Dandelion (Taraxacum mongolicum) Extract Alleviated H2O2-Induced Oxidative Damage: The Underlying Mechanism Revealed by Metabolomics and Lipidomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DE
2.3. Composition Analysis of DE
2.4. Cell Viability Assay
2.5. Hoechst 33342 Staining
2.6. In Vitro Antioxidant Measurement
2.7. Quantitative Real-Time PCR (RT-PCR) Measurement
2.8. Metabolomics and Lipidomics Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. LC-Q-TOF/MS Characterization of DE Composition
3.2. Effects of DE on H2O2-Induced Cytotoxicity
3.3. Effects of DE on ROS Generation
3.4. Effects of DE on MMP
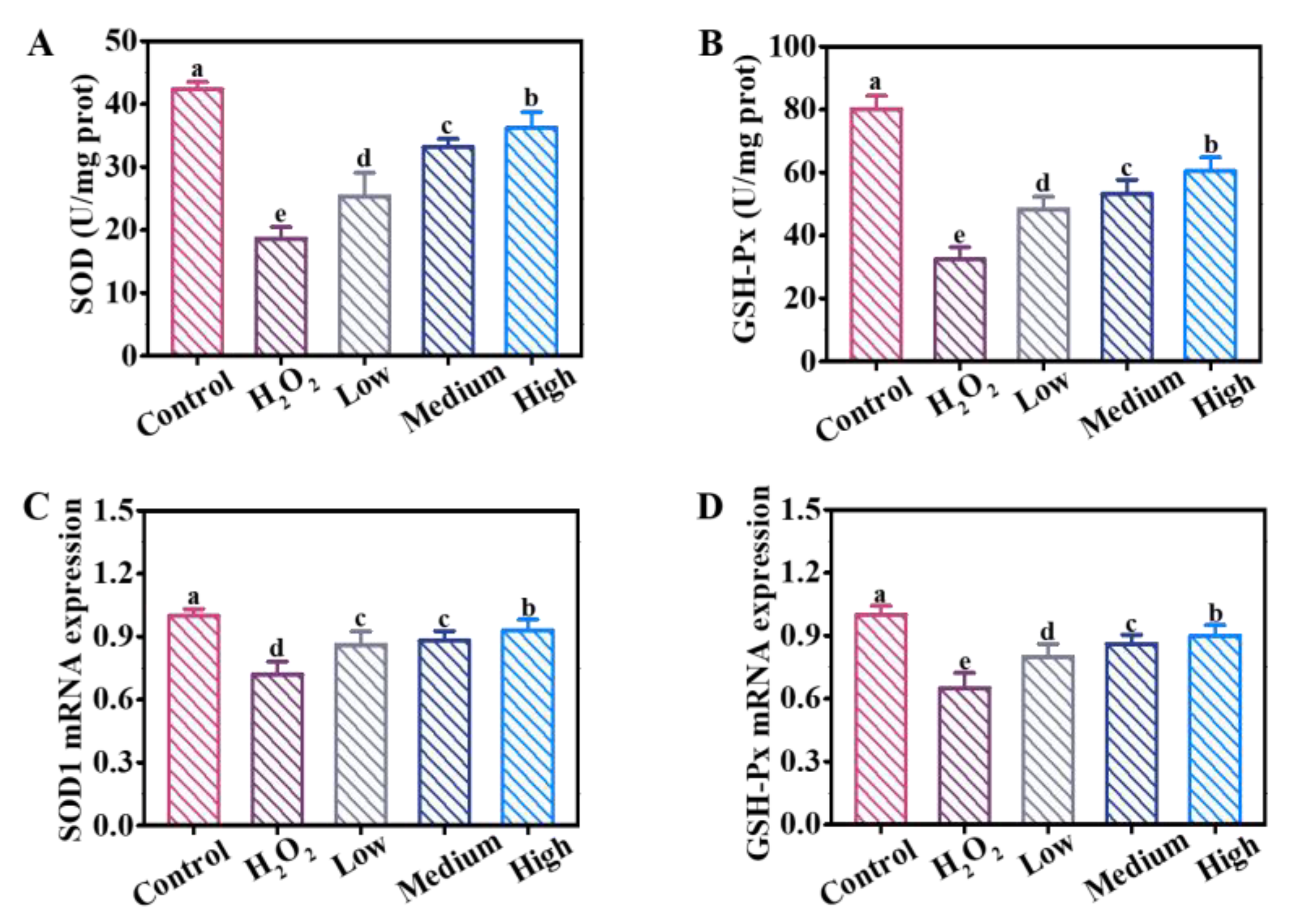
3.5. Effects of DE on Antioxidant-Related Enzyme Activities
3.6. Effects of DE on Metabolic Response
3.7. Effects of DE on Energy Metabolism
3.8. Effects of DE on Amino Acid Metabolism
3.9. Effects of DE on Other Metabolism Pathways
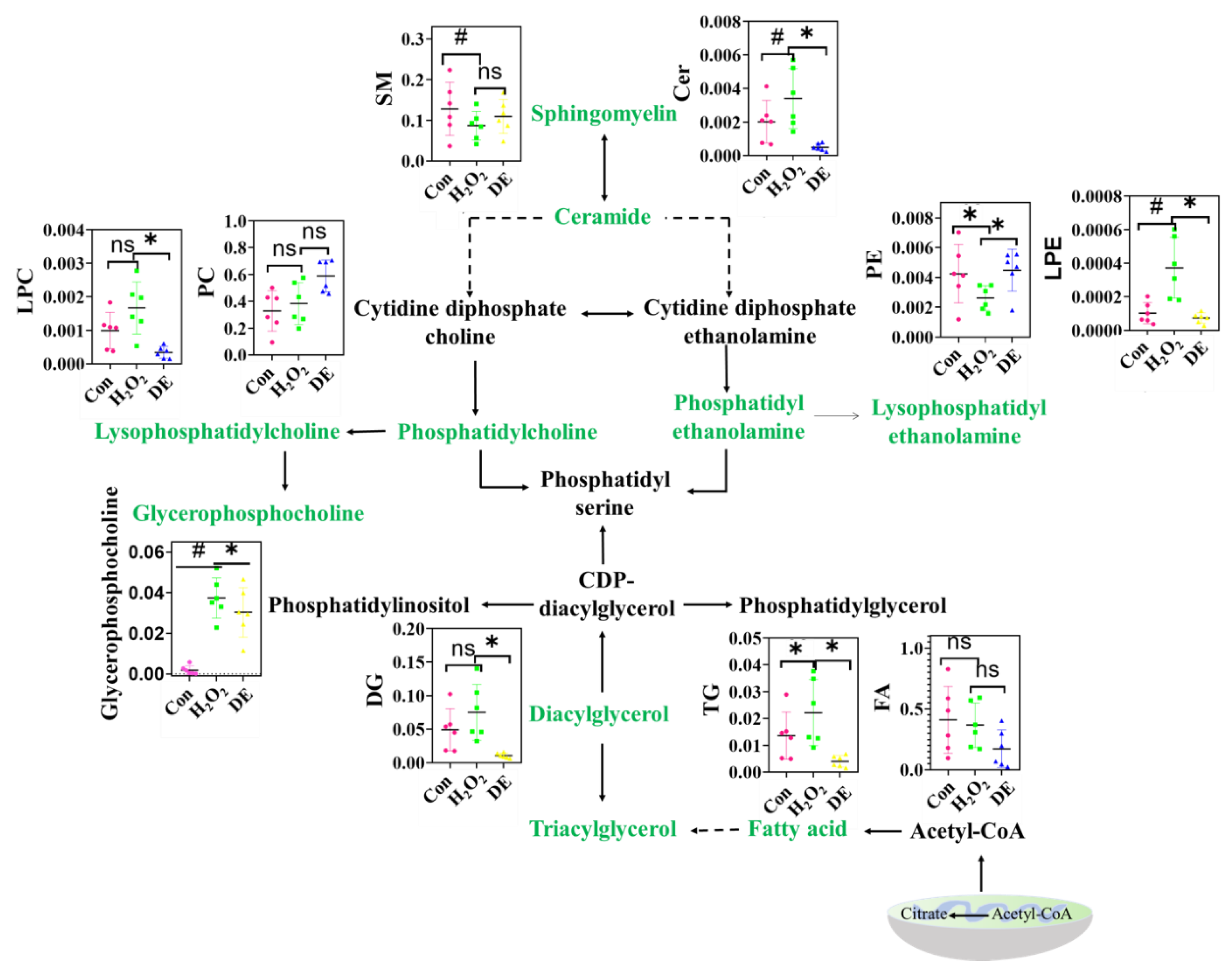
3.10. Effects of DE on Lipid Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lis, B.; Jedrejek, D.; Rywaniak, J.; Soluch, A.; Stochmal, A.; Olas, B. Flavonoid preparations from Taraxacum officinale L. fruits-A phytochemical, antioxidant and hemostasis studies. Molecules 2020, 25, 5402. [Google Scholar] [CrossRef]
- Grauso, L.; Emrick, S.; de Falco, B.; Lanzotti, V.; Bonanomi, G. Common dandelion: A review of its botanical, phytochemical and pharmacological profiles. Phytochem. Rev. 2019, 18, 1115–1132. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M.J. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Jedrejek, D.; Lis, B.; Rolnik, A.; Stochmal, A.; Olas, B. Comparative phytochemical, cytotoxicity, antioxidant and haemostatic studies of Taraxacum officinale root preparations. Food Chem. Toxicol. 2019, 126, 233–247. [Google Scholar] [CrossRef]
- Ge, B.J.; Zhao, P.; Li, H.T.; Sang, R.; Wang, M.; Zhou, H.Y.; Zhang, X.M. Taraxacum mongolicum protects against Staphylococcus aureus-infected mastitis by exerting anti-inflammatory role via TLR2-NF-kappaB/MAPKs pathways in mice. J. Ethnopharmacol. 2021, 268, 113595. [Google Scholar] [CrossRef]
