Preparation, Structural Characterization, and Stability of Low-Molecular-Weight Collagen Peptides–Calcium Chelate Derived from Tuna Bones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of TBCPLMW
2.3. Preparation of TBCPLMW-Ca
2.4. Amino Acid Composition Analysis
2.5. Molecular Weight Distribution
2.6. Structural Characterization
2.6.1. Fluorescence Spectroscopy Determination
2.6.2. Ultraviolet Spectroscopic Determination
2.6.3. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.6.4. Circular Dichroism (CD) Spectroscopy
2.7. Particle Size Distribution and Zeta Potential Analysis
2.8. Scanning Electron Microscopy
2.9. Stability Analysis of TBCPLMW-Ca
2.9.1. Acid–Base Stability
2.9.2. Thermal Stability
2.9.3. Simulated Digestion In Vitro
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation of TBCPLMW-Ca
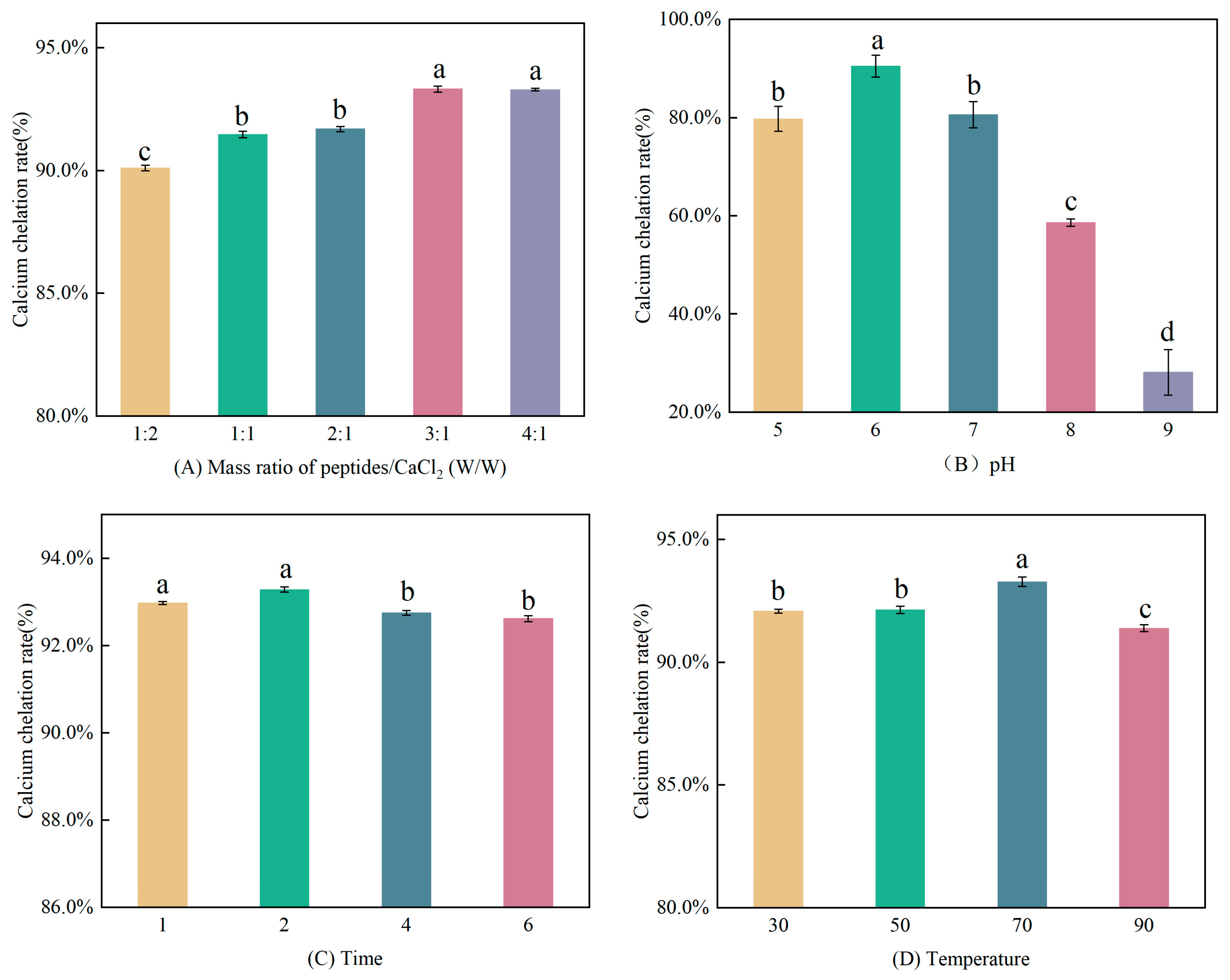
3.1.1. Single Factor Experiments
3.1.2. Response Surface Optimization
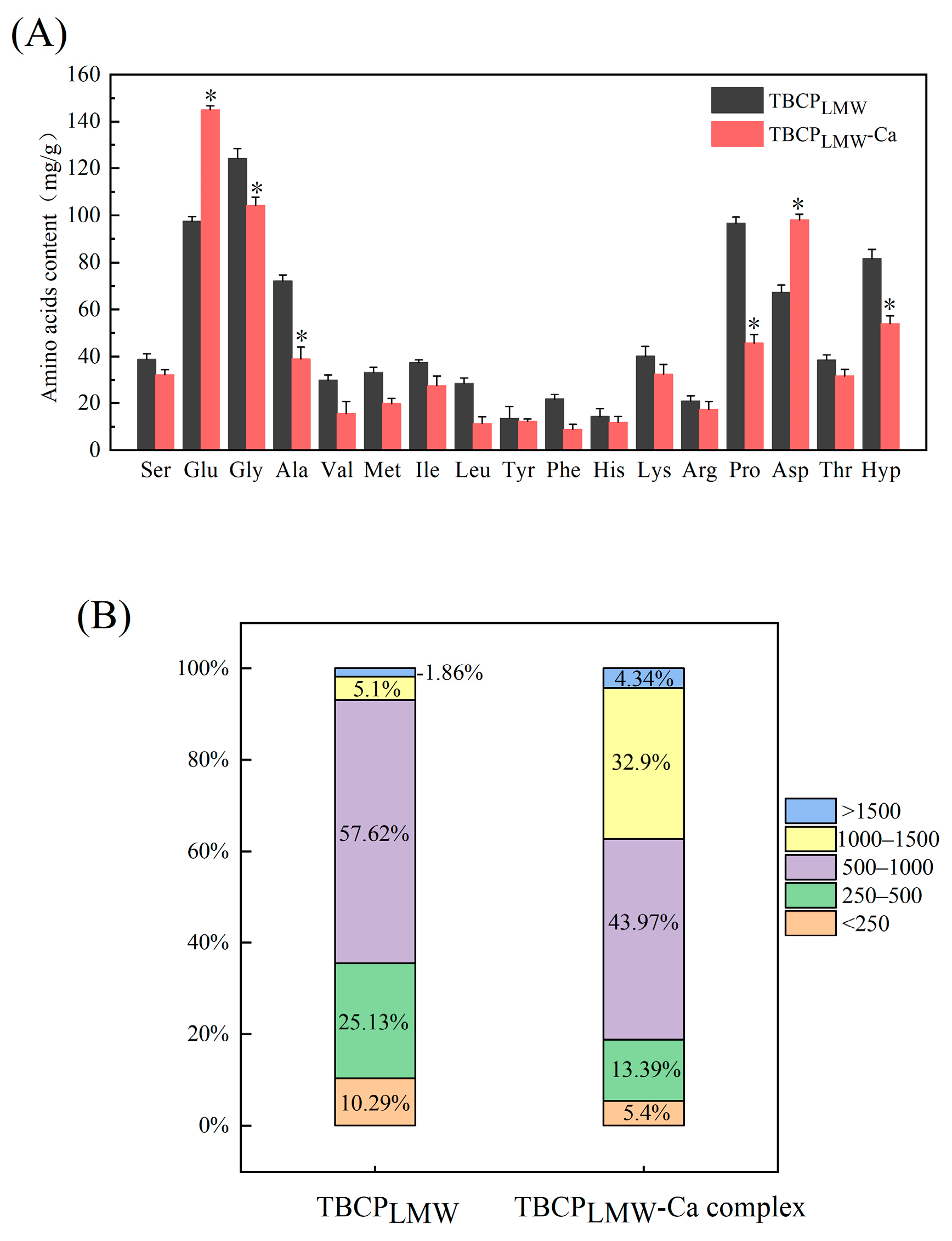
3.2. Amino Acid Composition
3.3. Molecular Weight Distribution
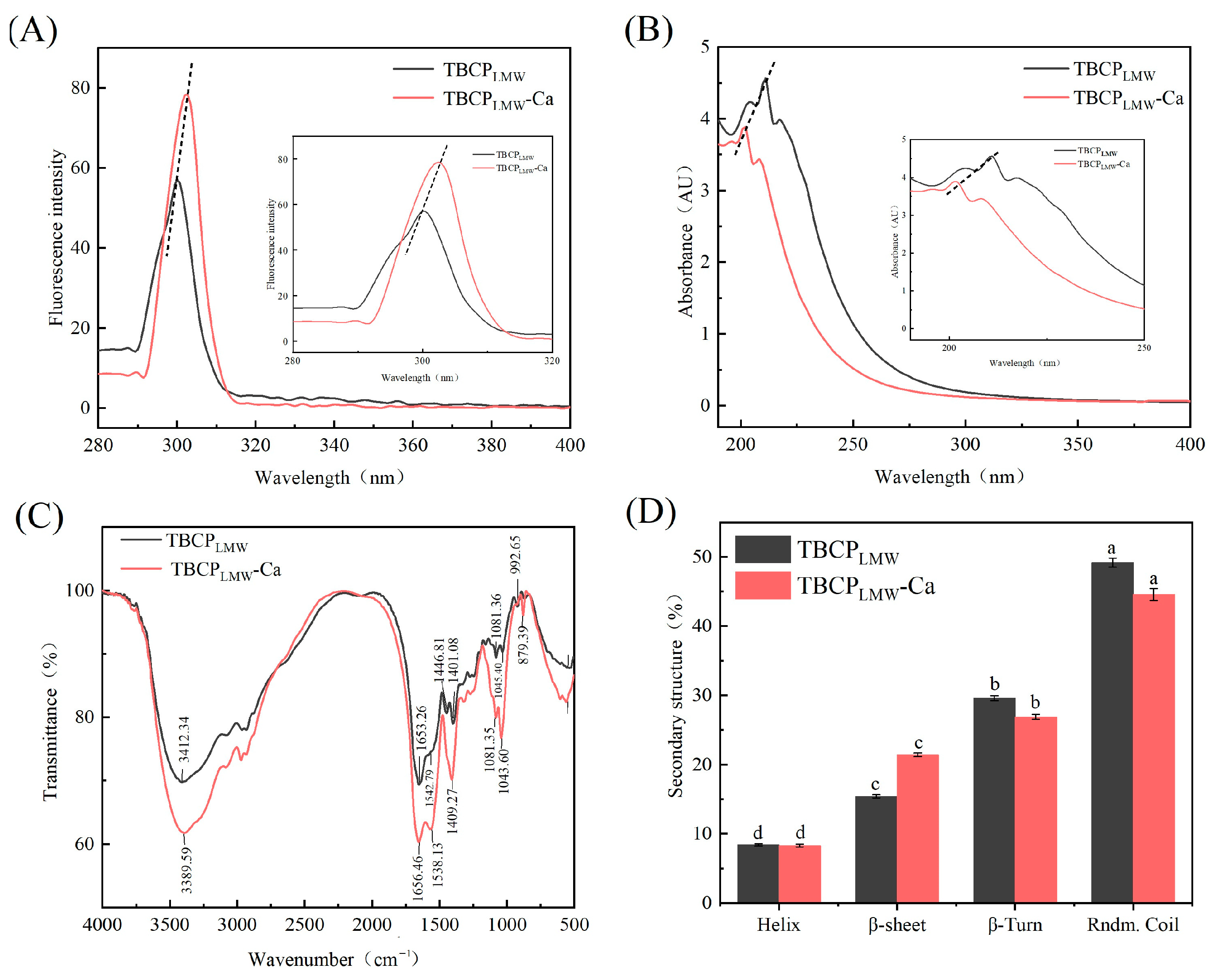
3.4. Structural Characterization
3.4.1. Fluorescence Spectroscopy
3.4.2. UV–Vis Absorption Spectroscopy Assay
3.4.3. Fourier-Transform Infrared Spectroscopy
3.4.4. Circular Dichroism
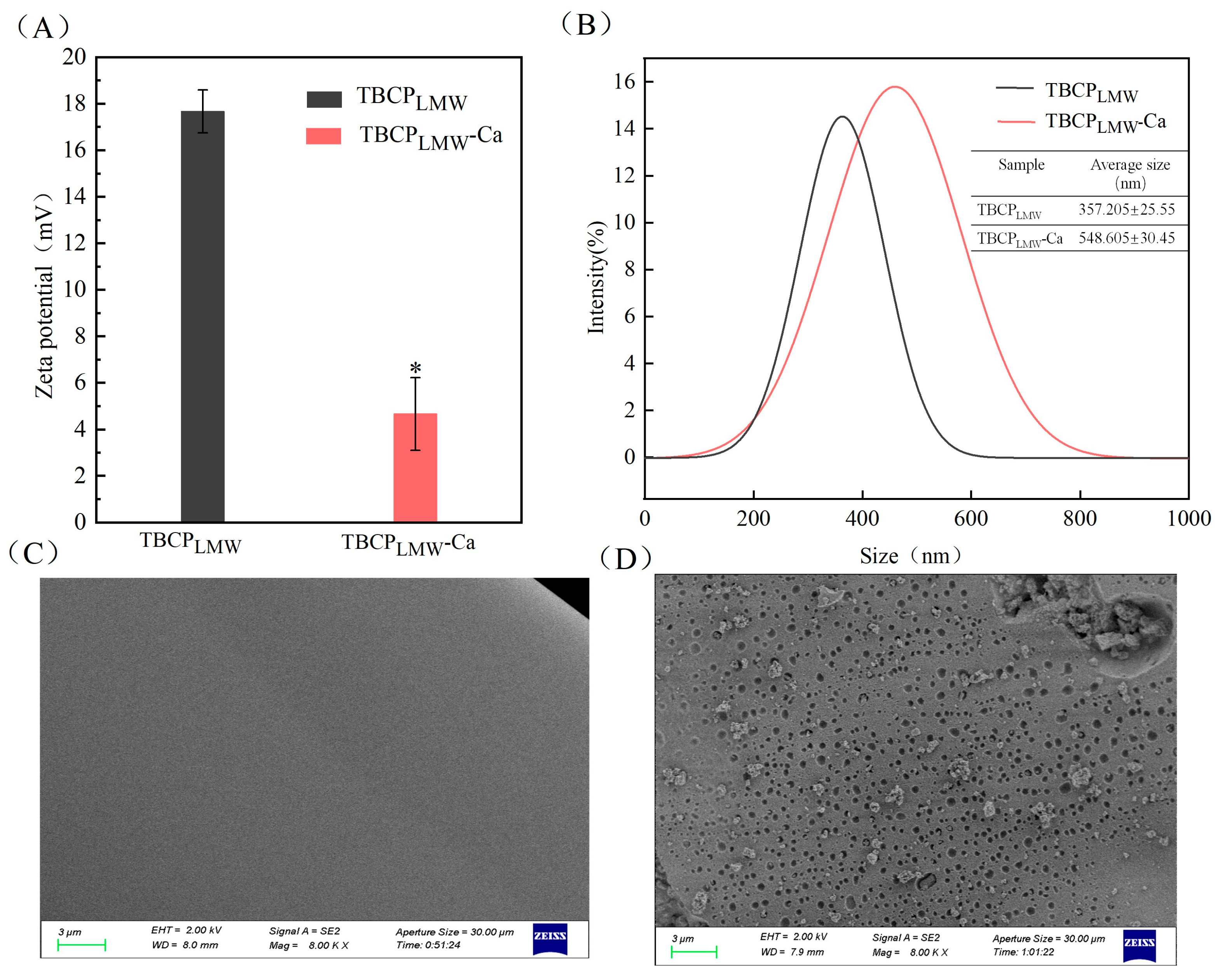
3.5. Zeta Potential and Particle Size Distribution
3.6. Scanning Electron Microscope
3.7. Stability
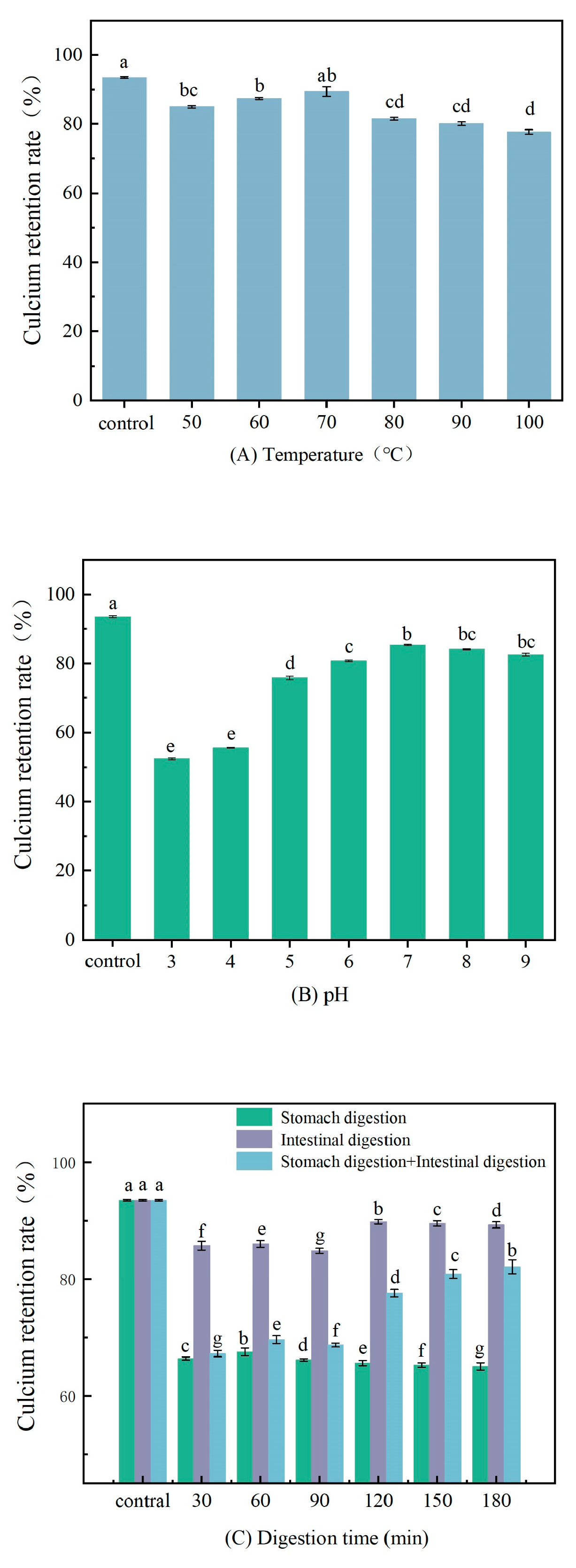
3.7.1. pH and Temperature
3.7.2. In Vitro Digestion Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhe, P.; Hou, H.; Zhang, K.; Li, B.F. Effect of Calcium-Binding Peptide from Pacific Cod (Gadus Macrocephalus) Bone on Calcium Bioavailability in Rats. Food Chem. 2017, 221, 373–378. [Google Scholar]
- Shaalan, A.A.M.; El-Sherbiny, M.; El-Abaseri, T.B.; Shoaeir, M.Z.; Abdel-Aziz, T.M.; Mohamed, M.I.; Zaitone, S.A.; Mohammad, H.M.F. Supplement with Calcium or Alendronate Suppresses Osteopenia Due to Long Term Rabeprazole Treatment in Female Mice: Influence on Bone Trap and Osteopontin Levels. Front. Pharmacol. 2020, 11, 583. [Google Scholar] [CrossRef]
