Effect of Different Roughage Sources in Fermented Total Mixed Ration and Energy Intake on Meat Quality, Collagen Solubility, Troponin T Degradation, and Fatty Acids of Native Thai Cattle Longissimus Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Experimental FTMR, Animals, Design, and Data Collection
2.3. Meat Quality Analysis
2.4. Collagen Analysis
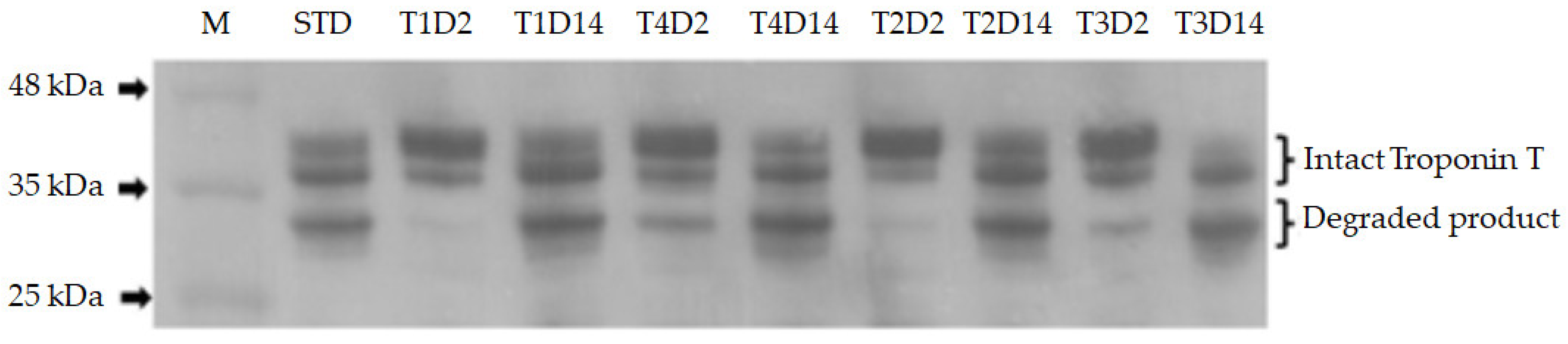
2.5. Troponin T Degradation Analysis
2.6. Fatty Acids Analysis
2.7. Statistical Analysis
3. Results
3.1. Meat Quality
3.2. Collagen Solubility
3.3. Troponin T Degradation
3.4. Fatty Acid Composition
4. Discussion
4.1. Meat Quality
4.2. Collagen Solubility
4.3. Troponin T Degradation
4.4. Fatty Acid Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Trade Negotiations. Beef and Beef Products. Available online: https://api.dtn.go.th/files/v3/613b2770ef41401f0822c2dc/download (accessed on 2 March 2022).
- Department of Livestock Development. Number of Livestock in Thailand. 2020. Available online: https://docimage.dld.go.th/FILEROOM/CABDLD_BOOKSHELF2/DRAWER26/GENERAL/DATA0000/00000082.PDF (accessed on 20 March 2022).
