Abstract
This work was carried out with the aim to investigate the microbiological, physicochemical, and sensory properties of an innovative yoghurt produced from ewe’s milk. Experimental yoghurt productions were performed with a commercial freeze-dried starter preparation and a natural milk starter culture (NMSC) of Streptococcus thermophilus and Lactobacillus delbrueckii. The two yoghurts did not differ for colour parameters, showing an average value of lightness, redness, and yellowness of 94.99, −3.74, and 9.37, respectively. The yoghurt produced using the NMSC as a fermenting agent was characterised by a significantly lower fat percentage and a higher antioxidant potential than commercial starters. Microbiological analysis confirmed the safety of the final product and a level of living lactic acid bacteria of 108 CFU/g. Sensory analysis revealed some differences among yoghurts regarding unpleasant odour, homogeneity, and persistence in the mouth, but the yoghurt processed with NMSC was more appreciated. Thus, the production of ewe’s yoghurt fermented by a selected multi-strain starter culture represents an interesting strategy to enlarge the functional ovine dairy product portfolio.
1. Introduction
Yoghurt is the most popular fermented milk product [1], and its unique properties are attributed to the symbiotic fermentation of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus [2,3]. The nutritional value of this fermented milk product determines its worldwide consumption [4]. Indeed, yoghurt consumption can help people meet their nutritional needs for various nutrients, such as calcium, potassium, phosphorus, and vitamins B2 and B12 [5]. For this reason, yoghurt is generally known to contribute to the overall quality of the diet [6] and provide health benefits [4]. In some Asian countries, yoghurt is even used in folk medicine [7]. This product can also lower the risk of cardiovascular diseases and support bone health, especially for the elderly [8,9]. Moreover, yoghurt-fermenting bacteria express functional lactase, helping the digestibility of yoghurt compared to milk in intolerant subjects [10]. More than twenty years ago, Kailasapathy et al. [11] provided the first evidence of the positive impact of yoghurt consumption on the gut microbiota, which is associated with a reduced risk of gastrointestinal disease. Thus, the FAO/WHO recommended daily yoghurt intake due to its rich nutritional profile and its therapeutic and general benefits on the human body [12].
Yoghurt is usually made from cow’s milk. However, in several European and non-European countries, the milk from other species, such as sheep or goats, is processed into yoghurt [2,13]. In countries where climatic and soil conditions are not favourable for cow farming, the use of different types of milk would be a suitable alternative to cow’s milk to produce yoghurt. These conditions characterise Sicily, a southern Italian island region where sheep breeding is more common than cattle breeding (https://www.istat.it/, accessed on 27 July 2023). Ewe’s milk has several beneficial attributes over cow’s milk, providing higher levels of proteins, lipids, minerals, and vitamins essential for human health [14]. Several studies suggested that high levels of protein and fat positively affect the production of fermented milk products [15,16,17], contributing to increased firmness, cohesiveness, and viscosity of the yoghurt [18]. In particular, the viscosity of yoghurt may be useful in preserving probiotic cultures throughout the gastrointestinal tract. They offer added protection to probiotics in the stomach [19], as well as during the commercial storage period [20]. Furthermore, ewe’s milk yoghurt exhibits desirable flavour properties, such as the creamy-sour attribute appreciated by many consumers [21].
This work was carried out with the aim to develop an ewe’s milk yoghurt adapted to cow’s milk yoghurt technology. The goal was to expand the dairy Sicilian portfolio by targeting modern consumers who are increasingly interested in functional foods with higher nutritional values and health benefits. A natural milk starter culture (NMSC) was created using selected strains of S. thermophilus and L. delbrueckii isolated from typical Sicilian ewe’s dairy products. The use of NMSC is considered important to better link the novel product with the Sicilian area and to avoid the taste homologation phenomena determined by commercial starter cultures [22]. The resulting yoghurts were analysed for hygienic safety, physicochemical traits, and antioxidant capacity. Moreover, the yoghurts were also assessed for their sensory properties to determine how appealing the product is to consumers.
2. Materials and Methods
2.1. Raw Materials and Starter Cultures
Yoghurt was prepared using bulk milk of the “Valle del Belíce” breed, an indigenous Sicilian sheep species. Raw milk was obtained from several dairy farms located in Agrigento province (Cammarata, Italy) and was then transformed at “Cooperativa Agricola Tumarrano” (Cammarata, Italy). The experimental plan of this study consisted of two different ewe’s milk yoghurt productions by applying the technology typically followed in the production of cow’s yoghurt. The first production (EY-1) was performed using the commercial freeze-dried starter culture YODX091 (ALCE s.r.l., Quistello, MN, Italy), composed of S. thermophilus and L. delbrueckii spp. bulgaricus (one strain each), while a second production (EY-2) was carried out with the addition of a multi-strain NMSC developed in this study.
2.2. Preparation of Natural Milk Starter Culture
The NMSC used in this study included the following strains: S. thermophilus (PON244) and L. delbrueckii (WT601). These strains, selected for their high dairy performances such as acidification capacity, autolysis kinetics, diacetyl production, and antibacterial activity belonged to the collection of the laboratory of agricultural microbiology at the University of Palermo and were previously isolated from traditional PDO Sicilian cheeses [23,24]. Briefly, both strains were refreshed in their optimal growth media. S. thermophilus PON244 was reactivated in M17 medium [25], while L. delbrueckii WT601 was reactivated in MRS medium [26]. Both strains were incubated at 40 °C for 48 h. After growth, strains were subjected to two consecutive washes in Ringer’s solution (Sigma-Aldrich, Milan, Italy). Each washing step was followed by centrifugation at 6000× g for 2 min using a Heraeus Biofuge Pico (Kendro Lab, Waltham, MA, USA) in order to remove any residue of growth medium. The washed cells were inoculated at 106 colony-forming units (CFU)/mL into ovine whole-fat UHT milk (Leeb Vital, Wartberg an der Krems, Austria) and incubated at 40 °C for 24 h. NMSC containing both S. thermophilus and L. delbrueckii at approximately 109 CFU/mL, as verified by plate count, was used for yoghurt production.
2.3. Yoghurt Production and Sample Collection
Yoghurts were prepared according to the production protocol indicated by Gaglio et al. [27]. Briefly, a volume of 100 L of raw milk was pasteurised at 72 °C for 15 s in a stainless-steel Comat PS 20351 pasteuriser (Bellizzi, SA, Italy) and cooled at 42 °C. The entire bulk milk was then transferred into an automatic Due Ci Inox s.r.l. yoghurt maker (Guastalla, RE, Italy). After cooling, starter cultures were directly inoculated, and the milk was stirred for 10 min. Fermentation took place at 38 °C for 6 h and was stopped at pH 4.5 [28]. Finally, the yoghurt was packaged into 120 mL plastic pots with an air-tight cap (FD Store s.r.l., Vignola, Italy) and stored at 4 °C for 3 d before distribution. Figure 1 provides an overview of the production protocol followed.