- Wang, G.; Liu, L.J.; Wang, Z.P.; Pei, X.; Tao, W.J.; Xiao, Z.P.; Liu, B.J.; Wang, M.Q.; Lin, G.; Ao, T.Y. Comparison of Inorganic and Organically Bound Trace Minerals on Tissue Mineral Deposition and Fecal Excretion in Broiler Breeders. Biol. Trace Elem. Res. 2019, 189, 224–232. [Google Scholar] [CrossRef] [PubMed]
- An, J.L.; Zhang, Y.X.; Ying, Z.W.; Li, H.; Liu, W.L.; Wang, J.R.; Liu, X.Q. The Formation, Structural Characteristics, Absorption Pathways and Bioavailability of Calcium-Peptide Chelates. Foods 2022, 11, 2762. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Q.; Zhou, Z.S.; Yao, X.T.; Fu, Y. Mineral-Chelating Peptides Derived from Fish Collagen: Preparation, Bioactivity and Bioavailability. LWT Food Sci. Technol. 2020, 134, 110209. [Google Scholar] [CrossRef]
- Luo, J.Q.; Yao, X.T.; Soladoye, O.P.; Zhang, Y.H.; Fu, Y. Phosphorylation modification of collagen peptides from fish bone enhances their calcium-chelating and antioxidant activity. LWT Food Sci. Technol. 2022, 155, 112978. [Google Scholar] [CrossRef]
- Luo, X.L.; Liu, W.X.; Zhao, M.J.; Liu, T.; Xiong, F.F.; Lei, L.; Jia, F.H.; Feng, F.Q. A Novel Atlantic Salmon (Salmo salar) Bone Collagen Peptide Delays Osteoarthritis Development by Inhibiting Cartilage Matrix Degradation and Anti-Inflammatory. Food Res. Int. 2022, 162, 112148. [Google Scholar] [CrossRef]
- Moety, G.A.F.A.; Ali, A.M.; Fouad, R.; Ramadan, W.; Belal, D.S.; Haggag, H.M. Amino Acid Chelated Iron Versus an Iron Salt in the Treatment of Iron Deficiency Anemia with Pregnancy: A Randomized Controlled Study. Eur. J. Gynecol. Reprod. Biol. 2017, 210, 242–246. [Google Scholar] [CrossRef]
- Pawlos, M.; Znamirowska, A.; Zagua, G.; Buniowska, M. Use of Calcium Amino Acid Chelate in the Production of Acid-Curd Goat Cheese. Foods 2020, 9, 994. [Google Scholar] [CrossRef]
- Guo, L.D.; Harnedy, P.A.; Li, B.F.; Hou, H.; Zhang, Z.H.; Zhao, X.; FitzGerald, R.J. Food Protein-Derived Chelating Peptides: Biofunctional Ingredients for Dietary Mineral Bioavailability Enhancement. Trends Food Sci. Technol. 2014, 37, 92–105. [Google Scholar] [CrossRef]
- Guo, L.D.; Harnedy, P.A.; O’Keeffe, M.B.; Zhang, L.; Li, B.F.; Hou, H.; FitzGerald, R.J. Fractionation and Identification of Alaska Pollock Skin Collagen-Derived Mineral Chelating Peptides. Food Chem. 2015, 173, 536–542. [Google Scholar] [CrossRef]