- Saithong, S.; Chatchawan, T.; Boonyanuwat, K. Thai indigenous cattle production provide a sustainable alternative for the benefit of small scale farmers, healthy food, and the environment. BAHGI e-J. 2011, 1, 21–26. [Google Scholar]
- Sethakul, J.; Opatpatanakit, Y.; Sivapirunthep, P.; Intrapornudom, P. Beef quality under production systems in Thailand: Preliminary Remarks. In Proceedings of the 13th AAAP Animal Science Congress, Hanoi, Vietnam, 22–26 September 2008. [Google Scholar]
- Duanyai, S.; Duanyai, S.; Tanasunthonsut, W.; Suwannee, P. Natural Beef Production; Research Report Project Code RDG5120012; Thailand Research Fund (TRF): Bangkok, Thailand, 2009. [Google Scholar]
- Somboonpanyakul, P. Nutritional value of beef. In Value of Thai Beef; Sethakul, J., Sivapirunthep, P., Eds.; Amarin Printing: Bangkok, Thailand, 2009; pp. 35–44. (In Thai) [Google Scholar]
- Chaokaur, A.; Nishida, T.; Phaowphaisal, I.; Sommart, K. Effects of feeding level on methane emissions and energy utilization of Brahman cattle in the tropics. Agric. Ecosyst. Environ. 2015, 199, 225–230. [Google Scholar] [CrossRef]
- Ogino, A.; Sommart, K.; Subepang, S.; Mitsumori, M.; Hayashi, K.; Yamashita, T.; Tanaka, Y. Environmental impacts of extensive and intensive beef production systems in Thailand evaluated by life cycle assessment. J. Clean. Prod. 2016, 112, 22–31. [Google Scholar]
- Kongphitee, K.; Sommart, K.; Phonbumrung, T.; Gunha, T.; Suzuki, T. Feed intake, digestibility and energy partitioning in beef cattle fed diets with cassava pulp instead of rice straw. Asian-Australas. J. Anim. Sci. 2018, 31, 1431. [Google Scholar] [CrossRef]
- Subepang, S.; Suzuki, T.; Phonbumrung, T.; Sommart, K. Enteric methane emissions, energy partitioning, and energetic efficiency of zebu beef cattle fed total mixed ration silage. Asian-Australas. J. Anim. Sci. 2019, 32, 548. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Joo, S.-T. Fatty acid profiles, meat quality, and sensory palatability of grain-fed and grass-fed beef from Hanwoo, American, and Australian crossbred cattle. Korean J. Food Sci. Anim. Resour. 2017, 37, 153. [Google Scholar] [CrossRef]
- Aquino, D.; Del Barrio, A.; Trach, N.X.; Hai, N.T.; Khang, D.N.; Toan, N.T.; Van Hung, N. Rice straw-based fodder for ruminants. In Sustainable Rice Straw Management; Springer: Cham, Switzerland, 2020; pp. 111–129. [Google Scholar]
- Kiyothong, K. Manual for Planting Napier Pakchong 1; The Department of Livestock Development: Bangkok, Thailand, 2014.
- Turano, B.; Tiwari, U.P.; Jha, R. Growth and nutritional evaluation of napier grass hybrids as forage for ruminants. Trop. Grassl.-Forrajes Trop. 2016, 4, 168–178. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Wu, F.; Qiu, X.; Yu, Z.; Niu, W.; He, Y.; Su, H.; Cao, B. Effects of dietary energy on growth performance, rumen fermentation and bacterial community, and meat quality of Holstein-Friesians bulls slaughtered at different ages. Animals 2019, 9, 1123. [Google Scholar] [CrossRef]
- Keller, M.; Kreuzer, M.; Reidy, B.; Scheurer, A.; Guggenbühl, B.; Luder, M.; Frank, J.; Giller, K. Effects on performance, carcass and meat quality of replacing maize silage and concentrate by grass silage and corn-cob mix in the diet of growing bulls. Meat Sci. 2022, 188, 108795. [Google Scholar]
- Zhu, X.; Liu, B.; Xiao, J.; Guo, M.; Zhao, S.; Hu, M.; Cui, Y.; Li, D.; Wang, C.; Ma, S. Effects of different roughage diets on fattening performance, meat quality, fatty acid composition, and rumen microbe in steers. Front. Nutr. 2022, 9, 885069. [Google Scholar]