- Kang, L.; Miao, M.S.; Song, Y.G.; Fang, X.Y.; Zhang, J.; Zhang, Y.N.; Miao, J.X. Total flavonoids of Taraxacum mongolicum inhibit non-small cell lung cancer by regulating immune function. J. Ethnopharmacol. 2021, 281, 114514. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, P.; Ying, J.; Dong, Z.; Chen, X.D. Mechanistic study on inhibition of porcine pancreatic alpha-amylase using the flavonoids from dandelion. Food Chem. 2021, 344, 128610. [Google Scholar] [CrossRef]
- Lis, B.; Rolnik, A.; Jedrejek, D.; Soluch, A.; Stochmal, A.; Olas, B. Dandelion (Taraxacum officinale L.) root components exhibit anti-oxidative and antiplatelet action in an in vitro study. J. Funct. Foods 2019, 59, 16–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.F.; Li, W.; Xu, G.Y.; Wang, K.R.; Li, L.; Luo, H.; Zou, L.; Wu, J.S. Updates and advances on pharmacological properties of Taraxacum mongolicum Hand.-Mazz and its potential applications. Food Chem. 2022, 373, 131380. [Google Scholar] [CrossRef]
- Jedrejek, D.; Kontek, B.; Lis, B.; Stochmal, A.; Olas, B. Evaluation of antioxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chem.-Biol. Interact. 2017, 262, 29–37. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Wang, Z.; Chen, J.; Yang, Y.; Dong, G. Dandelion extract alleviated lipopolysaccharide-induced oxidative stress through the Nrf2 pathway in bovine mammary epithelial cells. Toxins 2020, 12, 496. [Google Scholar] [CrossRef]
- Li, F.; Feng, K.L.; Yang, J.C.; He, Y.S.; Guo, H.; Wang, S.P.; Gan, R.Y.; Wu, D.T. Polysaccharides from dandelion (Taraxacum mongolicum) leaves: Insights into innovative drying techniques on their structural characteristics and biological activities. Int. J. Biol. Macromol. 2021, 167, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Abulikemu, A.; Lv, S.; Qi, Y.; Duan, J.; Zhang, J.; Chen, R.; Guo, C.; Li, Y.; Sun, Z. Oxidative stress- and mitochondrial dysfunction-mediated cytotoxicity by silica nanoparticle in lung epithelial cells from metabolomic perspective. Chemosphere 2021, 275, 129969. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Song, X.; Wang, Y.; Wang, J.; Su, S.; Zhu, J.; Geng, Y. Studies on protection of astaxanthin from oxidative damage induced by H2O2 in RAW 264.7 cells based on 1H NMR metabolomics. J. Agric. Food Chem. 2019, 67, 13568–13576. [Google Scholar] [CrossRef]
