Investigating Structural Defects in Extra Hard Cheese Produced from Low-Temperature Centrifugation of Milk
Abstract
:1. Introduction
2. Materials and Methods
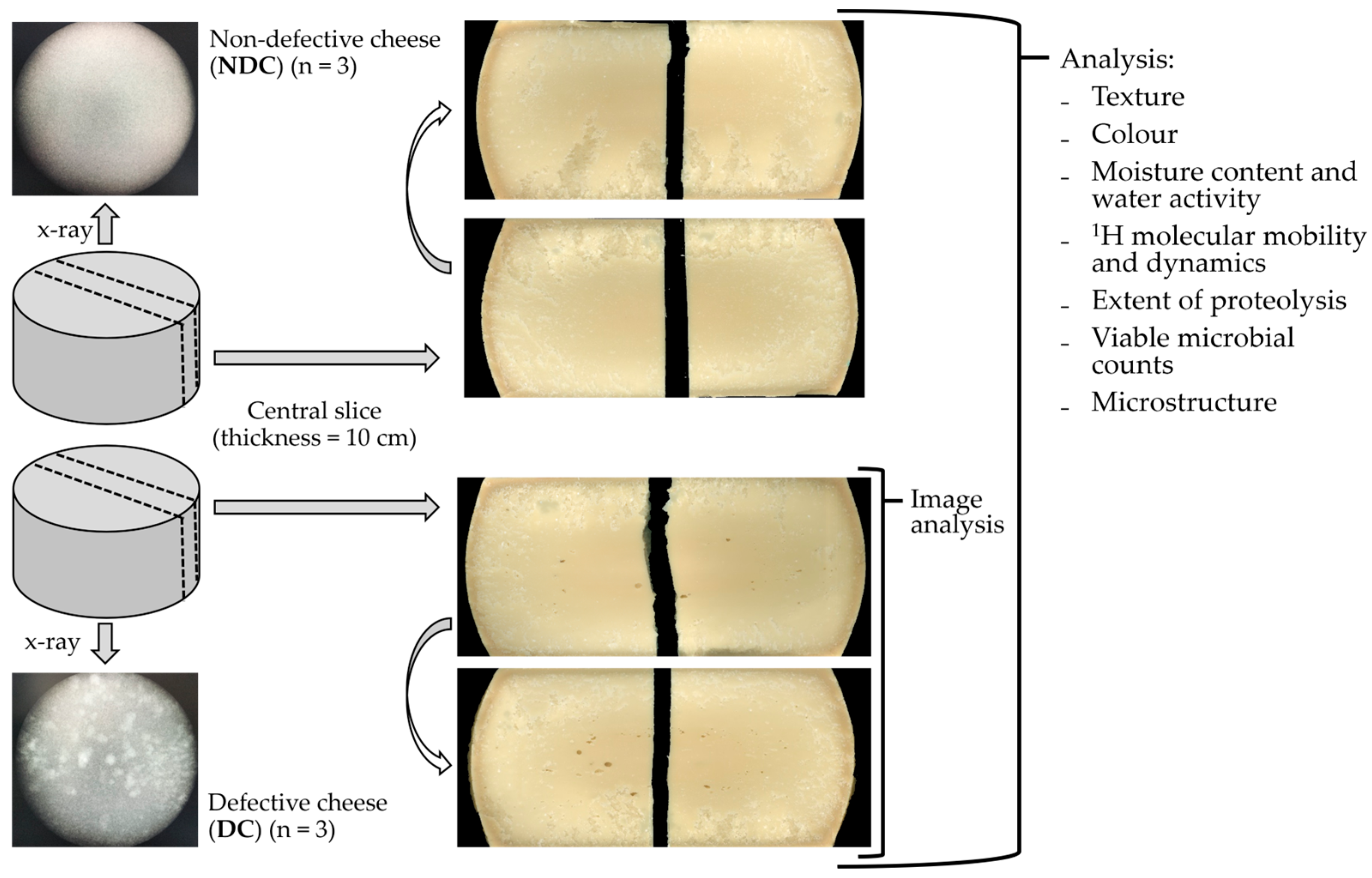
2.1. Cheese Production and Sampling
2.2. Image Analysis
2.3. Cheese Physico-Chemical Properties
2.3.1. Texture Analysis
2.3.2. Colorimetric Characteristics
2.3.3. Moisture Content and Water Activity
2.3.4. Low Resolution 1H NMR
2.4. Confocal Laser Scanning Microscopy
2.5. Capillary Zone Electrophoresis (CZE)
2.6. Microbiological Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Cheese Openings
3.2. Cheese Physico-Chemical Properties
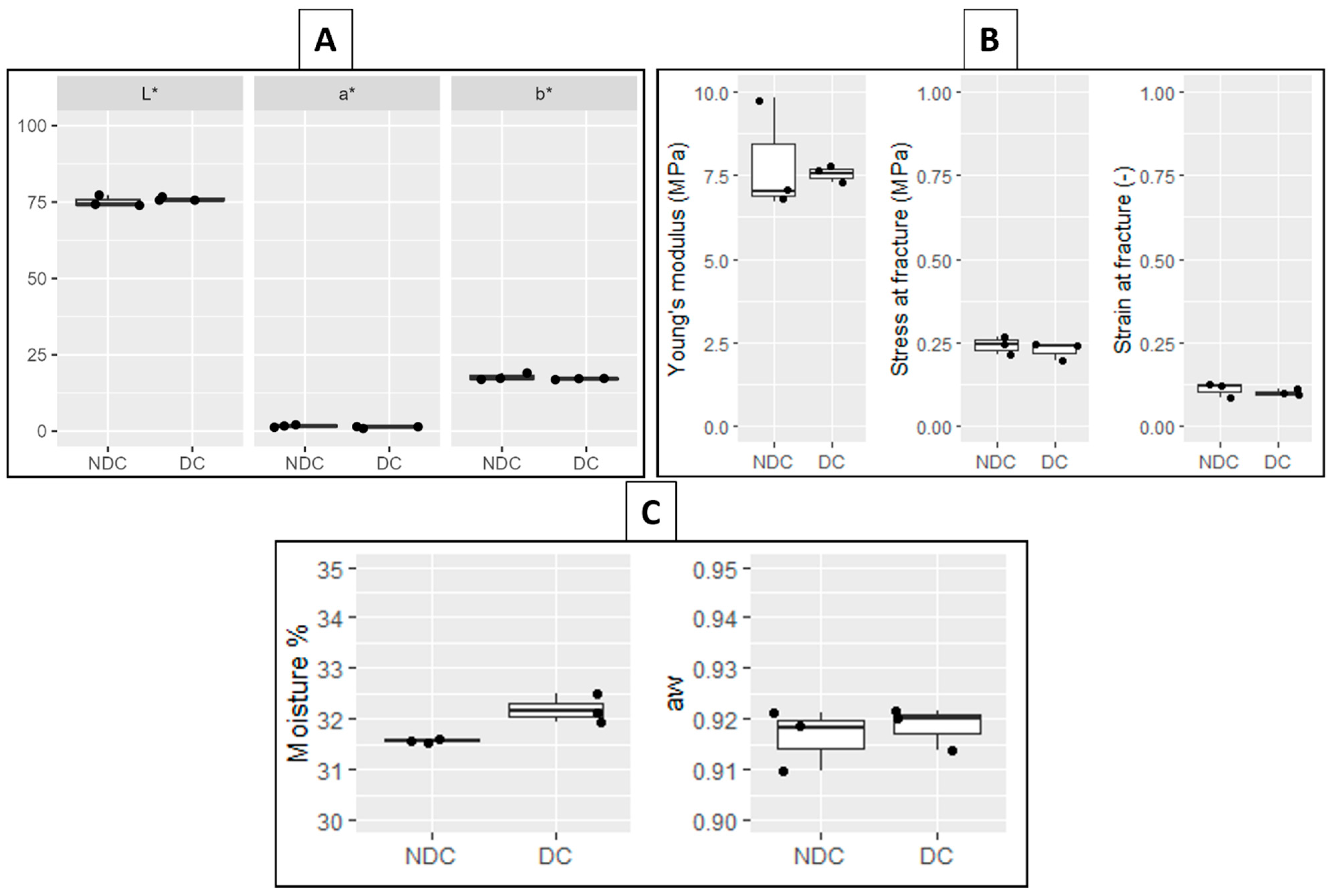
3.2.1. Cheese Colour and Texture
3.2.2. Cheese Moisture and Water Activity
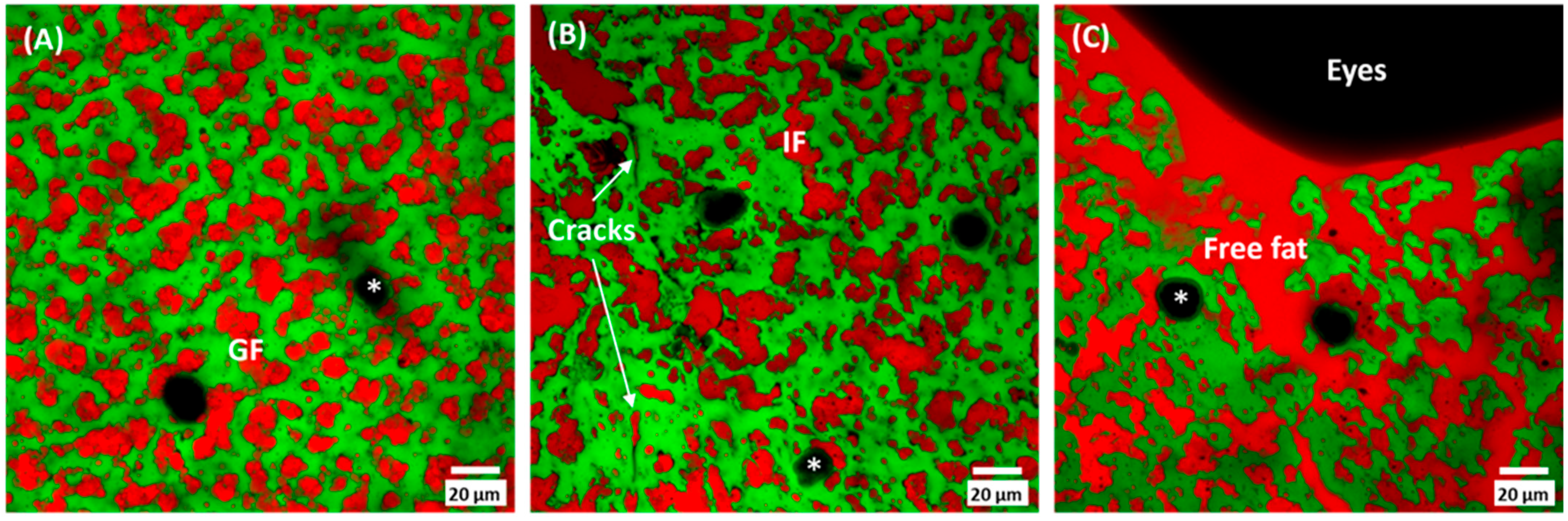
3.3. Cheese Microstructure
3.4. Cheese Proteolysis
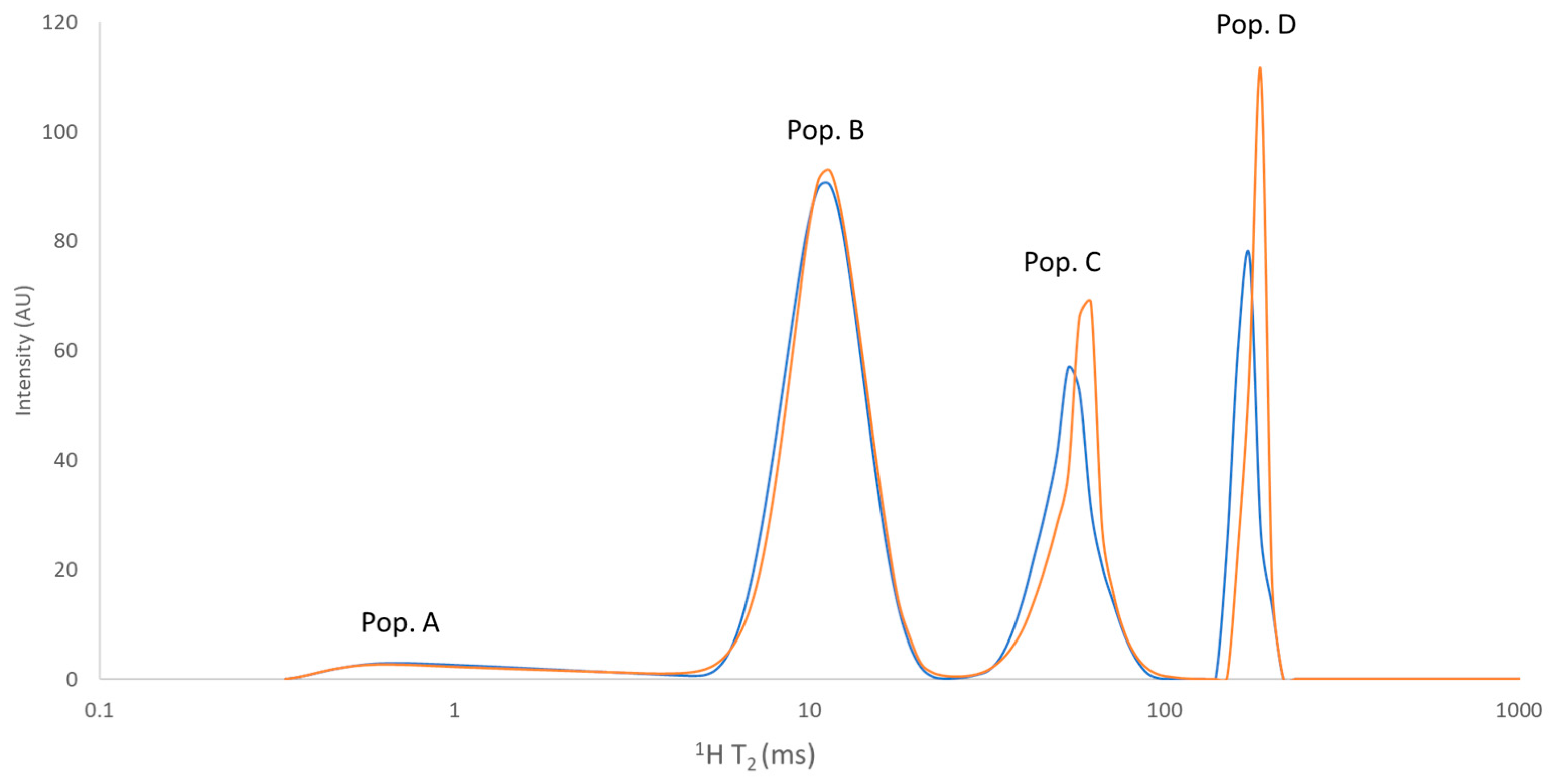
3.5. Low-Resolution 1H NMR Analyses
3.6. Microbiological Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clydesdale, F.M. Color as a Factor in Food Choice. Crit. Rev. Food Sci. Nutr. 1993, 33, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Nestle, M.; Wing, R.; Birch, L.; DiSogra, L.; Drewnowski, A.; Middleton, S.; Sigman-Grant, M.; Sobal, J.; Winston, M.; Economos, C. Behavioral and Social Influences on Food Choice. Nutr. Rev. 1998, 56, S50–S64. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. On the Relationship(s) Between Color and Taste/Flavor. Exp. Psychol. 2019, 66, 99–111. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science, 2nd ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Guggisberg, D.; Schuetz, P.; Winkler, H.; Amrein, R.; Jakob, E.; Fröhlich-Wyder, M.T.; Irmler, S.; Bisig, W.; Jerjen, I.; Plamondon, M.; et al. Mechanism and Control of the Eye Formation in Cheese. Int. Dairy J. 2015, 47, 118–127. [Google Scholar] [CrossRef]