- Nishino, N.; Harada, H.; Sakaguchi, E. Evaluation of fermentation and aerobic stability of wet brewers’ grains ensiled alone or in combination with various feeds as a total mixed ration. J. Sci. Food Agric. 2003, 83, 557–563. [Google Scholar]
- Li, Y.; Wang, F.; Nishino, N. Lactic acid bacteria in total mixed ration silage containing soybean curd residue: Their isolation, identification and ability to inhibit aerobic deterioration. Asian-Australas. J. Anim. Sci. 2016, 29, 516. [Google Scholar] [PubMed]
- Gunha, T.; Kongphitee, K.; Sommart, K. Feed intake, digestibility, growth performances and eating behavior of native Thai beef cattle fed diets differing in energy density using cassava pulp with rice straw. In Proceedings of the 1st International Conference on Tropical Animal Science and Production, Bangkok, Thailand, 26–29 July 2016; pp. 112–115. [Google Scholar]
- Kongphitee, K.; Sommart, K. Ensilage quality, digestibility and enteric methane emission of the fermented total mixed ration in Thai native beef cattle. In Proceedings of the 1st International Conference on Tropical Animal Science and Production, Bangkok, Thailand, 26–29 July 2016; pp. 116–120. [Google Scholar]
- Kim, M.; Choe, J.; Lee, H.J.; Yoon, Y.; Yoon, S.; Jo, C. Effects of aging and aging method on physicochemical and sensory traits of different beef cuts. Food Sci. Anim. Resour. 2019, 39, 54. [Google Scholar]
- Ho, C.; Stromer, M.; Robson, R. Identification of the 30 kDa polypeptide in post mortem skeletal muscle as a degradation product of troponin-T. Biochimie 1994, 76, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Koohmaraie, M.; Geesink, G. Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci. 2006, 74, 34–43. [Google Scholar]
- Bhat, Z.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Role of calpain system in meat tenderness: A review. Food Sci. Hum. Wellness 2018, 7, 196–204. [Google Scholar]
- Nishimura, T. Role of extracellular matrix in development of skeletal muscle and postmortem aging of meat. Meat Sci. 2015, 109, 48–55. [Google Scholar]
- Modzelewska-Kapituła, M.; Nogalski, Z.; Kwiatkowska, A. The influence of crossbreeding on collagen solubility and tenderness of Infraspinatus and Semimembranosus muscles of semi-intensively reared young bulls. Anim. Sci. J. 2016, 87, 1312–1321. [Google Scholar] [CrossRef]
- Purslow, P.P. New developments on the role of intramuscular connective tissue in meat toughness. Annu. Rev. Food Sci. Technol. 2014, 5, 133–153. [Google Scholar]
- Gilmore, L.A.; Walzem, R.L.; Crouse, S.F.; Smith, D.R.; Adams, T.H.; Vaidyanathan, V.; Cao, X.; Smith, S.B. Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high-and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. J. Nutr. 2011, 141, 1188–1194. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Goswami, S.; Mondal, S.A.; Dutta, D. Dietary fat, salt, and sugar: A clinical perspective of the social catastrophe. In Dietary Sugar, Salt and Fat in Human Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–91. [Google Scholar]
- Sommart, K.; Tangjitwattanachai, N.; Nitipot, P.; Tumdee, A.; Chokcharoen, S. Feed Innovation and Feeding for High Quality Beef Cattle; Research report project code RDG5820025; Thailand Research Fund (TRF): Bangkok, Thailand, 2017. [Google Scholar]
- The Working Committee of Thai Feeding Standard for Ruminant. Nutrient Requirement of Beef Cattle in Indochinese Peninsula; Klungnanavithaya Press: Khon Kaen, Thailand, 2010.