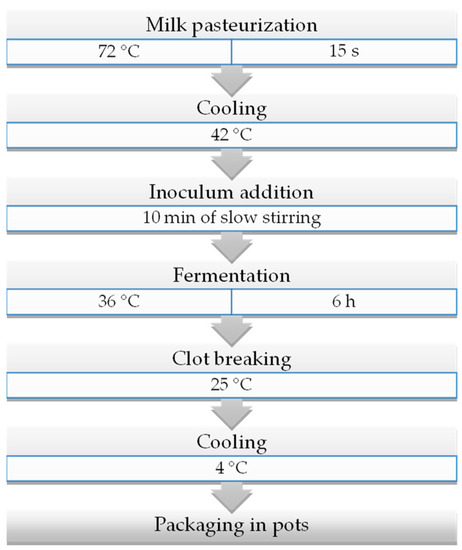
Figure 1.
Flow chart of ewe’s yoghurt.
Both trials (EY-1 and EY-2) were repeated after two weeks (two independent replicates). Raw milk (RM), pasteurised milk (PM), inoculated milk (IM), and yoghurt (Y) after 3 d of refrigerated (4 °C) storage were collected for analyses.
2.4. Microbiological Analysis
All samples collected before and after yoghurt production were microbiologically evaluated to enumerate total mesophilic microorganisms (TMM) by spreading on plate count agar (PCA), incubated aerobically at 30 °C for 72 h, and thermophilic rod and coccus-shaped lactic acid bacteria (LAB) after pouring on de Man-Rogosa-Sharpe (MRS) agar acidified to pH 5.4 with lactic acid (5 mol/L) and M17 agar, respectively, incubated anaerobically in anaerobic jars (AnaeroGen, Thermo Fisher Scientific, Waltham, MA, USA) at 42 °C for 48 h. Fungal growth was inhibited by adding chloramphenicol (1 mg/L) to both media. Raw and pasteurised milk samples were also analysed for the presence of the main four food-borne pathogens in compliance with Commission Regulation (EC) No 2073/2005 [29] on microbiological criteria for foodstuffs: Escherichia coli and coagulase-positive staphylococci (CPS) were analysed as process hygiene criteria applying ISO 16649-1 [30] and ISO 6888-2 [31], respectively; Salmonella spp. and Listeria monocytogenes were analysed as food safety criteria by spreading on Hektoen enteric agar (HEA) and on Listeria selective agar base (LSAB) added with SR0140E supplement, respectively, both incubated at 37 °C for 24 h. All media and supplements were purchased from Oxoid.
The plate count method was applied after serial decimal dilutions. Aliquots of 1 mL of liquid samples (RM, PM, and IM) were diluted with 9 mL of sterile 0.9% (v/v) Ringer’s solution, while 15 g of yoghurt just after production and after 24 h of refrigerated storage were first homogenised in 135 mL of sodium citrate (2% v/v) solution in a stomacher (Bag Mixer 400; Interscience, Saint Nom, France) for 2 min at the highest speed and then serially diluted as described for liquid samples. Bacterial enumerations were carried out in duplicate (technical repeats) for each trial. The results were expressed as CFU per mL (liquid samples) or gram (solid samples).
2.5. Physicochemical Analysis
Milk samples before processing were analysed for pH, lactose, fat, protein, and urea by the infrared method (Combi-Foss 6000, Foss Electric, Hiller’d, Denmark). The colour of milk and yoghurts was evaluated in duplicate using a Minolta Chroma Meter CR-300 (Minolta, Osaka, Japan), which measures values of lightness (L* = 0/100, from black to white), redness (a* = −a/+a, from green to red) and yellowness (b* = −b/+b, blue to yellow), according to the CIE L*a*b* system [32]. The values of L*, a*, and b* were used for the calculation of chroma [(a*2 + b*2)0.5], measuring the colour intensity or saturation, hue angle [tan−1(b*/a*)], as a measure of colour tone, and the whiteness index [100−((100−L*)2 + a*2 + b*2)0.5], according to Vargas et al. [33]. Total colour change (ΔE) after yoghurt production was calculated as ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]0.5, where ΔL*, Δa*, and Δb* are the differences of L*, a*, and b*, respectively, between milk and yoghurt.
All yoghurt samples were freeze-dried and analysed for centesimal chemical composition [dry matter (DM), protein (N × 6.38), fat, and ash content] in accordance with FIL-IDF standards [34,35,36].
2.6. Oxidation Products and Antioxidant Capacity
The extracts of lyophilised samples were prepared following the protocol described by Rashidinejad et al. [37] with minor modifications. Briefly, each lyophilised yoghurt sample (0.5 g) was dissolved in a 95% aqueous methanol solution (25 mL) with 1% (v/v) HCl. The suspension was mixed by vortex for about 30 s and then held at 40 °C in an ultrasonic water bath (LBS1 Sonicator; Falc Instruments, Treviglio, Italy) for 30 min, during which it was mixed by vortex for 5–10 s every 10 min. The resulting suspension was cooled, filtered with linen cloth, centrifuged at 7000× g at 9 °C for 10 min, and finally kept at −18 °C until analysis. Extracted samples were analysed in duplicate for antioxidant properties, measuring the antioxidant capacity and the indexes of primary and secondary lipid oxidation.
Total antioxidant capacity in extracted yoghurt samples was assessed by the Trolox equivalent antioxidant capacity (TEAC) assay, according to a published protocol [37], as modified by Bonanno et al. [38]. TEAC is a discoloration assay aimed at evaluating the radical scavenging ability of the samples with the use of the [2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid)] (ABTS) radical cation (ABTS•+) and Trolox as standards [39]. To obtain the ABTS radical cation, equal volumes of a 14 mM ABTS aqueous solution and 4.9 mM K2S2O8 were mixed and placed in the dark for 16 h at room temperature. ABTS radical cation solution was diluted in 5 mM PBS solution (phosphate buffered saline, pH 7.40) until it reached an absorbance of 0.795 (±0.020) at 734 nm using a Hach DR/4000 U spectrophotometer (Hach Company, Ames, Iowa, USA). The absorbance reading of a mixture of 150 µL of PBS with 2850 µL of a diluted ABTS radical cation solution was recorded at 734 nm immediately (as white sample at 0 min) and after incubation at 30 °C for 6 min (as white sample at 6 min). Similarly, the absorbance of the 150 µL solution of each extracted sample mixed with 2850 µL of the dilute ABTS radical cation solution was read at 734 nm after 6 min of incubation at 30 °C. The absorbance values were used to determine the percentage decrease of the absorbance due to decolorization, calculated by comparison with the absorbance obtained with PBS. Solutions of Trolox in PBS (0–2.5 mM) were used to construct the calibration curve (R2 = 0.99), and the results are expressed in mmol Trolox/kg DM.