- D’Incecco, P.; Gatti, M.; Hogenboom, J.A.; Bottari, B.; Rosi, V.; Neviani, E.; Pellegrino, L. Lysozyme Affects the Microbial Catabolism of Free Arginine in Raw-Milk Hard Cheeses. Food Microbiol. 2016, 57, 16–22. [Google Scholar] [CrossRef]
- Tabla, R.; Gómez, A.; Rebollo, J.E.; Molina, F.; Roa, I. Effectiveness of a Bacteriophage Cocktail in Reducing Cheese Early Blowing Caused by Escherichia Coli. LWT 2022, 153, 112430. [Google Scholar] [CrossRef]
- Julien, M.-C.; Dion, P.; Lafrenière, C.; Antoun, H.; Drouin, P. Sources of Clostridia in Raw Milk on Farms. Appl. Environ. Microbiol. 2008, 74, 6348–6357. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.M.M.; Driehuis, F.; Te Giffel, M.C.; De Jong, P.; Lankveld, J.M.G. Concentrations of Butyric Acid Bacteria Spores in Silage and Relationships with Aerobic Deterioration. J. Dairy Sci. 2007, 90, 928–936. [Google Scholar] [CrossRef]
- Langó, Z.; Heinonen-Tanski, H. Occurrence of Clostridium Tyrobutyricum in Cattle Slurry and Fresh Forage Grasses. Bioresour. Technol. 1995, 53, 189–191. [Google Scholar] [CrossRef]
- Ávila, M.; Gómez-Torres, N.; Hernández, M.; Garde, S. Inhibitory Activity of Reuterin, Nisin, Lysozyme and Nitrite against Vegetative Cells and Spores of Dairy-Related Clostridium Species. Int. J. Food Microbiol. 2014, 172, 70–75. [Google Scholar] [CrossRef]