- Gupta, S.V.K.; Smith, P.M.C.; Natera, S.H.A.; Roessner, U. Biochemical changes in two barley genotypes inoculated with a beneficial fungus Trichoderma harzianum Rifai T-22 grown in saline soil. Front. Plant Sci. 2022, 13, 908853. [Google Scholar] [CrossRef]
- Chen, Y.; Su, W.; Tie, S.; Cui, W.; Yu, X.; Zhang, L.; Hua, Z.; Tan, M. Orally deliverable sequence-targeted astaxanthin nanoparticles for colitis alleviation. Biomaterials 2022, 293, 121976. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Q.; Song, S.; Wang, H.; Tan, M. Enhanced cytotoxicity of cadmium by a sulfated polysaccharide from abalone. J. Agric. Food Chem. 2020, 68, 14996–15004. [Google Scholar] [CrossRef]
- Qu, J.; Ke, F.; Liu, Z.; Yang, X.; Li, X.; Xu, H.; Li, Q.; Bi, K. Uncovering the mechanisms of dandelion against triple-negative breast cancer using a combined network pharmacology, molecular pharmacology and metabolomics approach. Phytomedicine 2022, 99, 153986. [Google Scholar] [CrossRef]
- Chen, J.; Gong, D.; Liu, X.; Sun, G.; Sun, W. Quality and antioxidant activity evaluation of dandelion by HPLC with five-wavelength fusion fingerprint. New J. Chem. 2021, 45, 9856–9863. [Google Scholar] [CrossRef]
- Chen, Y.; Tie, S.; Zhang, X.; Zhang, L.; Tan, M. Preparation and characterization of glycosylated protein nanoparticles for astaxanthin mitochondria targeting delivery. Food Funct. 2021, 12, 7718–7727. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Hua, Z.; Xing, S.; Li, J.; Fei, S.; Tan, M. ROS-triggered self-disintegrating and pH-responsive astaxanthin nanoparticles for regulating the intestinal barrier and colitis. Biomaterials 2023, 292, 121937. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, L.; Zaky, A.A.; Liu, R.; Wang, H.; Abd El-Aty, A.M.; Tan, M. Protein coronas formed by three blood proteins and food-borne carbon dots from roast mackerel: Effects on cytotoxicity and cellular metabolites. Int. J. Biol. Macromol. 