The oxidation of yoghurt fat was estimated by determining in duplicate the peroxide value (POV, mEq O2/kg fat), expressing the primary lipid oxidation [40], and the thiobarbituric acid reactive substances (TBARS, μg malonylaldehyde (MDA)/kg DM) as secondary lipid oxidation products, in line with the method described by Tarladgis et al. [41] and modified by Mele et al. [42]. Briefly, TBARS analysis was conducted on 2 g of lyophilised yoghurt, which was mixed with an 8 mL aqueous solution of phosphate buffer at pH 7 by vortex. After that, 2 mL of a 30% (v/v) aqueous solution of trichloroacetic acid was added, and the resulting solution was vortexed for about 5 s and filtered with Whatman No. 1 filter paper. Five millilitres of the filtered solution were mixed with an equal volume of 0.02 M thiobarbituric acid aqueous solution, placed in a hot water bath at 90 °C for 20 min, and then refrigerated. After centrifugation at 4500× g for 5 min, the absorbance of the supernatant at 530 nm was read spectrophotometrically. Solutions of 1,1,3,3-tetramethoxypropane at concentrations between 0.016 and 0.165 µg/mL were read to construct the calibration curve (R2 = 0.99).
2.7. Sensory Evaluation
EY-1 and EY-2 yoghurts were sensory evaluated by a panel of 19 judges composed of 10 males and 9 females aged between 25 and 62 years old. The evaluation of odours, tastes, and appearance was performed according to a list of eleven attributes: odour intensity and unpleasant odours (odour perception); sweet, acid, bitter, persistence, and off flavour (taste sensation); and colour, homogeneity, spontaneous syneresis, and viscosity (appearance and texture). The judges were also asked to give an overall evaluation in terms of satisfaction with the final product, considering the scores of all attributes [20,43,44]. To this purpose, the members of the descriptive panel used a sensory 9-rating scale as reported by Gaglio et al. [27], where 0 corresponds to the lowest score of the character and 9 to the highest value. Sensory assessment was performed in individual temperature-controlled cabs (20 °C). A commercial white cow’s yoghurt (CCY) (Muller, Fischach, Germania) purchased at a retail market was used as a control. Each yoghurt (35 mL) was served at 7 °C using plastic cups coded with an alphanumeric random code. Water was used for rinsing between samples that were randomly presented as described by Akalın et al. [45].
2.8. Statistical Analyses
The data were analysed using XLStat software version 2020.3.1 for Excel software (Addinsoft, New York, NY, USA). A statistical analysis of bacterial counts was performed after logarithmic conversion of the data. Results are given as means ± standard deviation (SD). Differences between the means were determined by Tukey’s multiple range post hoc test. p values of < 0.05 were deemed to be significant.
The effect of starter culture on the physicochemical traits of yoghurt was evaluated statistically using the generalised linear model (GLM) procedure in SAS 9.2 software (SAS Institute Inc., Campus Drive, Cary, NC, USA).
3. Results and Discussion
3.1. Microbiological Monitoring
The results of microbiological analyses of raw milk samples from EY-1 and EY-2 productions are shown in Figure 2.
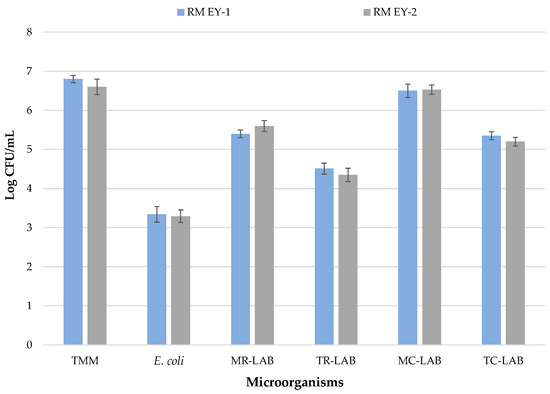
Figure 2.
Microbiological loads of milk samples before pasteurization. Results indicate mean values and SD of four determinations (carried out in duplicate for two independent productions). Abbreviations: EY-1, production performed with freeze-dried commercial starter culture (starter formulation YODOX91); EY-2, production performed with a multi-strain milk starter culture consisting of S. thermophilus and L. delbrueckii; RM, raw milk; TMM, total mesophilic microorganisms; E., Escherichia; LAB, lactic acid bacteria; MR, mesophilic rod; TR, thermophilic rod; MC, mesophilic cocci; TC, thermophilic cocci.
In both productions, RM samples show no statistically significant differences (p > 0.05) for the microbial groups investigated. These samples show levels of TMM at about 107 log CFU/mL in EY-1 and EY-2. Similar levels were registered by Gaglio and co-workers [27], who analysed raw cow’s milk before transformation into yoghurt, while higher TMM levels have been detected in raw cow’s milk in other studies [46,47]. The high TMM cell densities found in raw milk are not surprising since this food matrix, thanks to its high concentrations and variety of nutrients and its neutral pH, represents the ideal growth medium for a wide variety of microorganisms [48]. Raw milk microbiota is very heterogeneous [49] and can be made up of pathogenic and spoilage microorganisms as well as bacteria of technological importance [50,51]. Regarding the specific search for undesired bacteria, plate counts did not detect L. monocytogenes or Salmonella spp. To date, the regulation (EC) No 2073/2005, which includes microbiological parameters [29], indicates the complete absence of these microorganisms for the human consumption of milk. Indeed, none of the samples show the presence of CPS.
The absence of staphylococci, potential contaminants in raw milk samples, is evidence of good hygiene practices applied during milking, conveyance, and storage [52], because bacterial cross-contamination of milk can arise as a result of many factors [53]. Nevertheless, plate counts revealed the presence of E. coli at levels of 3.34 and 3.29 CFU/mL in EY-1 and EY-2 production, respectively. Similar results were found by Caro et al. [54] on samples of raw ewe’s milk used to make “Castellano” cheese. E. coli represents an indicator of faecal contamination [55], and such contamination during the milking process is quite common [56,57,58]. For this reason, a sanitization treatment becomes mandatory if the final product has a short maturation period (<2 months) [59].
LAB were dominant in raw milk samples; levels of mesophilic rod and cocci LABs were almost superimposable to those of TMM (log 5.4 and 6.5 CFU/mL, respectively, in EY-1; log 5.6 and 6.53 CFU/mL, respectively, in EY-2). Similar results were previously registered by Samelis et al. [60] for raw milk used for Greek hard cheese production.
Thermophilic LAB were detected at levels lower than mesophilic LAB since log 105 CFU/mL were recorded for thermophilic cocci. Thermophilic rods were even lower (log 104 CFU/mL) in both productions. Garofalo et al. [61] registered a similar trend for raw ewe’s bulk milk collected in a different area (Palermo province) of Sicily. The microbial ecology of raw milk consists of a complex interaction among indigenous LAB, which play different roles during cheese-making [62], and they are generally high in number [63].