- Gómez-Torres, N.; Ávila, M.; Gaya, P.; Garde, S. Prevention of Late Blowing Defect by Reuterin Produced in Cheese by a Lactobacillus Reuteri Adjunct. Food Microbiol. 2014, 42, 82–88. [Google Scholar] [CrossRef]
- Martínez-Cuesta, M.C.; Requena, T.; Peláez, C. Use of a Bacteriocin-Producing Transconjugant as Starter in Acceleration of Cheese Ripening. Int. J. Food Microbiol. 2001, 70, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Elwell, M.W.; Barbano, D.M. Use of Microfiltration to Improve Fluid Milk Quality1,2. J. Dairy Sci. 2006, 89, E20–E30. [Google Scholar] [CrossRef] [PubMed]
- Alinovi, M.; Mucchetti, G.; Tidona, F. Application of NIR Spectroscopy and Image Analysis for the Characterisation of Grated Parmigiano-Reggiano Cheese. Int. Dairy J. 2019, 92, 50–58. [Google Scholar] [CrossRef]
- Felix da Silva, D.; Larsen, F.H.; Hougaard, A.B.; Ipsen, R. The Influence of Raw Material, Added Emulsifying Salt and Spray Drying on Cheese Powder Structure and Hydration Properties. Int. Dairy J. 2017, 74, 27–38. [Google Scholar] [CrossRef]
- Felix da Silva, D.; Hirschberg, C.; Ahrné, L.; Hougaard, A.B.; Ipsen, R. Cheese Feed to Powder: Effects of Cheese Age, Added Dairy Ingredients and Spray Drying Temperature on Properties of Cheese Powders. J. Food Eng. 2018, 237, 215–225. [Google Scholar] [CrossRef]
- D’Incecco, P.; Bancalari, E.; Gatti, M.; Ranghetti, A.; Pellegrino, L. Low-Temperature Centrifugation of Milk for Manufacture of Raw Milk Cheeses: Impact on Milk Debacterization and Cheese Yield. LWT 2020, 118, 108789. [Google Scholar] [CrossRef]