2022, 216, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Tie, S.; Li, J.; Su, W.; Tan, M. A smart cauliflower-like carrier for astaxanthin delivery to relieve colon inflammation. J. Control. Release 2022, 342, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dong, L.; Du, H.; Bao, Z.; Lin, S. Potential mechanisms underlying the protective effects of Tricholoma matsutake singer peptides against LPS-induced inflammation in RAW264.7 macrophages. Food Chem. 2021, 353, 129452. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Cui, J.; Wu, X.; Li, Y.; Wu, W.; Zhang, X. Ochratoxin A induces reprogramming of glucose metabolism by switching energy metabolism from oxidative phosphorylation to glycolysis in human gastric epithelium GES-1 cells in vitro. Toxicol. Lett. 2020, 333, 232–241. [Google Scholar] [CrossRef]
- Ryu, J.K.; Choi, H.B.; McLarnon, J.G. Combined minocycline plus pyruvate treatment enhances effects of each agent to inhibit inflammation, oxidative damage, and neuronal loss in an excitotoxic animal model of Huntington’s disease. Neuroscience 2006, 141, 1835–1848. [Google Scholar] [CrossRef]
- Hu, X.; Chandler, J.D.; Park, S.; Liu, K.; Fernandes, J.; Orr, M.; Smith, M.R.; Ma, C.; Kang, S.M.; Uppal, K.; et al. Low-dose cadmium disrupts mitochondrial citric acid cycle and lipid metabolism in mouse lung. Free Radic. Biol. Med. 2019, 131, 209–217. [Google Scholar] [CrossRef]
- Oh, T.J.; Kim, I.G.; Park, S.Y.; Kim, K.C.; Shim, H.W. NAD-dependent malate dehydrogenase protects against oxidative damage in Escherichia coli K-12 through the action of oxaloacetate. Environ. Toxicol. Pharmacol. 2002, 11, 9–14. [Google Scholar] [CrossRef]
- Qu, X.; Gao, H.; Sun, J.; Tao, L.; Zhang, Y.; Zhai, J.; Song, Y.; Hu, T.; Li, Z. Identification of key metabolites during cisplatin-induced acute kidney injury using an HPLC-TOF/MS-based non-targeted urine and kidney metabolomics approach in rats. Toxicology 2020, 431, 152366. [Google Scholar] [CrossRef]
- Yue, B.; Zhang, X.; Li, W.; Wang, J.; Sun, Z.; Niu, R. Fluoride exposure altered metabolomic profile in rat serum. Chemosphere 2020, 258, 127387. [Google Scholar] [CrossRef]