- Cai, X.X.; Lin, J.P.; Wang, S.Y. Novel Peptide with Specific Calcium-Binding Capacity from Schizochytrium Sp. Protein Hydrolysates and Calcium Bioavailability in Caco-2 Cells. Mar. Drugs 2016, 15, 3. [Google Scholar] [CrossRef]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A Review on Recent Advances and Applications of Fish Collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef]
- Wu, J.H.; Cai, X.X.; Tang, M.R.; Wang, S.Y. Novel Calcium-Chelating Peptides from Octopus Scraps and Their Corresponding Calcium Bioavailability. J. Sci. Food Agric. 2019, 99, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.B.; Lin, S.Y.; Han, W.W.; Jiang, P.F.; Zhu, B.W.; Sun, N. Calcium Delivery System Assembled by a Nanostructured Peptide Derived from the Sea Cucumber Ovum. J. Agric. Food Chem. 2019, 67, 12283–12292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, J.W.; Hou, H.; Zhang, H.W.; Li, B.F. Purification and Characterization of a Novel Calcium-Biding Decapeptide from Pacific Cod (Gadus macrocephalus) Bone: Molecular Properties and Calcium Chelating Modes. J. Funct. Foods 2019, 52, 670–679. [Google Scholar] [CrossRef]
- Teng, H.; Qian, Y.W.; Fan, X.Y.; Cao, H.; Tian, Y.T.; Chen, L. Nutritional Properties of Europen Eel (Anguilla anguilla) Bone Peptide-Calcium and Its Apoptosis Effect on Caco-2 Cells. Food Sci. Hum. Well. 2022, 11, 1482–1490. [Google Scholar] [CrossRef]
- He, J.L.; Guo, H.; Zhang, M.; Wang, M.; Sun, L.P.; Zhuang, Y.L. Purification and Characterization of a Novel Calcium-Binding Heptapeptide from the Hydrolysate of Tilapia Bone with Its Osteogenic Activity. Foods 2022, 11, 468. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, X.J.; Hao, G.X.; Zhang, M.; Weng, W.Y. Preparation and Bioavailability of Calcium-Chelating Peptide Complex from Tilapia Skin Hydrolysates. J. Sci. Food Agric. 2017, 97, 4898–4903. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.K.; Zhu, X.; Li, Q.Q.; Fan, Y.; Cheng, D.; Li, B.F. A Novel Calcium-Binding Peptide from Antarctic Krill Protein Hydrolysates and Identification of Binding Sites of Calcium-Peptide Complex. Food Chem. 2018, 243, 389–395. [Google Scholar] [CrossRef]