- D’Incecco, P.; Bettera, L.; Bancalari, E.; Rosi, V.; Sindaco, M.; Gobbi, S.; Candotti, P.; Nazzicari, N.; Limbo, S.; Gatti, M.; et al. High-Speed Cold Centrifugation of Milk Modifies the Microbiota, the Ripening Process and the Sensory Characteristics of Raw-Milk Hard Cheeses. Food Res. Int. 2023, 172, 113102. [Google Scholar] [CrossRef]
- D’Incecco, P.; Pellegrino, L.; Hogenboom, J.A.; Cocconcelli, P.S.; Bassi, D. The Late Blowing Defect of Hard Cheeses: Behaviour of Cells and Spores of Clostridium Tyrobutyricum throughout the Cheese Manufacturing and Ripening. LWT 2018, 87, 134–141. [Google Scholar] [CrossRef]
- Bettera, L.; Alinovi, M.; Mondinelli, R.; Mucchetti, G. Ripening of Nostrano Valtrompia PDO Cheese in Different Storage Conditions: Influence on Chemical, Physical and Sensory Properties. Foods 2020, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Hort, J.; Grys, G.; Woodman, J. The Relationships between the Chemical, Rheological and Textural Properties of Cheddar Cheese. Lait 1997, 77, 587–600. [Google Scholar] [CrossRef]
- Hort, J.; Le Grys, G. Developments in the Textural and Rheological Properties of UK Cheddar Cheese during Ripening. Int. Dairy J. 2001, 11, 475–481. [Google Scholar] [CrossRef]
- IDF Standard 4A; IDF Cheese and Processed Cheese Products. Determination of the Total Solids Content. IDF: Vilnius, Lithuania, 1982.
- Alinovi, M.; Tidona, F.; Monti, L.; Francolino, S.; Brusa, G.; Ghiglietti, R.; Locci, F.; Giraffa, G. Physicochemical and Rheological Characteristics of Crescenza Cheese Made with 40% of Recombined Milk during Manufacture and Storage. Int. J. Dairy Technol. 2022, 75, 643–652. [Google Scholar] [CrossRef]
- Alinovi, M.; Corredig, M.; Mucchetti, G.; Carini, E. Water Status and Dynamics of High-Moisture Mozzarella Cheese as Affected by Frozen and Refrigerated Storage. Food Res. Int. 2020, 137, 109415. [Google Scholar] [CrossRef]