Inoculated starter cultures drove the fermentation process during yoghurt manufacture. Therefore, microbiological analysis included the detection of thermophilic LAB cocci and rods representing the starter cultures inoculated. Figure 3 shows the results of the microbial growth from pasteurised milk to final yoghurts.
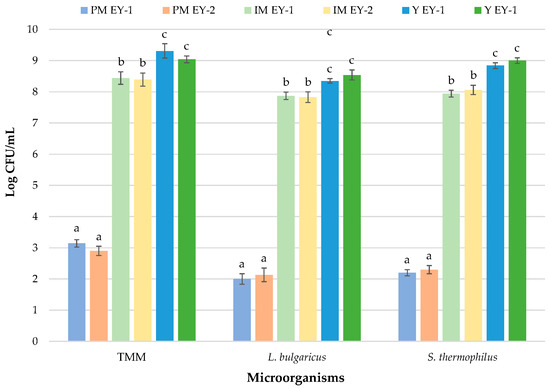
Figure 3.
Growth of starter cultures during yoghurt production. Units are log CFU/mL for liquid samples and log CFU/g for solid samples. Results indicate mean values ±SD of four plate counts (carried out in duplicate for two independent productions). a, b, c = p < 0.05. Abbreviations: TMM, total mesophilic microorganisms; S., Streptococcus; L., Lactobacillus; PM, pasteurised milk; IM, inoculated milk; Y, yoghurt; EY-1, production of ewe’s yoghurt performed with freeze-dried commercial starter culture (starter formulation YODOX91); EY-2, production of ewe’s yoghurt performed with a multi-strain milk starter culture consisting of S. thermophilus and L. delbrueckii.
After pasteurization, the levels of TMM in milk were recorded at approximately three log cycles in comparison to RM samples. Pasteurization treatment is necessary to guarantee the hygiene and security of the final products [64]. Figure 3 clearly highlights a decrease of approximately three log cycles for TMM in PM samples. The main aim of thermal treatment (72 °C for 15 s) is to sanitise raw milk from any potential trace contaminants it may have. A sudden and consistent drop was also registered for the levels of LAB and E. coli. In particular, E. coli was undetectable after the application of the thermal treatment. Although pasteurization causes a drastic reduction in microbial levels, this treatment does not determine the complete elimination of the indigenous microbiota [65]. This treatment leads to a reduction of at least 90% of the bacterial population, and usually only thermoduric bacteria survive [66].
Immediately after starter addition, the levels of S. thermophilus and L. delbrueckii in the yoghurt were approximately log 108 CFU/g in both productions. It is widely known that starter microorganisms should be added at levels of 107 CFU/g of viable bacteria since they have the primary task to produce lactic acid from lactose [67]. S. thermophilus was observed at slightly higher levels than L. delbrueckii in IM of both productions, with no statistically significant differences (p > 0.05). These results are in line with those argued in the study of Güler-Akin (2005) [68]. After fermentation, the final yoghurts show count loads one log cycle higher than inoculums (S. thermophilus and L. delbrueckii were detected at 8.84 and 8.35 CFU/g, respectively, in EY-1 and 9.00 and 8.54 in EY-2). Similar results can be found in the work of Shazly et al. [69], who produced probiotic yoghurt from ewe’s milk. Birollo et al. [70] stated that the high levels of viable LAB in fermented milks are correlated with consumers’ health benefits.
3.2. Physicochemical Traits of Ewe’s Yoghurt
Chemical composition and colour parameters of milk used for the production of yoghurts (Table 1) show some fluctuations, especially for protein, casein, urea, and yellowness, that can be attributed to the different feeding systems of the origin farms of milk [71]. However, milk parameters fully reflect those characterizing sheep milk from Valle del Belìce sheep [72].

Table 1.
Composition of milk used for yoghurt production.
Regarding the physicochemical traits of the yoghurts produced with the different types of starters (Table 2), the differences in protein content can be attributed to the different starting milk composition since the protein percentage changes from milk to yoghurt occurred at similar levels (about +20%) for both production lines.

Table 2.
Physicochemical traits of ewe’s yoghurt.
Compared to milk fat, yoghurt fat shows a greater reduction with the use of NMSC than commercial starter, suggesting an effect of more intense bacterial activity in degrading fatty acids as an energy source [27].
None of the colour parameters of yoghurts (Table 2) were significantly affected by the starter culture, but the use of NMSC induced a significant increase in the total colour change. This result can be explained by the larger change from milk to yoghurt observed in the EY-2 yoghurt than in the EY-1 yoghurt for lightness and yellow index, even if these differences between yoghurts did not emerge at a statistically significant level; these results can be imputable to the microbial activity carried out by the bacteria constituting the selected starter culture.
3.3. Oxidation and Antioxidant Activity of Ewe’s Yoghurt
The antioxidant capacity is defined as the action of all the antioxidant molecules present in the food matrix [73]. Authors such as Jaster et al. [74] and Guz et al. [75] describe yoghurt as a potential carrier of antioxidant compounds derived from its enrichment with a matrix containing bioactive molecules such as polyphenols. However, milk and its derivatives constitute a complex mixture of enzymatic systems, proteins, minerals, and vitamins, and all these substances together confer antioxidant capacity [76]. The antioxidant characteristics of milk and dairy products were extensively studied. Thus, studies on cow’s milk and cow’s yoghurt have shown that the fermented version of milk products has a higher antioxidant capacity than milk [77]; this result can be linked to the antioxidant properties of peptides produced from milk protein by the enzymatic activity of inoculated microorganisms [78]. In this regard, there is no study on non-fortified yoghurt made from ewe’s milk. The present study demonstrates that the application of selected bacteria can improve the antioxidant properties of ewe’s yoghurt, contributing to enhanced health benefits for consumers [79].
The antioxidant activity and the primary and secondary lipid oxidation values of the yoghurts processed in this study are reported in Table 3.

Table 3.
Oxidation products and antioxidant capacity of ewe’s yoghurt.
The antioxidant activity of yoghurts was evaluated by the TEAC assay in order to verify the effects of the use of selected strains. A better antioxidant capacity emerged in the EY-2 yoghurt (15.41 mmol TE/kg DM) produced with the selected starters S. thermophilus PON244 and L. delbrueckii WT601; presumably these microorganisms were able to perform more intense proteolytic activity and release peptides with antioxidant properties [76,78]. Future studies on sheep yoghurt should evaluate its antioxidant capacity during storage, since some studies have stated that this property decreases over time [80]. Moreover, since the antioxidant properties of this type of dairy product can derive from different events, more antioxidant assays are opportune.
