Winters, M.; Panayotides, D.; Bayrak, M.; Remont, G.; Viejo, C.G.; Liu, D.; Le, B.; Liu, Y.; Luo, J.; Zhang, P.; et al. Defined co-cultures of yeast and bacteria modify the aroma, crumb and sensory properties of bread. J. Appl. Microbiol. 2019, 127, 778–793. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.; Xi, J.; Jin, Y.; Chen, Y.; Guo, L.; Jin, Z.; Xu, X. Improving bread aroma using low-temperature sourdough fermentation. Food Biosci. 2020, 37, 100704. [Google Scholar] [CrossRef]
- Dhurat, R.; Sharma, A.; Surve, R.; McCoy, J.; Kovacevic, M.; Goren, A.; Tan, Y.; Zou, Y.; Goldust, M.; Situm, M.; et al. Novel yeast extract is superior to colloidal oatmeal in providing rapid itch relief. J. Cosmet. Dermatol. 2021, 20, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, S.; Wada, S.; Yamashita, M.; Morita, M.; Aoi, W.; Naito, Y.; Higashi, A. Torula yeast (Candida utilis)-derived glucosylceramide contributes to dermal elasticity in vitro. J. Food Biochem. 2019, 43, e12847. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, E.-J.; Lee, K.-E.; Nho, Y.-H.; Ryu, J.; Kim, S.Y.; Yoo, J.K.; Kang, S.; Seo, S.W. Lipid extract derived from newly isolated Rhodotorula toruloides LAB-07 for cosmetic applications. Comput. Struct. Biotechnol. J. 2023, 21, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Production of mannosylerythritol lipids and their application in cosmetics. Appl. Microbiol. Biotechnol. 2013, 97, 4691–4700. [Google Scholar] [CrossRef]
- Santamaría, P.; González-Arenzana, L.; Garijo, P.; Gutiérrez, A.R.; López, R. Nitrogen sources added to must: Effect on the fermentations and on the Tempranillo red wine quality. Fermentation 2020, 6, 79. [Google Scholar] [CrossRef]
- Prusova, B.; Humaj, J.; Sochor, J.; Baron, M. Formation, losses, preservation and recovery of aroma compounds in the winemaking process. Fermentation 2022, 8, 93. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, F.; Wang, W.; Liu, Y.; Wang, J.; Sun, J.; Mu, J.; Gao, Z. Effects of spontaneous fermentation on the microorganisms diversity and volatile compounds during “Marselan” from grape to wine. Lwt-Food Sci. Technol. 2020, 134, 110193. [Google Scholar] [CrossRef]
- Peng, L.; Liu, G.; Fei, Y.; Yu, J.; Liu, R.; Bai, W.; Wang, L.; Jia, A. Research progress of ester-producing yeast and its key enzymes for esters synthesis. Food Ferment. Ind. 2020, 46, 275–282. [Google Scholar]
| Yeast | Source | Flavoring Substance | Aroma Characteristics | Main Applications | Literature |
|---|---|---|---|---|---|
| Pichia jadinii, Torulaspora delbrueckii, and Kluyveromyces lactis | Agricultural Microbial Culture Collection (CCMA) | Phenethyl acetate, etc. | Honey aroma | honey wine | Van [17] |
| Torulaspora delbrueckii, Saccharomyces cerevisiae and Saccharomyces bayanus | the Department of Microbiology collection; Grape spontaneous fermentation juice | 2-phenylethanol, diethyl succinate, 2,3-butanediol, etc. | Rose fragrance | Sottil [18] | |
| T.delbrueckii YH178 and YH179 | Honeybee guts | ----- | Sweet and grassy fragrance | Barry [19] | |
| Pichia, Candida, and Issatchenkia | Indigenous yeast community | Terpenes and ethyl esters | Flowery and fruity aromas | plum wine | Chen [20] |
| Candida glabrata | Wine-producing area | Ethanol, isoamyl alcohol, ethyl nonanoic linalool, β-damascenone, terpene compounds, as well as phenyl acetate, ethyl benzoate, etc. | Rose aroma, tropical fruit aroma | wine | Han [21] |