- Cui, Y.W.; Yang, L.H.; Lu, W.B.; Yang, H.C.; Zhang, Y.Q.; Zhou, X.M.; Ma, Y.J.; Feng, J.L.; Shen, Q. Effect of Steam Explosion Pretreatment on the Production of Microscale Tuna Bone Power by Ultra-Speed Pulverization. Food Chem. 2021, 347, 129011. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Mad-Ali, S.; Senphan, T.; Sookchoo, P. Biocalcium Powder from Precooked Skipjack Tuna Bone: Production and Its Characteristics. J. Food Biochem. 2017, 41, e12412. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.X.; Bao, Z.J.; Cui, P.B.; Wang, S.; Lin, S.Y. Calcium Binding to Herring Egg Phosphopeptides: Binding Characteristics, Conformational Structure and Intermolecular Forces. Food Chem. 2020, 310, 125867. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, B.F.; Chen, Q.R.; Zhang, Z.H.; Zhao, X.; Hou, H. Functional Calcium Binding Peptides from Pacific Cod (Gadus macrocephalus) Bone: Calcium Bioavailability Enhancing Activity and Anti-Osteoporosis Effects in the Ovariectomy-Induced Osteoporosis Rat Model. Nutrients 2018, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Aenglong, C.; Ngasakul, N.; Limpawattana, M.; Sukketsiri, W.; Chockchaisawasdee, S.; Stathopoulos, C.; Tanasawet, S.; Klaypradit, W. Characterization of Novel Calcium Compounds from Tilapia (Oreochromis niloticus) by-Products and Their Effects on Proliferation and Differentiation of MC3T3-E1 Cells. J. Funct. Foods 2023, 100, 105361. [Google Scholar] [CrossRef]
- Zhang, H.R.; Qi, L.W.; Wang, X.D.; Guo, Y.J.; Liu, J.Q.; Xu, Y.; Liu, C.J.; Zhang, C.H.; Richel, A. Preparation of a Cattle Bone Collagen Peptide–Calcium Chelate by the Ultrasound Method and Its Structural Characterization, Stability Analysis, and Bioactivity on MC3T3-E1 Cells. Food Funct. 2023, 14, 978–989. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, Z.; Xu, H.Y.; Li, X.Y.; Hao, X.D. Preparation of Sheep Bone Collagen Peptide-Calcium Chelate Using Enzymolysis-Fermentation Methodology and Its Structural Characterization and Stability Analysis. RSC Adv. 2020, 10, 11624–11633. [Google Scholar] [CrossRef]