Lipid oxidation was evaluated by POV and TBARS analyses. Foods with a low peroxide value are generally perceived as having high oxidative stability and a long shelf life [81]. However, the number of peroxides is often affected [82] and increases with storage time [83,84]. In this study, the level of primary lipid oxidation in yoghurts produced using different starters was compared. Higher values were found for EY-2 yoghurt (0.43 O2/kg fat), presumably due to a greater microbial activity than that of EY-1, which led to an anticipated oxidation of the product. On the contrary, for secondary lipid oxidation, EY-2 yoghurt shows the lowest value (0.074 MDA/kg DM), and this is attributable to its higher antioxidant capacity than EY-1. In the literature, no other study reports the values of primary and secondary lipid oxidation of ewe’s yoghurt; however, the values observed in this study were analogous to those reported for fresh sheep cheese [61].
3.4. Ewe’s Yogurt Sensory Evaluation
The panellists scored the three yoghurt samples as objects of evaluation (EY-1, EY-2, and CCY), and the results for all attributes are given in the form of a spider plot (Figure 4). It is well known that the sensory attributes of dairy products are strictly associated with the milk composition, starter cultures, heat treatment, and storage conditions [85]. For this reason, the sensory evaluation of a new dairy product is mandatory before its commercialization in the marketplace [86]. The scores of the assessors for odour intensity, colour, sweetness, acidity, and bitterness were not statistically different (p > 0.05) among samples. These results are encouraging because they suggest that ovine milk yoghurts meet the basic requirements of consumers, which, according to Routray et al. [17], are colour, odour, and flavour. These attributes represent the most significant sensory factors used for the promotional marketing of a new dairy product [87]. Statistically significant differences (p < 0.0001) observed for unpleasant odour, homogeneity, and persistence in the mouth may be attributable to the type of milk used. Indeed, sheep’s milk is different from cow’s milk in terms of gross composition and single constituents. For example, the lipid fraction contains a higher percentage of volatile, short-chain fatty acids, such as rancid flavour notes [88]. The fat content also serves as substrate for important flavour-generating reactions performed by microorganisms, but it also contributes to the body and texture of cheese, which explains the differences (p < 0.01) observed between spontaneous syneresis, viscosity, and off-flavour. Bonanno et al. [89] stated in their work that the differences observed are induced by the breed, the nature of the feed consumed, and even the time of milking (i.e., morning vs. evening). In addition, the use of sheep milk is well known to enrich the nutritional properties of the final product because of the high levels of total solids and nutrients [90].
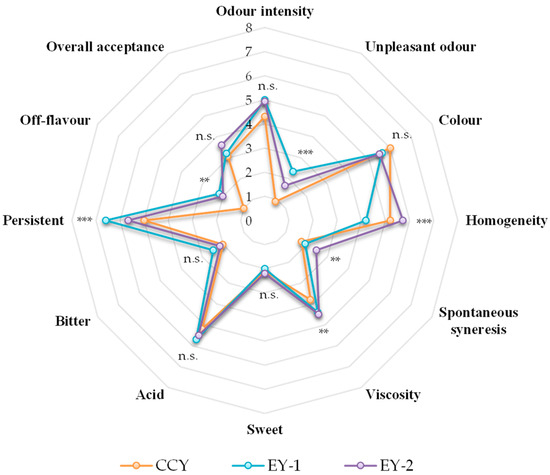
Figure 4.
Spider diagram of sensory evaluation of yoghurts. Abbreviations: CCY, commercial cow’s yoghurt; EY-1, production of ewe’s yoghurt performed with freeze-dried commercial starter culture (starter formulation YODOX91); EY-2, production of ewe’s yoghurt performed with a multi-strain milk starter culture consisting of S. thermophilus and L. delbrueckii; ** p < 0.01; *** p < 0.001; n.s., not significant (p > 0.05).
Finally, the panel was also asked to express their preference between the samples, and even if no difference was noticed between all samples, EY-2 received a higher score (3.6). This means that LAB strains consistently impacted the organoleptic characteristics of the final yoghurts.
4. Conclusions
The results of this work show that selected starter cultures can be used to create new and safe products from ewe’s milk, such as yoghurt, with good antioxidant properties. The consumers also liked the sheep’s milk yoghurt, which is essential for its market success. This work explored a novel dairy product for Sicily that can benefit the sheep farmers by preserving the native breeds and biodiversity. In a future perspective, sheep yoghurt can help face the increasing phenomenon of abandonment of inland areas affecting mountain provinces of the main island.
Author Contributions
Conceptualization, R.G. and L.S.; methodology, R.G. and H.E.; software, M.T.; validation, R.G. and L.S.; formal analysis, G.G., M.P. and G.B.; investigation, G.G., M.P. and G.B.; resources, M.T.S.; data curation, G.G. and M.P.; writing—original draft preparation, G.G. and M.P.; writing—review and editing, A.B., R.G., H.E. and L.S.; project administration, B.P. and M.T.S.; funding acquisition, M.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PO FESR 2014/2020 Azione 1.1.5 Progetto TRAIPROL@C-CUP G79J18000650007 R.S. Maria Teresa Sardina.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mckinley, M.C. The nutrition and health benefits of yoghurt. Int. J. Dairy Technol. 2005, 58, 1–12. [Google Scholar] [CrossRef]
- Serafeimidou, A.; Zlatanos, S.; Kritikos, G.; Tourianis, A. Change of fatty acid profile, including conjugated linoleic acid (CLA) content, during refrigerated storage of yogurt made of cow and sheep milk. J. Food Compost. Anal. 2013, 31, 24–30. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT Food Sci. Technol. 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Weerathilake, W.A.D.V.; Rasika, D.M.D.; Ruwanmali, J.K.U.; Munasinghe, M.A.D.D. The evolution, processing, varieties and health benefits of yogurt. Int. J. Sci. Res. Publ. 2014, 4, 1–10. [Google Scholar]
- Aznar, L.A.M.; Ral, P.C.; Anta, R.M.O.; Martín, J.J.D.; Baladia, E.; Basulto, J.; Serrat, S.B.; Altaba, I.I.; López-Sobaler, A.M.; Manera, M.; et al. Scientific evidence about the role of yogurt and other fermented milks in the healthy diet for the Spanish population. Nutr. Hosp. 2013, 28, 2039–2089. [Google Scholar]
- Panahi, S.; Fernandez, M.A.; Marette, A.; Tremblay, A. Yogurt, diet quality and lifestyle factors. Eur. J. Cli. Nutr. 2017, 71, 573–579. [Google Scholar] [CrossRef]
- Oh, Y.; Osato, M.S.; Han, X.; Bennett, G.; Hong, W.K. Folk yoghurt kills Helicobacter pylori. J. Appl. Microbiol. 2002, 93, 1083–1088. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D. Consumption of yogurt and the incident risk of cardiovascular disease: A meta-analysis of nine cohort studies. Nutrients 2017, 9, 315. [Google Scholar] [CrossRef]