| Hanseniaspora opuntiae, Hansenula uvarum, and Hanseniaspora opuntiae | Citrus wine and citrus orchard | Phenylethyl alcohol, 1-pentanol, ethyl acetate, isoamyl acetate, and phenethyl acetate; ethyl hexanoate, ethyl octanoate, and ethyl decanoate | Honey, rose, taste, pineapple, pear, and floral scent | orange wine | Hu [22] |
| W. versatilis, C. sorbosivorans, and S. etchellsii | Sauce residue | Ethyl esters and alcohols, furanone and maltol, pyrazine and phenyl ethyl alcohol | Fruity, mellow, sweet, and caramel aromas | Fermentation of sauces and wine | Wang [23] |
| Wickerhamomyces anomalus Y13, Saccharomycopsis fibuligera Y18, and Torulaspora delbrueckii Y22 | Dumplings | 2-pentylfuran, ethanol, hexanal and 1-hexanol, ethyl acetate, 2-heptanone and 2-nonanone, hexyl formate | Pineapple, varnish, balsamic vinegar; sweet fruit flavor, flower flavor, fruit flavor, peach flavor, aromatic fruit flavor | Flour product fermentation | Li [24] |
| Meyerozyma guilliermondii | Laobaigan Jiuqu | Higher fatty acid/acid ester, ethyl palmitate, ethyl linoleate | Fruity, sweet, rose fragrance | Laobaigan flavor liquor | Fu [25] |
| Debaryomyceshansenii | Fu brick tea | --- | --- | Fu brick tea fermentation | Xu [26] |
| Zygosaccharomyces bailii and Pichia kudriavzevii | Liquor fermented grains | Ethyl acetate and 3-methyl butyl acetate | --- | wine | Li [27] |
| Strain | Generating Substances | Key Pathways/Genes/Enzymes | Literature |
|---|---|---|---|
| Saccharomyces cerevisiae | Farnesene | strain improvement steps including mutagenesis, optimization of native metabolism, and enzyme engineering; overexpressed ADA, PK, PTA, and NADH-HMGr, and deleted RHR2 to generate AMR-5. | Meadows [102] |
| Saccharomyces cerevisiae | 2-phenylethanol | overexpression of genes for catalytic enzymes, Aro9 and Aro10, and Aro80 transcription factor. | Kim [103] |
| Saccharomyces cerevisiae | β-ionone | β-carotene biosynthesis genes (crtI, crtE, and crtYB); carotenoid-cleavage dioxygenase from raspberry (RiCCD1) | Beekwilder [104] |
| Saccharomyces cerevisiae | Santalols | Replace its innate promotor with PHXT1; the genes related to santalol biosynthesis were overexpressed under the control of GAL promotors; GAL4 (a transcriptional activator of GAL promotors) and PGM2 (a yeast phosphogluco-mutase) were overexpressed. | Zha [105] |
| Schizosaccharomyces pombe | Vanillin | 3-dehydroshikimate dehydratase, an aromatic carboxylic acid reductase (ACAR), and an O-methyltransferase; Knockout of the host alcohol dehydrogenase ADH6 | Hansen [106] |
| Pichia pastoris | sesquiterpenoid (+)-nootkatone | overexpression of a P. pastoris alcohol dehydrogenase and truncated hydroxy-methylglutaryl-CoA reductase (tHmg1p). | Wriessnegger [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Li, K.; Chen, H.; Li, Z. Reviewing the Source, Physiological Characteristics, and Aroma Production Mechanisms of Aroma-Producing Yeasts. Foods 2023, 12, 3501. https://doi.org/10.3390/foods12183501
Chen L, Li K, Chen H, Li Z. Reviewing the Source, Physiological Characteristics, and Aroma Production Mechanisms of Aroma-Producing Yeasts. Foods. 2023; 12(18):3501. https://doi.org/10.3390/foods12183501
Chicago/Turabian StyleChen, Li, Ke Li, Huitai Chen, and Zongjun Li. 2023. "Reviewing the Source, Physiological Characteristics, and Aroma Production Mechanisms of Aroma-Producing Yeasts" Foods 12, no. 18: 3501. https://doi.org/10.3390/foods12183501
APA StyleChen, L., Li, K., Chen, H., & Li, Z. (2023). Reviewing the Source, Physiological Characteristics, and Aroma Production Mechanisms of Aroma-Producing Yeasts. Foods, 12(18), 3501. https://doi.org/10.3390/foods12183501