- Suzuki, R.; Sato, Y.; Fukaya, M.; Suzuki, D.; Yoshizawa, F. Energy metabolism profile of the effects of amino acid treatment on hepatocytes: Phenylalanine and phenylpyruvate inhibit glycolysis of hepatocytes. Nutrition 2021, 82, 111042. [Google Scholar] [CrossRef]
- Li, L.; Lin, L.M.; Deng, J.; Lin, X.L.; Li, Y.M.; Xia, B.H. The therapeutic effects of Prunella vulgaris against fluoride-induced oxidative damage by using the metabolomics method. Environ. Toxicol. 2021, 36, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Anesi, A.; Rubert, J.; Oluwagbemigun, K.; Orozco-Ruiz, X.; Nothlings, U.; Breteler, M.M.B.; Mattivi, F. Metabolic profiling of human plasma and urine, targeting tryptophan, tyrosine and branched chain amino acid pathways. Metabolites 2019, 9, 261. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Chen, T.; Zhang, Q.; Zhang, H.; Zhu, Z.; Chai, Y.; Zhang, J. Metabolomic study of the protective effect of Gandi capsule for diabetic nephropathy. Chem.-Biol. Interact. 2019, 314, 108815. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jang, A. Correlations between the levels of the bioactive compounds and quality traits in beef loin and round during cold storage. Food Control 2021, 120, 107491. [Google Scholar] [CrossRef]
- Sandner, G.; König, A.; Wallner, M.; Weghuber, J. Functional foods-dietary or herbal products on obesity: Application of selected bioactive compounds to target lipid metabolism. Curr. Opin. Food Sci. 2020, 34, 9–20. [Google Scholar] [CrossRef]
- Ji, X.; Shi, S.; Liu, B.; Shan, M.; Tang, D.; Zhang, W.; Zhang, Y.; Zhang, L.; Zhang, H.; Lu, C.; et al. Bioactive compounds from herbal medicines to manage dyslipidemia. Biomed. Pharmacother. 2019, 118, 109338. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Liao, Z.; Feng, B.; Wang, T.; Shan, B.; Zeng, Q.; Song, J.; Song, Y. Pulsatilla chinensis saponins cause liver injury through interfering ceramide/sphingomyelin balance that promotes lipid metabolism dysregulation and apoptosis. Phytomedicine 2020, 76, 153265. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, N.; Lu, H.; Zhu, L. Disturbed phospholipid metabolism by three polycyclic aromatic hydrocarbons in Oryza sativa. Environ. Pollut. 2021, 283, 117073. [Google Scholar] [CrossRef]