- Chaosap, C.; Sitthigripong, R.; Sivapirunthep, P.; Pungsuk, A.; Adeyemi, K.D.; Sazili, A.Q. Myosin heavy chain isoforms expression, calpain system and quality characteristics of different muscles in goats. Food Chem. 2020, 321, 126677. [Google Scholar] [PubMed]
- Chaosap, C.; Sivapirunthep, P.; Takeungwongtrakul, S.; Zulkifli, R.B.M.; Sazili, A.Q. Effects of Zn-L-Selenomethionine on Carcass Composition, Meat Characteristics, Fatty Acid Composition, Glutathione Peroxidase Activity, and Ribonucleotide Content in Broiler Chickens. Food Sci. Anim. Resour. 2020, 40, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Polyorach, S.; Lukkananukool, A.; Sommart, K.; Chaosap, C. Effects of fermented total mixed ration (FTMR) using rice straw and napier Pachong1 as a roughage sources on carcass quality of Thai native beef cattle. In Proceedings of the 6th Meat Science Technoly Congress, Bangkok, Thailand, 18–19 June 2018. [Google Scholar]
- Mancini, R.; Hunt, M. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [PubMed]
- Suman, S.P.; Joseph, P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar]
- O’Sullivan, A.; O’Sullivan, K.; Galvin, K.; Moloney, A.; Troy, D.; Kerry, J. Grass silage versus maize silage effects on retail packaged beef quality. J. Anim. Sci. 2002, 80, 1556–1563. [Google Scholar] [CrossRef][Green Version]
- Ku, M.J.; Mamuad, L.; Nam, K.C.; Cho, Y.I.; Kim, S.H.; Choi, Y.S.; Lee, S.S. The effects of total mixed ration feeding with high roughage content on growth performance, carcass characteristics, and meat quality of Hanwoo steers. Food Sci. Anim. Resour. 2021, 41, 45. [Google Scholar]
- Kang, D.H.; Chung, K.Y.; Park, B.H.; Kim, U.H.; Jang, S.S.; Smith, Z.K.; Kim, J. Effects of feeding high-energy diet on growth performance, blood parameters, and carcass traits in Hanwoo steers. Anim. Biosci. 2022, 35, 1545. [Google Scholar]
- Hughes, J.M.; Clarke, F.M.; Purslow, P.P.; Warner, R.D. Meat color is determined not only by chromatic heme pigments but also by the physical structure and achromatic light scattering properties of the muscle. Compr. Rev. Food Sci. Food Saf. 2020, 19, 44–63. [Google Scholar]
- Huff-Lonergan, E. Fresh meat water-holding capacity. In Improving the Sensory and Nutritional Quality of Fresh Meat; Woodhead Publishing: Cambridge, UK, 2009; pp. 147–160. [Google Scholar]
- He, L.; Yang, J.; Chen, W.; Zhou, Z.; Wu, H.; Meng, Q. Growth performance, carcass trait, meat quality and oxidative stability of beef cattle offered alternative silages in a finishing ration. Animal 2018, 12, 657–666. [Google Scholar] [CrossRef]
- Kim, C.J.; Lee, E.S. Effects of quality grade on the chemical, physical and sensory characteristics of Hanwoo (Korean native cattle) beef. Meat Sci. 2003, 63, 397–405. [Google Scholar] [CrossRef]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and Flavor Chemistry Characteristics of Australian Beef: Influence of Intramuscular Fat, Feed, and Breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef] [PubMed]