- Wu, H.H.; Liu, Z.Y.; Zhao, Y.H.; Zeng, M.Y. Enzymatic Preparation and Characterization of Iron-Chelating Peptides from Anchovy (Engraulis japonicus) Muscle Protein. Food Res. Int. 2012, 48, 435–441. [Google Scholar] [CrossRef]
- Wu, W.M.; He, L.C.; Liang, Y.H.; Yue, L.L.; Peng, W.M.; Jin, G.F.; Ma, M.H. Preparation Process Optimization of Pig Bone Collagen Peptide-Calcium Chelate Using Response Surface Methodology and Its Structural Characterization and Stability Analysis. Food Chem. 2019, 284, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.; Osatomi, K.; Yoshida, A.; Osako, K.; Yamaguchi, A.; Hara, K. Biochemical Properties of Acid-Soluble Collagens Extracted from the Skins of Underutilised Fishes. Food Chem. 2008, 108, 49–54. [Google Scholar] [CrossRef]
- Huang, W.; Lan, Y.Q.; Liao, W.W.; Lin, L.; Liu, G.; Xu, H.M.; Xue, J.P.; Guo, B.Y.; Cao, Y.; Miao, J.Y. Preparation, Characterization and Biological Activities of Egg White Peptides-Calcium Chelate. LWT Food Sci. Technol. 2021, 149, 112035. [Google Scholar] [CrossRef]
- Lin, S.T.; Hu, X.; Li, L.H.; Yang, X.Q.; Chen, S.J.; Wu, Y.Y.; Yang, S.L. Preparation, Purification and Identification of Iron-Chelating Peptides Derived from Tilapia (Oreochromis niloticus) Skin Collagen and Characterization of the Peptide-Iron Complexes. LWT Food Sci. Technol. 2021, 149, 111796. [Google Scholar] [CrossRef]
- Zhang, H.R.; Zhao, L.Y.; Shen, Q.S.; Qi, L.W.; Jiang, S.; Guo, Y.J.; Zhang, C.H.; Richel, A. Preparation of Cattle Bone Collagen Peptides-Calcium Chelate and Its Structural Characterization and Stability. LWT Food Sci. Technol. 2021, 144, 111264. [Google Scholar] [CrossRef]
- Lin, Y.L.; Cai, X.X.; Wu, X.P.; Lin, S.Q.; Wang, S.Y. Fabrication of Snapper Fish Scales Protein Hydrolysate-Calcium Complex and the Promotion in Calcium Cellular Uptake. J. Funct. Foods 2020, 65, 103717. [Google Scholar] [CrossRef]
- Yang, L.H.; Guo, Z.L.; Wei, J.P.; Han, L.; Yu, Q.L.; Chen, H.Y.; Chen, Y.X.; Zhang, W. Extraction of Low Molecular Weight Peptides from Bovine Bone Using Ultrasound-Assisted Double Enzyme Hydrolysis: Impact on the Antioxidant Activities of the Extracted Peptides. LWT Food Sci. Technol. 2021, 146, 111470. [Google Scholar] [CrossRef]