- Bochkov, V.N.; Oskolkova, O.V.; Birukov, K.G.; Levonen, A.L.; Binder, C.J.; Stöckl, J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 2010, 12, 1009–1059. [Google Scholar] [CrossRef]
- Lopez, D.H.; Bestard-Escalas, J.; Garate, J.; Maimo-Barcelo, A.; Fernandez, R.; Reigada, R.; Khorrami, S.; Ginard, D.; Okazaki, T.; Fernandez, J.A.; et al. Tissue-selective alteration of ethanolamine plasmalogen metabolism in dedifferentiated colon mucosa. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2018, 1863, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, Q.; Wang, H.; Tan, M. Metallothionein attenuated arsenic-induced cytotoxicity: The underlying mechanism reflected by metabolomics and lipidomics. J. Agric. Food Chem. 2021, 69, 5372–5380. [Google Scholar] [CrossRef] [PubMed]
- Chaffai, R.; Cherif, A. The cadmium-induced changes in the polar and neutral lipid compositions suggest the involvement of triacylglycerol in the defense response in maize. Physiol. Mol. Biol. Plants 2020, 26, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Arambourou, H.; Llorente, L.; Moreno-Ocio, I.; Herrero, O.; Barata, C.; Fuertes, I.; Delorme, N.; Mendez-Fernandez, L.; Planello, R. Exposure to heavy metal-contaminated sediments disrupts gene expression, lipid profile, and life history traits in the midge Chironomus riparius. Water Res. 2020, 168, 115165. [Google Scholar] [CrossRef]
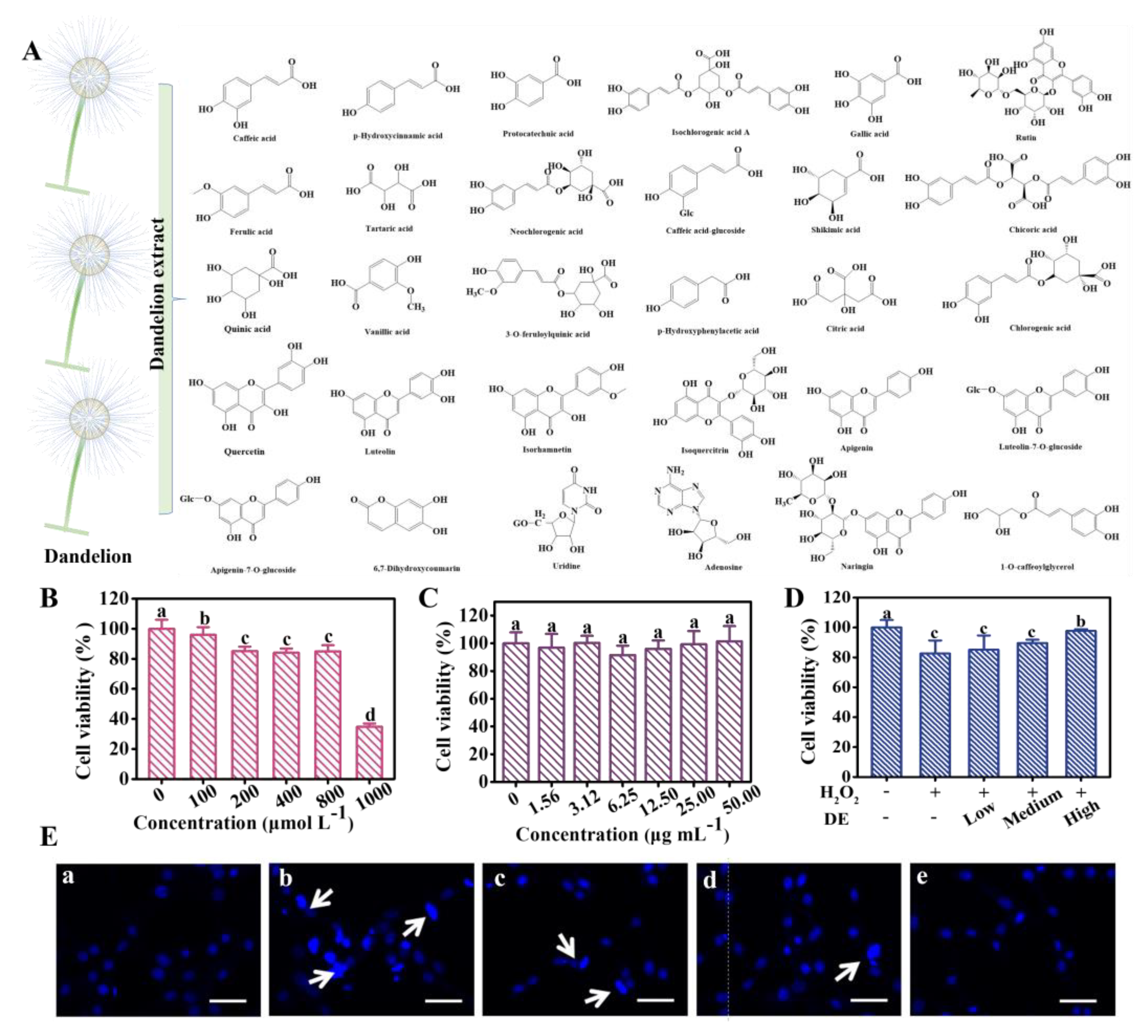




| No | Component | MS (m/z, Da) | MS2 (m/z, Da) |
|---|---|---|---|
| 1 | Caffeic acid | 179.0332[M-H]− | 135.0357/117.0245 |
| 2 | p-hydroxycinnamic acid | 163.0288[M-H]− | 119.0324/91.0246 |
| 3 | Protocatechuic acid | 153.0089[M-H]− | 109.0047/91.0025 |
| 4 | Isochlorogenic acid A | 515.1178[M-H]− | 353.0776 |
| 5 | Gallic acid | 169.0143[M-H]− | 125.0124 |
| 6 | Rutin | 609.1449[M-H]− | 301.0214/255.0103 |
| 7 | Ferulic acid | 193.0502[M-H]− | 134.0203/115.0097 |
| 8 | Tartaric acid | 149.0092[M-H]− | 87.0014 |
| 9 | Neochlorogenic acid | 353.0854[M-H]− | 179.0247/93.0251 |
| 10 | Caffeic acid-glucoside | 341.0816[M-H]− | 281.0649/179.0137 |
| 11 | Shikimic acid | 173.0421[M-H]− | 110.8724 |
| 12 | Chicoric acid | 473.0642[M-H]− | 219.0043/179.0247 |