- Weatherly, B.H.; Lorenzen, C.L.; Savell, J.W. Determining optimal aging times for beef subprimals. J. Anim. Sci. 1998, 76 (Suppl. S1), 598. [Google Scholar]
- Li, L.; Zhu, Y.; Wang, X.; He, Y.; Cao, B. Effects of different dietary energy and protein levels and sex on growth performance, carcass characteristics and meat quality of F1 Angus × Chinese Xiangxi yellow cattle. J. Anim. Sci. Biotechnol. 2014, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Han, Z.; Arbab, A.A.I.; Yang, Y.; Yang, Z. Effect of Aging Time on Meat Quality of Longissimus Dorsi from Yunling Cattle: A New Hybrid Beef Cattle. Animals 2020, 10, 1897. [Google Scholar] [CrossRef]
- Schonfeldt, H.; Strydom, P. Effect of age and cut on cooking loss, juiciness and flavour of South African beef. Meat Sci. 2011, 87, 180–190. [Google Scholar] [CrossRef]
- Vaskoska, R.; Ha, M.; Naqvi, Z.B.; White, J.D.; Warner, R.D. Muscle, Ageing and Temperature Influence the Changes in Texture, Cooking Loss and Shrinkage of Cooked Beef. Foods 2020, 9, 1289. [Google Scholar] [CrossRef]
- Jayasooriya, S.D.; Torley, P.J.; D’Arcy, B.R.; Bhandari, B.R. Effect of high power ultrasound and ageing on the physical properties of bovine Semitendinosus and Longissimus muscles. Meat Sci. 2007, 75, 628–639. [Google Scholar] [CrossRef]
- Martinez, H.A.; Miller, R.K.; Kerth, C.; Wasser, B.E. Prediction of beef tenderness and juiciness using consumer and descriptive sensory attributes. Meat Sci. 2023, 205, 109292. [Google Scholar] [CrossRef]
- Aberle, E.D.; Reeves, E.S.; Judge, M.D.; Hunsley, R.E.; Perry, T.W. Palatability and Muscle Characteristics of Cattle with Controlled Weight Gain: Time on a High Energy Diet. J. Anim. Sci. 1981, 52, 757–763. [Google Scholar] [CrossRef]
- Chaosap, C.; Lukkananukool, A.; Polyorach, S.; Sommart, K.; Sivapirunthep, P.; Limsupavanich, R. Effects of Dietary Energy Density in a Fermented Total Mixed Ration Formulated with Different Ratios of Rice Straw and Cassava Pulp on 2- or 14-Day-Aged Meat Quality, Collagen, Fatty Acids, and Ribonucleotides of Native Thai Cattle Longissimus Muscle. Foods 2022, 11, 2046. [Google Scholar] [CrossRef]
- Silva, C.; Rego, O.; Simões, E.; Rosa, H. Consumption of high energy maize diets is associated with increased soluble collagen in muscle of Holstein bulls. Meat Sci. 2010, 86, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczak, B.; Iwańska, E.; Spychaj, A.; Danyluk, B.; Montowska, M.; Grześ, B.; Banach, J.K.; Żywica, R.; Pospiech, E. An analysis of the influence of various tenderising treatments on the tenderness of meat from Polish Holstein-Friesian bulls and the course of changes in collagen. Meat Sci. 2019, 158, 107906. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P. Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Nonsee, M.; Lukkananukool, A.; Polyorach, S.; Sommart, K.; Sazili, A.Q.; Chaosap, C. Degradation of troponin-T associated with calpain/calpastatin genes expression in native Thai beef cattle fed different levels of energy. Int. J. Agric. Technol. 2018, 14, 1525–1534. [Google Scholar]