- Wang, L.; Ding, Y.Y.; Zhang, X.X.; Li, Y.F.; Wang, R.; Luo, X.H.; Li, Y.N.; Li, J.; Chen, Z.X. Isolation of a Novel Calcium-Binding Peptide from Wheat Germ Protein Hydrolysates and the Prediction for Its Mechanism of Combination. Food Chem. 2018, 239, 416–426. [Google Scholar] [CrossRef]
- Wang, L.; Ding, Y.Y.; Zhang, X.X.; Li, Y.F.; Wang, R.; Luo, X.H.; Li, Y.N.; Li, J.; Chen, Z.X. Effect of Electron Beam on the Functional Properties and Structure of Defatted Wheat Germ Proteins. J. Food Eng. 2017, 202, 9–17. [Google Scholar] [CrossRef]
- Liu, G.; Sun, S.W.; Guo, B.Y.; Miao, B.C.; Luo, Z.; Xia, Z.M.; Ying, D.Y.; Liu, F.; Guo, B.; Tang, J.; et al. Bioactive Peptide Isolated from Casein Phosphopeptides Promotes Calcium Uptake In Vitro and In Vivo. Food Funct. 2018, 9, 2251–2260. [Google Scholar] [CrossRef]
- Nara, M.; Morii, H.; Tanokura, M. Coordination to Divalent Cations by Calcium-Binding Proteins Studied by FTIR Spectroscopy. Biochim. Biophys. Acta 2013, 1828, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, A.; Chen, Y.; Zhang, X.Y.; Li, S.H.; Chen, Y. Preparation of Cucumber Seed Peptide-Calcium Chelate by Liquid State Fermentation and Its Characterization. Food Chem. 2017, 229, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.H.; Goto, Y.J.; Réfrégiers, M.; Kardos, J. Accurate Secondary Structure Prediction and Fold Recognition for Circular Dichroism Spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, X.J.; Yagoub, A.G.; Chen, L.; Wahia, H.; Osae, R.; Zhou, C.S. Structure and Stability of Low Molecular Weight Collagen Peptide (Prepared from White Carp Skin)—Calcium Complex. LWT Food Sci. Technol. 2021, 136, 110335. [Google Scholar] [CrossRef]
- Hu, G.H.; Wang, D.B.; Sun, L.N.; Su, R.N.; Corazzin, M.; Sun, X.Y.; Dou, L.; Zhang, M.; Zhao, L.H.; Su, L.; et al. Isolation, Purification and Structure Identification of a Calcium-Binding Peptide from Sheep Bone Protein Hydrolysate. Foods 2022, 11, 2655. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.W.; Liu, S.J.; Liu, X.R.; Duan, S.; Xiao, S.Y.; Yang, Z.N.; Cao, Y.; Miao, J.Y. The Purification, Identification and Bioactivity Study of a Novel Calcium-Binding Peptide from Casein Hydrolysate. Foods 2019, 10, 7724–7732. [Google Scholar] [CrossRef]