- D’Incecco, P.; Limbo, S.; Hogenboom, J.; Rosi, V.; Gobbi, S.; Pellegrino, L. Impact of Extending Hard-Cheese Ripening: A Multiparameter Characterization of Parmigiano Reggiano Cheese Ripened up to 50 Months. Foods 2020, 9, 268. [Google Scholar] [CrossRef]
- Carini, S.; Casadei, S. Contributo Alla Conoscenza Della Respirazione e Dei Metabolismi Ossidativi Nei Propionici. Sci Tec. Lait Casearia 1970, 21, 365–378. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Council of the European Union. Regulation (EU) No 1151/2012 of the European Parliament and of the Council of 21 November 2012 on Quality Schemes for Agricultural Products and Foodstuffs. Off. J. Eur. Union 2012, 343, 1–29. [Google Scholar]
- Innocente, N.; Corradini, C. Use of an Image-Analysis Technique in the Quality Control of Montasio Cheese. Sci. E Tec. Latt. Casearia 1998, 49, 82–94. [Google Scholar]
- Rinaldi, M.; Chiavaro, E.; Massini, R. Pecorino of Appennino Reggiano Cheese: Evaluation of Ripening Time Using Selected Physical Properties. Ital. J. Food Sci. 2010, 22, 54. [Google Scholar]
- Romani, S.; Sacchetti, G.; Pittia, P.; Pinnavaia, G.G.; Dalla Rosa, M. Physical, Chemical, Textural and Sensorial Changes of Portioned Parmigiano Reggiano Cheese Packed under Different Conditions. Food Sci. Technol. Int. 2002, 8, 203–211. [Google Scholar] [CrossRef]
- Sherveglieri, V.; Bhandari, M.P.; Carmona, E.N.; Betto, G.; Soprani, M.; Malla, R.; Sberveglieri, G. Spectrocolorimetry and Nanowire Gas Sensor Device S3 for the Analysis of Parmigiano Reggiano Cheese Ripening. In Proceedings of the 2017 ISOCS/IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Montreal, QC, Canada, 28–31 May 2017; pp. 1–3. [Google Scholar]