- Steen, D.; Claeys, E.; Uytterhaegen, L.; De Smet, S.; Demeyer, D. Early post-mortem conditions and the calpain/calpastatin system in relation to tenderness of double-muscled beef. Meat Sci. 1997, 45, 307–319. [Google Scholar] [CrossRef]
- Blank, C.P.; Russell, J.; Lonergan, S.M.; Hansen, S.L. Influence of feed efficiency classification and growing and finishing diet type on meat tenderness attributes of beef steers1. J. Anim. Sci. 2017, 95, 2986–2992. [Google Scholar] [CrossRef]
- McDonagh, M.B.; Herd, R.M.; Richardson, E.C.; Oddy, V.H.; Archer, J.A.; Arthur, P.F. Meat quality and the calpain system of feedlot steers following a single generation of divergent selection for residual feed intake. Aust. J. Exp. Agric. 2001, 41, 1013–1021. [Google Scholar] [CrossRef]
- Randby, Å.; Aass, L.; Haug, A. Fatty acid profile and intramuscular fat concentration of Musculus longissimus thoracis in bulls fed grass silage harvested at one of three maturity stages, either with or without concentrate supplementation. Acta Agric. Scand. Sect. A—Anim. Sci. 2021, 70, 78–90. [Google Scholar] [CrossRef]
- De Smet, S.; Webb, E.; Claeys, E.; Uytterhaegen, L.; Demeyer, D. Effect of dietary energy and protein levels on fatty acid composition of intramuscular fat in double-muscled Belgian Blue bulls. Meat Sci. 2000, 56, 73–79. [Google Scholar] [CrossRef]
- Jaturasitha, S.; Norkeaw, R.; Vearasilp, T.; Wicke, M.; Kreuzer, M. Carcass and meat quality of Thai native cattle fattened on Guinea grass (Panicum maxima) or Guinea grass–legume (Stylosanthes guianensis) pastures. Meat Sci. 2009, 81, 155–162. [Google Scholar] [CrossRef]
- Chaiwang, N.; Jaturasitha, S.; Sringarm, K.; Wicke, M.; Kreuzer, M. Comparison of the Fatty Acid Profiles of the Meat of Crossbreds with 75% Charolais Blood Proportion and Thai Indigenous Upland Cattle. Chiang Mai Univ. J. Nat. Sci. 2015, 14, 199–205. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zuo, S.; Peng, S.; Wang, Z.; Zhang, Y.; Luo, H. Untargeted and Targeted Metabolomics Profiling of Muscle Reveals Enhanced Meat Quality in Artificial Pasture Grazing Tan Lambs via Rescheduling the Rumen Bacterial Community. J. Agric. Food Chem. 2021, 69, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Nogoy, K.M.; Sun, B.; Shin, S.; Lee, Y.; Li, X.; Choi, S.H.; Park, S. Fatty Acid Composition of Grain- and Grass-Fed Beef and Their Nutritional Value and Health Implication. Food Sci. Anim. Resour. 2022, 42, 18–33. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Jump, D.B.; Depner, C.M.; Tripathy, S. Omega-3 fatty acid supplementation and cardiovascular disease. J. Lipid Res. 2012, 53, 2525–2545. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; Matthan, N.R.; Lamon-Fava, S.; Lecker, J.L.; Lichtenstein, A.H. Reduction in dietary omega-6 polyunsaturated fatty acids: Eicosapentaenoic acid plus docosahexaenoic acid ratio minimizes atherosclerotic lesion formation and inflammatory response in the LDL receptor null mouse. Atherosclerosis 2009, 204, 147–155. [Google Scholar] [CrossRef]