- Ke, H.L.; Ma, R.J.; Liu, X.Y.; Xie, Y.P.; Chen, J.F. Highly Effective Peptide-Calcium Chelate Prepared from Aquatic Products Processing Wastes: Stickwater and Oyster Shells. LWT Food Sci. Technol. 2022, 168, 113947. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Wang, B.; Xie, N.N. Degradation and Antioxidant Activities of Peptides and Zinc-Peptide Complexes during In Vitro Gastrointestinal Digestion. Food Chem. 2015, 173, 733–740. [Google Scholar] [CrossRef]
| Levels | Factors | |||
|---|---|---|---|---|
| A: Peptide/Calcium Mass Ratio | B: pH | C: Time (h) | D: Temperature (°C) | |
| −1 | 2.5:1 | 5.0 | 1.5 | 60 |
| 0 | 3.0:1 | 6.0 | 2 | 70 |
| 1 | 3.5:1 | 7.0 | 2.5 | 80 |
| A: Peptide/Calcium Mass Ratio | B: pH | C: Time (h) | D: Temperature (°C) | Calcium-Chelating Capacity (%) | |
|---|---|---|---|---|---|
| 1 | 2.50 | 5.00 | 2.00 | 70.00 | 82.31 |
| 2 | 3.50 | 5.00 | 2.00 | 70.00 | 84.88 |
| 3 | 2.50 | 7.00 | 2.00 | 70.00 | 86.35 |
| 4 | 3.50 | 7.00 | 2.00 | 70.00 | 89.26 |
| 5 | 3.00 | 6.00 | 1.50 | 60.00 | 90.56 |
| 6 | 3.00 | 6.00 | 2.50 | 60.00 | 91.78 |
| 7 | 3.00 | 6.00 | 1.50 | 80.00 | 89.35 |
| 8 | 3.00 | 6.00 | 2.50 | 80.00 | 90.14 |
| 9 | 2.50 | 6.00 | 2.00 | 60.00 | 90.59 |
| 10 | 3.50 | 6.00 | 2.00 | 60.00 | 92.27 |
| 11 | 2.50 | 6.00 | 2.00 | 80.00 | 89.48 |
| 12 | 3.50 | 6.00 | 2.00 | 80.00 | 90.09 |
| 13 | 3.00 | 5.00 | 1.50 | 70.00 | 84.26 |
| 14 | 3.00 | 7.00 | 1.50 | 70.00 | 88.07 |
| 15 | 3.00 | 5.00 | 2.50 | 70.00 | 83.19 |
| 16 | 3.00 | 7.00 | 2.50 | 70.00 | 87.42 |
| 17 | 2.50 | 6.00 | 1.50 | 70.00 | 90.54 |
| 18 | 3.50 | 6.00 | 1.50 | 70.00 | 92.43 |
| 19 | 2.50 | 6.00 | 2.50 | 70.00 | 91.76 |
| 20 | 3.50 | 6.00 | 2.50 | 70.00 | 93.18 |
| 21 | 3.00 | 5.00 | 2.00 | 60.00 | 82.93 |
| 22 | 3.00 | 7.00 | 2.00 | 60.00 | 86.31 |
| 23 | 3.00 | 5.00 | 2.00 | 80.00 | 80.79 |
| 24 | 3.00 | 7.00 | 2.00 | 80.00 | 85.22 |
| 25 | 3.00 | 6.00 | 2.00 | 70.00 | 94.26 |
| 26 | 3.00 | 6.00 | 2.00 | 70.00 | 94.54 |
| 27 | 3.00 | 6.00 | 2.00 | 70.00 | 94.01 |
| 28 | 3.00 | 6.00 | 2.00 | 70.00 | 93.85 |