- Aprea, E.; Romanzin, A.; Corazzin, M.; Favotto, S.; Betta, E.; Gasperi, F.; Bovolenta, S. Effects of Grazing Cow Diet on Volatile Compounds as Well as Physicochemical and Sensory Characteristics of 12-Month-Ripened Montasio Cheese. J. Dairy Sci. 2016, 99, 6180–6190. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, G.; Ferlito, J.; Pasini, G.; Contiero, B.; Gottardo, F. Application of Near-Infrared Spectroscopy as an Alternative to Chemical and Color Analysis to Discriminate the Production Chains of Asiago d’Allevo Cheese. J. Agric. Food Chem. 2009, 57, 11449–11454. [Google Scholar] [CrossRef]
- Marchesini, G.; Balzan, S.; Segato, S.; Novelli, E.; Andrighetto, I. Colour Traits in the Evaluation of the Ripening Period of Asiago Cheese. Ital. J. Anim. Sci. 2009, 8, 411–413. [Google Scholar] [CrossRef]
- Noël, Y.; Zannoni, M.; Hunter, E.A. Texture of Parmigiano Reggiano Cheese: Statistical Relationships between Rheological and Sensory Variates. Le Lait 1996, 76, 243–254. [Google Scholar] [CrossRef]
- Pellegrino, L.; Battelli, G.; Resmini, P.; Ferranti, P.; Barone, F.; Addeo, F. Effects of Heat Load Gradient Occurring in Moulding on Characterization and Ripening of Grana Padano. Lait 1997, 77, 217–228. [Google Scholar] [CrossRef]
- D’Incecco, P.; Limbo, S.; Faoro, F.; Hogenboom, J.; Rosi, V.; Morandi, S.; Pellegrino, L. New insight on crystal and spot development in hard and extra-hard cheeses: Association of spots with incomplete aggregation of curd gran-ules. J. Dairy Sci. 2016, 99, 6144–6156. [Google Scholar] [CrossRef]