| Trait | Roughage (R) | Energy Intake (E) | RMSE | p Value | |||
|---|---|---|---|---|---|---|---|
| NG | RS | 1.5M | ad lib | R | E | ||
| L* | 36.93 | 35.44 | 35.86 | 36.52 | 2.80 | 0.287 | 0.622 |
| a* | 16.72 | 17.05 | 17.01 | 16.76 | 1.75 | 0.691 | 0.763 |
| b* | 9.11 | 10.17 | 9.96 | 9.32 | 2.26 | 0.335 | 0.550 |
| Drip loss (%) | 1.87 | 1.59 | 2.09 | 1.37 | 0.47 | 0.285 | 0.017 |
| Trait | Roughage (R) | Energy Intake (E) | Aging (A) | RMSE | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NG | RS | 1.5M | ad lib | 2 days | 14 days | R | E | A | ||
| pH | 5.42 | 5.43 | 5.44 | 5.41 | 5.44 | 5.39 | 0.10 | 0.831 | 0.416 | 0.140 |
| Cooking loss (%) | 23.32 | 21.49 | 22.33 | 22.48 | 19.10 | 25.61 | 3.02 | 0.076 | 0.884 | <0.0001 |
| Shear force (kg) | 7.96 | 7.65 | 8.39 | 7.21 | 9.33 | 6.28 | 1.65 | 0.579 | 0.037 | <0.0001 |
| Collagen | Roughage (R) | Energy Intake (E) | Aging (A) | RMSE | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NG | RS | 1.5M | ad lib | 2 days | 14 days | R | E | A | ||
| Soluble | 0.22 | 0.21 | 0.21 | 0.23 | 0.20 | 0.24 | 0.09 | 0.710 | 0.488 | 0.176 |
| Insoluble | 2.91 | 3.10 | 3.23 | 2.78 | 3.10 | 2.91 | 0.77 | 0.462 | 0.096 | 0.478 |
| Total | 3.13 | 3.32 | 3.44 | 3.01 | 3.29 | 3.15 | 0.84 | 0.523 | 0.141 | 0.616 |
| Solubility (%) | 7.39 | 6.14 | 5.86 | 7.67 | 5.93 | 7.59 | 2.06 | 0.084 | 0.015 | 0.022 |
| Troponin T | Roughage (R) | Energy Intake (E) | Aging (A) | RMSE | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NG | RS | 1.5M | ad lib | 2 days | 14 days | R | E | A | ||
| Intact 1 | 0.07 | 0.06 | 0.06 | 0.07 | 0.06 | 0.06 | 0.02 | 0.297 | 0.171 | 0.774 |
| Degraded product 1 | 0.04 | 0.03 | 0.03 | 0.04 | 0.02 | 0.05 | 0.01 | 0.213 | 0.379 | <0.0001 |
| % Degraded product 2 | 32.66 | 31.06 | 33.23 | 30.48 | 21.65 | 42.07 | 5.67 | 0.452 | 0.203 | <0.0001 |
| Trait | Roughage (R) | Energy Intake (E) | RMSE | p Value | |||
|---|---|---|---|---|---|---|---|
| NG | RS | 1.5M | ad lib | R | E | ||
| Saturated fatty acid (SFA) | |||||||
| Capric acid (C10:0) | 8.18 | 5.89 | 6.46 | 7.61 | 3.50 | 0.247 | 0.553 |
| Lauric acid (C12:0) | 10.18 | 6.58 | 7.07 | 9.69 | 4.46 | 0.160 | 0.299 |
| Myristic acid (C14:0) | 184.21 | 163.03 | 135.85 | 181.36 | 42.91 | 0.047 | 0.075 |
| Pentadecylic acid (C15:0) | 15.15 | 8.12 | 10.87 | 12.40 | 4.10 | 0.008 | 0.505 |
| Palmitic acid (C16:0) | 901.45 | 818.50 | 723.33 | 996.62 | 294.45 | 0.608 | 0.114 |
| Margaric acid (C17:0) | 27.55 | 23.17 | 24.23 | 26.48 | 6.64 | 0.243 | 0.543 |
| Stearic acid (C18:0) | 385.85 | 282.91 | 327.96 | 340.80 | 59.93 | 0.008 | 0.699 |
| Arachidic (C20:0) | 2.40 | 1.55 | 1.95 | 2.01 | 0.55 | 0.015 | 0.856 |
| Monounsaturated fatty acid (MUFA) | |||||||
| Myristoleic acid (C14:1) | 60.05 | 53.60 | 47.79 | 65.86 | 55.53 | 0.526 | 0.097 |
| Pentadecenoic acid (C15:1) | 5.57 | 4.24 | 4.39 | 5.42 | 1.88 | 0.212 | 0.334 |