| 29 | 3.00 | 6.00 | 2.00 | 70.00 | 93.73 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value (Prob > F) | Significant |
|---|---|---|---|---|---|---|
| Models | 447.41 | 14 | 31.96 | 103.68 | <0.0001 | ** |
| A | 12.16 | 1 | 12.16 | 39.45 | <0.0001 | ** |
| B | 49.09 | 1 | 49.09 | 159.25 | <0.0001 | ** |
| C | 0.43 | 1 | 0.43 | 1.38 | 0.2596 | |
| D | 5.84 | 1 | 5.84 | 18.94 | 0.0007 | ** |
| AB | 0.029 | 1 | 0.029 | 0.094 | 0.7640 | |
| AC | 0.055 | 1 | 0.055 | 0.18 | 0.6785 | |
| AD | 0.001 | 1 | 0.001 | 0.004 | 0.9506 | |
| BC | 0.044 | 1 | 0.044 | 0.14 | 0.7109 | |
| BD | 0.28 | 1 | 0.28 | 0.89 | 0.3604 | |
| CD | 0.046 | 1 | 0.046 | 0.15 | 0.7044 | |
| A2 | 4.81 | 1 | 4.81 | 15.62 | 0.0014 | ** |
| B2 | 365.38 | 1 | 365.38 | 1185.35 | <0,0001 | ** |
| C2 | 7.07 | 1 | 7.07 | 22.94 | 0.0003 | ** |
| D2 | 42.68 | 1 | 42.68 | 138.48 | <0.0001 | ** |
| Residual | 4.32 | 14 | 0.31 | |||
| Lack of Fit | 3.89 | 10 | 0.39 | 3.67 | 0.1110 | Not significant |
| Pure Error | 0.42 | 4 | 0.11 | |||
| Cor Total | 451.73 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Zhou, Y.; Ma, M.; Zhao, Y.; Xiang, X.; Shu, C.; Zheng, B. Preparation, Structural Characterization, and Stability of Low-Molecular-Weight Collagen Peptides–Calcium Chelate Derived from Tuna Bones. Foods 2023, 12, 3403. https://doi.org/10.3390/foods12183403
Zhong Y, Zhou Y, Ma M, Zhao Y, Xiang X, Shu C, Zheng B. Preparation, Structural Characterization, and Stability of Low-Molecular-Weight Collagen Peptides–Calcium Chelate Derived from Tuna Bones. Foods. 2023; 12(18):3403. https://doi.org/10.3390/foods12183403
Chicago/Turabian StyleZhong, Yaqi, Yufang Zhou, Mingzhu Ma, Yadong Zhao, Xingwei Xiang, Conghan Shu, and Bin Zheng. 2023. "Preparation, Structural Characterization, and Stability of Low-Molecular-Weight Collagen Peptides–Calcium Chelate Derived from Tuna Bones" Foods 12, no. 18: 3403. https://doi.org/10.3390/foods12183403
APA StyleZhong, Y., Zhou, Y., Ma, M., Zhao, Y., Xiang, X., Shu, C., & Zheng, B. (2023). Preparation, Structural Characterization, and Stability of Low-Molecular-Weight Collagen Peptides–Calcium Chelate Derived from Tuna Bones. Foods, 12(18), 3403. https://doi.org/10.3390/foods12183403