| 13 | Quinic acid | 191.0558[M-H]− | 93.0247 |
| 14 | Vanillic acid | 167.0345[M-H]− | 152.0217/108.0125 |
| 15 | 3-O-Feruloylquinic acid | 367.0124[M-H]− | 193.0415/111.0386 |
| 16 | p-hydroxyphenylacetic acid | 151.0288[M-H]− | 107.0124 |
| 17 | Citric acid | 191.0187[M-H]− | 87.0018/67.0135 |
| 18 | Chlorogenic acid | 353.0834[M-H]− | 191.0347/127.0298, |
| 19 | Quercetin | 301.0324[M-H]− | 150.9876/107.0032 |
| 20 | Luteolin | 285.0395[M-H]− | 150.9941/133.0127, |
| 21 | Isorhamnetin | 315.0506[M-H]− | 300.0162/271.0034 |
| 22 | Isoquercitrin | 463.0864[M-H]− | 301.0245/150.9934 |
| 23 | Apigenin | 269.0452[M-H]− | 269.0353, 117.0264 |
| 24 | Luteolin-7-O-glucoside | 447.0928[M-H]− | 285.0301 |
| 25 | Apigenin-7-O-glucoside | 431.0972[M+H]+ | 269.0357 |
| 26 | 6,7-Dihydroxycoumarin | 177.0124[M-H]− | 149.0132/133.0178 |
| 27 | Uridine | 243.0614[M+H]+ | 152.0237/110.0162 |
| 28 | Adenosine | 268.1024[M+H]+ | 136.0542 |
| 29 | Naringin | 581.1832[M+H]+ | 435.1224 |
| 30 | 1-O-caffeoylglycerol | 253.0653[M-H]− | 179.0102/133.0164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Fei, S.; Yu, X.; Tan, M. Dandelion (Taraxacum mongolicum) Extract Alleviated H2O2-Induced Oxidative Damage: The Underlying Mechanism Revealed by Metabolomics and Lipidomics. Foods 2023, 12, 3314. https://doi.org/10.3390/foods12173314
Chen Y, Fei S, Yu X, Tan M. Dandelion (Taraxacum mongolicum) Extract Alleviated H2O2-Induced Oxidative Damage: The Underlying Mechanism Revealed by Metabolomics and Lipidomics. Foods. 2023; 12(17):3314. https://doi.org/10.3390/foods12173314
Chicago/Turabian StyleChen, Yannan, Siyuan Fei, Xiaoting Yu, and Mingqian Tan. 2023. "Dandelion (Taraxacum mongolicum) Extract Alleviated H2O2-Induced Oxidative Damage: The Underlying Mechanism Revealed by Metabolomics and Lipidomics" Foods 12, no. 17: 3314. https://doi.org/10.3390/foods12173314
APA StyleChen, Y., Fei, S., Yu, X., & Tan, M. (2023). Dandelion (Taraxacum mongolicum) Extract Alleviated H2O2-Induced Oxidative Damage: The Underlying Mechanism Revealed by Metabolomics and Lipidomics. Foods, 12(17), 3314. https://doi.org/10.3390/foods12173314