- Laird, E.; Molloy, A.M.; McNulty, H.; Ward, M.; McCarroll, K.; Hoey, L.; Hughes, C.F.; Cunningham, C.; Strain, J.J.; Casey, M.C. Greater yogurt consumption is associated with increased bone mineral density and physical function in older adults. Osteoporos. Int. 2017, 28, 2409–2419. [Google Scholar] [CrossRef]
- Shaukat, A.; Levitt, M.D.; Taylor, B.C.; MacDonald, R.; Shamliyan, T.A.; Kane, R.L.; Wilt, T.J. Systematic review: Effective management strategies for lactose intolerance. Ann. Intern. Med. 2010, 152, 797–803. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO/WHO: Rome, Italy, 2001. [Google Scholar]
- Gyawali, R.; Feng, X.; Chen, Y.P.; Lorenzo, J.M.; Ibrahim, S.A. A review of factors influencing the quality and sensory evaluation techniques applied to Greek yogurt. J. Dairy Res. 2022, 89, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep milk: Physicochemical characteristics and relevance for functional food development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Mohameed, H.A.; Abu-Jdayil, B.; Al-Shawabkeh, A. Effect of solids concentration on the rheology of labneh (concentrated yogurt) produced from sheep milk. J. Food Eng. 2004, 61, 347–352. [Google Scholar] [CrossRef]
- Güler, Z.; Gürsoy-Balcı, A.C. Evaluation of volatile compounds and free fatty acids in set types yogurts made of ewes’, goats’ milk and their mixture using two different commercial starter cultures during refrigerated storage. Food Chem. 2011, 127, 1065–1071. [Google Scholar] [CrossRef]
- Routray, W.; Mishra, H.N. Scientific and technical aspects of yogurt aroma and taste: A review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 208–220. [Google Scholar] [CrossRef]
- Jørgensen, C.E.; Abrahamsen, R.K.; Rukke, E.O.; Hoffmann, T.K.; Johansen, A.G.; Skeie, S.B. Processing of high-protein yoghurt–A review. Int. Dairy J. 2019, 88, 42–59. [Google Scholar] [CrossRef]
- Karimi, R.; Mortazavian, A.M.; Da Cruz, A.G. Viability of probiotic microorganisms in cheese during production and storage: A review. Dairy Sci. Technol. 2011, 9, 283–308. [Google Scholar] [CrossRef]
- Prasanna, P.H.P.; Charalampopoulos, D. Encapsulation of Bifidobacterium longum in alginate-dairy matrices and survival in simulated gastrointestinal conditions, refrigeration, cow milk and goat milk. Food Biosci. 2018, 21, 72–79. [Google Scholar] [CrossRef]
- Vianna, F.S.; Canto, A.C.; da Costa-Lima, B.R.; Salim, A.P.A.; Costa, M.P.; Balthazar, C.F.; Oliveira, B.R.; Rachid, R.P.; Franco, R.M.; Conte-Junior, C.A.; et al. Development of new probiotic yoghurt with a mixture of cow and sheep milk: Effects on physicochemical, textural and sensory analysis. Small Rumin. Res. 2017, 149, 154–162. [Google Scholar] [CrossRef]
- Mariani, M.; Cerdan, C.; Peri, I. Origin food schemes and the paradox of reducing diversity to defend it. Sociol. Ruralis 2021, 61, 465–490. [Google Scholar] [CrossRef]
- Gaglio, R.; Francesca, N.; Di Gerlando, R.; Cruciata, M.; Guarcello, R.; Portolano, B.; Moschetti, G.; Settanni, L. Identification, typing and investigation of the dairy characteristics of lactic acid bacteria isolated from “Vastedda della valle del Belìce” cheeses. Dairy Sci. Technol. 2014, 94, 157–180. [Google Scholar] [CrossRef]
- Busetta, G.; Garofalo, G.; Mangione, G.; Botta, L.; Franciosi, E.; Di Gerlando, R.; Todaro, M.; Licitra, G.; Scatassa, M.L.; Gaglio, R.; et al. Polyphasic characterization of microbiota of “mastredda”, a traditional wooden tool used during the production of PDO Provola dei Nebrodi cheese. Appl. Sci. 2021, 11, 8647. [Google Scholar] [CrossRef]
- Terzaghi, B.E.; Sandine, W. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 1975, 29, 807–813. [Google Scholar] [CrossRef] [PubMed]
- De Man, J.C.; Rogosa, D.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Microbiol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Gaglio, R.; Gentile, C.; Bonanno, A.; Vintaloro, L.; Perrone, A.; Mazza, F.; Barbaccia, P.; Settanni, L.; Di Grigoli, A. Effect of saffron addition on the microbiological, physicochemical, antioxidant and sensory characteristics of yoghurt. Int. J. Dairy Technol. 2019, 72, 208–217. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R2073&from=EN (accessed on 28 July 2023).
- ISO (International Organization for Standardization). Microbiology of the Food Chain–Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia Coli–Part 3: Detection and Most Probable Number Technique Using 5-bromo-4-chloro-3-indolyl-β-D-Glucuronide; International Standardization Organization (ISO): Geneva, Switzerland, 2018. [Google Scholar]
- ISO (International Organization for Standardization). Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Technique Using Baird-Parker Agar Medium—Amendment 2: Inclusion of an Alternative Confirmation Test Using RPFA Stab Method; International Standardization Organization (ISO): Geneva, Switzerland, 1999. [Google Scholar]
- CIE (Commission International de l’Eclairage). Colorimetry. Volume CIE 15.2; Commission International de l’Eclairage: Vienna, Austria, 1986. [Google Scholar]
- Vargas, M.; Cháfer, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Physicochemical and sensory characteristics of yoghurt produced from mixtures of cows’ and goats’ milk. Int. Dairy J. 2008, 18, 1146–1152. [Google Scholar] [CrossRef]
- FIL-IDF 27; Determination of the Ash Content of Processed Cheese Products. International Dairy Federation: Brussels, Belgium, 1964.
- FIL-IDF 4A; Determination of the Total Solids Content. International Dairy Federation: Brussels, Belgium, 1982.
- FIL-IDF 5B; Determination of Fat Content-Gravimetric Method. International Dairy Federation: Brussels, Belgium, 1986.
- Rashidinejad, A.; Birch, J.E.; Sun-Waterhouse, D.; Everett, D.W. Effects of catechin on the phenolic content and antioxidant properties of low-fat cheese. Int. J. Food Sci. Technol. 2013, 48, 2448–2455. [Google Scholar] [CrossRef]
- Bonanno, A.; Di Grigoli, A.; Vitale, F.; Di Miceli, G.; Todaro, M.; Alabiso, M.; Gargano, M.L.; Venturella, G.; Anike, F.N.; Isikhuemhenal, O.S. Effects of feeding diets supplemented with medicinal mushrooms myceliated grains on some production, health and oxidation traits of dairy ewes. Int. J. Med. Mushrooms 2019, 21, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- FIL-IDF 74A; Determination of the Peroxide Value. International Dairy Federation: Brussels, Belgium,, 1991.