| Palmitoleic acid (C16:1) | 184.67 | 161.93 | 158.73 | 187.86 | 34.60 | 0.244 | 0.147 |
| Heptadecenoic acid (C17:1) | 19.39 | 18.60 | 18.77 | 19.22 | 2.83 | 0.612 | 0.778 |
| Oleic acid (C18:1n9c) | 1189.62 | 1029.70 | 1103.79 | 1115.52 | 131.91 | 0.044 | 0.872 |
| Erucic acid (C20:1n9) | 4.18 | 7.12 | 7.27 | 4.04 | 6.88 | 0.441 | 0.403 |
| Erucic acid (C22:1n9) | 19.02 | 16.39 | 19.27 | 16.14 | 6.99 | 0.497 | 0.424 |
| Nervonic acid (C24:1) | 4.09 | 4.11 | 4.52 | 3.69 | 2.14 | 0.988 | 0.488 |
| Polyunsaturated fatty acid (PUFA) | |||||||
| Linoleic acid (C18:2n6t) | 77.31 | 69.01 | 74.85 | 71.46 | 25.31 | 0.552 | 0.808 |
| α-Linolenic acid (C18:3n6) | 3.20 | 2.01 | 2.65 | 2.56 | 1.27 | 0.109 | 0.889 |
| Arachidic acid (C20:2) | 9.79 | 8.40 | 10.08 | 8.11 | 3.96 | 0.524 | 0.374 |
| Arachidonic acid (C20:4n6) | 4.49 | 2.56 | 3.84 | 3.30 | 1.50 | 0.044 | 0.517 |
| Docosahexaenoic acid (C22:6n3) | 10.62 | 16.71 | 13.45 | 13.87 | 4.81 | 0.037 | 0.874 |
| SFA | 1534.97 | 1279.74 | 1237.73 | 1576.98 | 362.13 | 0.214 | 0.111 |
| MUFA | 1486.60 | 1295.69 | 1364.55 | 1417.75 | 158.91 | 0.046 | 0.548 |
| PUFA | 105.41 | 98.79 | 104.89 | 99.30 | 34.98 | 0.730 | 0.773 |
| Total fatty acid | 3126.99 | 2674.23 | 2707.17 | 3094.04 | 451.74 | 0.088 | 0.141 |
| PUFA:SFA | 0.07 | 0.08 | 0.09 | 0.07 | 0.03 | 0.855 | 0.209 |
| ω 3 | 10.62 | 16.71 | 13.45 | 13.87 | 4.81 | 0.037 | 0.874 |
| ω 6 | 84.99 | 73.68 | 81.35 | 77.32 | 27.47 | 0.457 | 0.791 |
| ω 6:ω 3 | 8.50 | 4.45 | 6.41 | 6.55 | 1.74 | 0.001 | 0.888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukkananukool, A.; Polyorach, S.; Sommart, K.; Chaosap, C. Effect of Different Roughage Sources in Fermented Total Mixed Ration and Energy Intake on Meat Quality, Collagen Solubility, Troponin T Degradation, and Fatty Acids of Native Thai Cattle Longissimus Muscle. Foods 2023, 12, 3402. https://doi.org/10.3390/foods12183402
Lukkananukool A, Polyorach S, Sommart K, Chaosap C. Effect of Different Roughage Sources in Fermented Total Mixed Ration and Energy Intake on Meat Quality, Collagen Solubility, Troponin T Degradation, and Fatty Acids of Native Thai Cattle Longissimus Muscle. Foods. 2023; 12(18):3402. https://doi.org/10.3390/foods12183402
Chicago/Turabian StyleLukkananukool, Achara, Sineenart Polyorach, Kritapon Sommart, and Chanporn Chaosap. 2023. "Effect of Different Roughage Sources in Fermented Total Mixed Ration and Energy Intake on Meat Quality, Collagen Solubility, Troponin T Degradation, and Fatty Acids of Native Thai Cattle Longissimus Muscle" Foods 12, no. 18: 3402. https://doi.org/10.3390/foods12183402
APA StyleLukkananukool, A., Polyorach, S., Sommart, K., & Chaosap, C. (2023). Effect of Different Roughage Sources in Fermented Total Mixed Ration and Energy Intake on Meat Quality, Collagen Solubility, Troponin T Degradation, and Fatty Acids of Native Thai Cattle Longissimus Muscle. Foods, 12(18), 3402. https://doi.org/10.3390/foods12183402