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L., Jr. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Mele, M.; Contarini, G.; Cercaci, L.; Serra, A.; Buccioni, A.; Povolo, M.; Conte, G.; Funaro, A.; Banni, S.; Lercker, G.; et al. Enrichment of pecorino cheese with conjugated linoleic acid by feeding dairy ewes with extruded linseed: Effect on fatty acid and triglycerides composition and on oxidative stability. Int. Dairy J. 2011, 21, 365–372. [Google Scholar] [CrossRef]
- Fadela, C.; Abderrahim, C.; Ahmed, B. Sensorial and physico-chemical characteristics of yoghurt manufactured with ewe’s and skim milk. World J. Dairy Food Sci. 2009, 4, 136–140. [Google Scholar]
- Megalemou, K.; Sioriki, E.; Lordan, R.; Dermiki, M.; Nasopoulou, C.; Zabetakis, I. Evaluation of sensory and in vitro anti-thrombotic properties of traditional Greek yogurts derived from different types of milk. Heliyon 2017, 3, e00227. [Google Scholar] [CrossRef] [PubMed]
- Akalın, A.S.; Unal, G.; Dinkci, N.A.Y.I.L.; Hayaloglu, A.A. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. J. Dairy Sci. 2012, 95, 3617–3628. [Google Scholar] [CrossRef]
- Mallet, A.; Guéguen, M.; Kauffmann, F.; Chesneau, C.; Sesboué, A.; Desmasures, N. Quantitative and qualitative microbial analysis of raw milk reveals substantial diversity influenced by herd management practices. Int. Dairy J. 2012, 27, 13–21. [Google Scholar] [CrossRef]
- Porcellato, D.; Aspholm, M.; Skeie, S.B.; Monshaugen, M.; Brendehaug, J.; Mellegård, H. Microbial diversity of consumption milk during processing and storage. Int. J. Food Microbiol. 2018, 266, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Perin, L.M.; Pereira, J.G.; Bersot, L.S.; Nero, L.A. The microbiology of raw milk. In Raw Milk; Nero, L.A., De Carvalho, A.F., Eds.; Academic Press: Cambridge, UK, 2019; pp. 45–64. [Google Scholar]
- Celano, G.; Calasso, M.; Costantino, G.; Vacca, M.; Ressa, A.; Nikoloudaki, O.; De Palo, P.; Calabrese, F.M.; Gobbetti, M.; De Angelis, M. Effect of Seasonality on Microbiological Variability of Raw Cow Milk from Apulian Dairy Farms in Italy. Microbiol. Spectr. 2022, 10, e0051422. [Google Scholar] [CrossRef]
- Franciosi, E.; Settanni, L.; Carlin, S.; Cavazza, A.; Poznanski, E. A factory-scale application of secondary adjunct cultures selected from lactic acid bacteria during Puzzone di Moena cheese ripening. J. Dairy Sci. 2008, 91, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Montel, M.C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Kumari, A.; Varma, A.K.; Sahu, S.; Akbar, M.A. Impact of applying hygienic practices at farm on bacteriological quality of raw milk. Vet. World 2014, 7, 754–758. [Google Scholar] [CrossRef][Green Version]
- Coorevits, A.N.; De Jonghe, V.; Vandroemme, J.; Reekmans, R.; Heyrman, J.; Messens, W.; De Vos, P.; Heyndrickx, M. Comparative analysis of the diversity of aerobic spore-forming bacteria in raw milk from organic and conventional dairy farms. Syst. Appl. Microbiol. 2008, 31, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Caro, I.; García-Armesto, M.R. Occurrence of Shiga toxin-producing Escherichia coli in a Spanish raw ewe’s milk cheese. Int. J. Food Microbiol. 2007, 116, 410–413. [Google Scholar] [CrossRef]
- Odonkor, S.T.; Ampofo, J.K. Escherichia coli as an indicator of bacteriological quality of water: An overview. Microbiol. Res. 2013, 4, e2. [Google Scholar] [CrossRef]
- Dontorou, C.; Papadopoulou, C.; Filioussis, G.; Economou, V.; Apostolou, I.; Zakkas, G.; Salamoura, A.; Kansouzidou, A.; Levidiotou, S. Isolation of Escherichia coli O157: H7 from foods in Greece. Int. J. Food Microbiol. 2003, 82, 273–279. [Google Scholar] [CrossRef]
- Caro, I.; Fernández-Barata, V.M.; Alonso-Llamazares, A.; García-Armesto, M.R. Detection, occurrence, and characterization of Escherichia coli O157: H7 from raw ewe’s milk in Spain. J. Food Prot. 2006, 69, 920–924. [Google Scholar] [CrossRef]
- Condoleo, R.; Palumbo, R.; Mezher, Z.; Bucchini, L.; Taylor, R.A. Microbial risk assessment of Escherichia coli shiga-toxin producers (STEC) in raw sheep’s milk cheeses in Italy. Food Control 2022, 137, 108951. [Google Scholar] [CrossRef]
- Park, Y.W.; Albenzio, M.; Sevi, A.; Haenlein, G.F.W. Milk quality standards and controls. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; Park, Y.W., Haenlein, G.F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 261–287. [Google Scholar]
- Samelis, J.; Lianou, A.; Kakouri, A.; Delbes, C.; Rogelj, I.; Bogovič-Matijašić, B.; Montel, M.C. Changes in the microbial composition of raw milk induced by thermization treatments applied prior to traditional Greek hard cheese processing. J. Food Prot. 2009, 72, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, G.; Ponte, M.; Greco, C.; Barbera, M.; Mammano, M.M.; Fascella, G.; Greco, G.; Salsi, G.; Orlando, S.; Alfonzo, A.; et al. Improvement of Fresh Ovine “Tuma” Cheese Quality Characteristics by Application of Oregano Essential Oils. Antioxidants 2023, 12, 1293. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, E.; Settanni, L.; Cologna, N.; Cavazza, A.; Poznanski, E. Microbial analysis of raw cows’ milk used for cheese-making: Influence of storage treatments on microbial composition and other technological traits. World J. Microbiol. Biotechnol. 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Moghaddam, N.R.; Jafari, S.M. Pasteurization in the food industry. In Thermal Processing of Food Products by Steam and Hot Water; Jafari, S.M., Ed.; Woodhead Publishing: Cambridge, UK, 2023; pp. 247–273. [Google Scholar]
- Grappin, R.; Beuvier, E. Possible implications of milk pasteurization on the manufacture and sensory quality of ripened cheese. Int. Dairy J. 1997, 7, 751–761. [Google Scholar] [CrossRef]
- Martin, N.H.; Boor, K.J.; Wiedmann, M. Symposium review: Effect of post-pasteurization contamination on fluid milk quality. J. Dairy Sci. 2018, 101, 861–870. [Google Scholar] [CrossRef]
- Parente, E.; Cogan, T.M.; Powell, I.B. Starter cultures: General aspects. In Cheese; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: Cambridge, UK, 2017; pp. 201–226. [Google Scholar]
- Güler-Akin, M.B. The effects of different incubation temperatures on the acetaldehyde content and viable bacteria counts of bio-yogurt made from ewe’s milk. Int. J. Dairy Technol. 2005, 58, 174–179. [Google Scholar] [CrossRef]
- Shazly, A.B.; Khattab, M.S.; Fouad, M.T.; Abd El Tawab, A.M.; Saudi, E.M.; El-Aziz, M.A. Probiotic Yoghurt Made from Milk of Ewes Fed a Diet Supplemented with Spirulina platensis or Fish Oil. Ann. Microbiol. 2022, 72, 29. [Google Scholar] [CrossRef]
- Birollo, G.A.; Reinheimer, J.A.; Vinderola, C.G. Viability of lactic acid microflora in different types of yoghurt. Food Res. Int. 2000, 33, 799–805. [Google Scholar] [CrossRef]
- Gannuscio, R.; Ponte, M.; Di Grigoli, A.; Maniaci, G.; Di Trana, A.; Bacchi, M.; Alabiso, M.; Bonanno, A.; Todaro, M. Feeding dairy ewes with fresh or dehydrated sulla (Sulla coronarium L.) Forage. 1. Effects on feed utilization, milk production, and oxidative status. Animals 2022, 12, 2317. [Google Scholar] [CrossRef]
- Todaro, M.; Bonanno, A.; Scatassa, M.L. The quality of Valle del Belice sheep’s milk and cheese produced in the hot summer season in Sicily. Dairy Sci. Technol. 2014, 94, 225–239. [Google Scholar] [CrossRef][Green Version]
- Ghiselli, A.; Natella, F.; Guidi, A.; Montanari, L.; Fantozzi, P.; Scaccini, C. Beer increases plasma antioxidant capacity in humans. J. Nutr. Biochem. 2000, 11, 76–80. [Google Scholar] [CrossRef]
- Jaster, H.; Arend, G.D.; Rezzadori, K.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C. Enhancement of antioxidant activity and physicochemical properties of yogurt enriched with concentrated strawberry pulp obtained by block freeze concentration. Food Res. Int. 2018, 104, 119–125. [Google Scholar] [CrossRef]
- Guz, E.A.; Novitskaya, E.G.; Kalenik, T.K.; Levochkina, L.V.; Piekoszewski, W. The influence of vegetable puree containing carotenoids on the nutrient composition and structure of milk yoghurt. Int. J. Dairy Technol. 2018, 71, 89–95. [Google Scholar] [CrossRef]
- Park, Y.W.; Nam, M.S. Bioactive peptides in Milk and dairy products: A review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Songisepp, E.; Kulisaar, T.; Hütt, P.; Elias, P.; Brilene, T.; Zilmer, M.; Mikelsaar, M. A new probiotic cheese with antioxidative and antimicrobial. J. Dairy Sci. 2004, 87, 17–23. [Google Scholar] [CrossRef]
- Zulueta, A.; Maurizi, A.; Frígola, A.; Esteve, M.J.; Coli, R.; Burini, G. Antioxidant capacity of cow milk, whey and deproteinized milk. Int. Dairy J. 2009, 19, 380–385. [Google Scholar] [CrossRef]
- Di Trana, A.; Bonanno, A.; Cecchini, S.; Giorgio, D.; Di Grigoli, A.; Claps, S. Effects of Sulla forage (Sulla coronarium L.) on the oxidative status and milk polyphenol content in goats. J. Dairy Sci. 2015, 98, 37–46. [Google Scholar] [CrossRef]
- De Carvalho, M.W.; Arriola, N.D.A.; Pinto, S.S.; Verruk, S.; Fritzen-Freire, C.B.; Prudencio, E.S.; Amboni, R.D.D.C. Stevia-fortified yoghurt: Stability, antioxidant activity and in vitro digestion behavior. Int. J. Dairy Technol. 2019, 72, 57–64. [Google Scholar] [CrossRef]
- Anwar, F.; Hussain, A.I.; Iqbal, S.; Bhanger, M.I. Enhancement of the oxidative stability of some vegetable oils by blending with Moringa oleifera oil. Food Chem. 2007, 103, 81–91. [Google Scholar] [CrossRef]
- Akal, C.; Yetisemiyen, A. Use of whey powder and skim milk powder for the production of fermented cream. Food Sci. Technol. 2016, 36, 616–621. [Google Scholar] [CrossRef][Green Version]
- Okullo, J.B.L.; Omujal, F.; Agea, J.G.; Vuzi, P.C.; Namutebi, A.; Okello, J.B.A.; Nyanzi, S.A. Physico-chemical characteristics of Shea butter (Vitellaria paradoxa C.F. Gaertn.) oil from the Shea districts of Uganda. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 2070–2084. [Google Scholar] [CrossRef]
- Nadeem, M.; Abdullah, M.; Hussain, I.; Inayat, S.; Javid, A.; Zahoor, Y. Antioxidant potential of Moringa oleifera leaf extract for the stabilisation of butter at refrigeration temperature. Czech J. Food Sci. 2013, 31, 2–9. [Google Scholar] [CrossRef]
- Aktar, T. Physicochemical and sensory characterisation of different yoghurt production methods. Int. Dairy J. 2022, 125, 105245. [Google Scholar] [CrossRef]
- Garofalo, G.; Busetta, G.; Maniaci, G.; Sardina, M.T.; Portolano, B.; Badalamenti, N.; Maggio, A.; Bruno, M.; Gaglio, R.; Settanni, L. Development of “Quadrello di Ovino”, a novel fresh ewe’s cheese. Foods 2021, 11, 25. [Google Scholar] [CrossRef]
- El Zubeir, I.E.M.; Basher, M.A.E.; Alameen, M.H.; Mohammed, M.A.S.; Shuiep, E.S. The processing properties, chemical characteristics and acceptability of yoghurt made from non bovine milks. Livest. Res. Rural. Dev. 2012, 24, 3. [Google Scholar]
- Hutkins, R.W. Microbiology and Technology of Fermented Foods; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bonanno, A.; Di Grigoli, A.; Todaro, M.; Alabiso, M.; Vitale, F.; Di Trana, A.; Giorgio, D.; Settanni, L.; Gaglio, R.; Laddomada, B.; et al. Improvement of oxidative status, milk and cheese production, and food sustainability indexes by addition of durum wheat bran to dairy cows’ diet. Animals 2019, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Raynal-Ljutovac, K.; Lagriffoul, G.; Paccard, P.; Guillet, I.; Chilliard, Y. Composition of goat and sheep milk products: An update. Small Rumin. Res. 2008, 79, 57–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).