- Vélez, M.A.; Bergamini, C.V.; Ramonda, M.B.; Candioti, M.C.; Hynes, E.R.; Perotti, M.C. Influence of Cheese Making Technologies on Plasmin and Coagulant Associated Proteolysis. LWT—Food Sci. Technol. 2015, 64, 282–288. [Google Scholar] [CrossRef]
- McSweeney, P.L.H. Biochemistry of Cheese Ripening. Int. J. Dairy Techol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.-T.; Bachmann, H.-P.; Casey, M.G. Interaction between Propionibacteria and Starter/Non-Starter Lactic Acid Bacteria in Swiss-Type Cheeses. Lait 2002, 82, 1–15. [Google Scholar] [CrossRef]
- Bordoni, A.; Picone, G.; Babini, E.; Vignali, M.; Danesi, F.; Valli, V.; Di Nunzio, M.; Laghi, L.; Capozzi, F. NMR Comparison of in Vitro Digestion of Parmigiano Reggiano Cheese Aged 15 and 30 Months. Magn. Reson. Chem. 2011, 49, S61–S70. [Google Scholar] [CrossRef]
- De Angelis Curtis, S.; Curini, R.; Delfini, M.; Brosio, E.; D’Ascenzo, F.; Bocca, B. Amino Acid Profile in the Ripening of Grana Padano Cheese: A NMR Study. Food Chem. 2000, 71, 495–502. [Google Scholar] [CrossRef]
- Chen, Y.; MacNaughtan, W.; Jones, P.; Yang, Q.; Foster, T. The State of Water and Fat during the Maturation of Cheddar Cheese. Food Chem. 2020, 303, 125390. [Google Scholar] [CrossRef]
- Song, Y.-Q. A 2D NMR Method to Characterize Granular Structure of Dairy Products. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 4, 324–334. [Google Scholar] [CrossRef]
- Vermeir, L.; Declerck, A.; To, C.M.; Kerkaert, B.; Van der Meeren, P. Water and Oil Signal Assignment in Low-moisture Mozzarella as Determined by Time-domain NMR T2 Relaxometry. Magn. Reson. Chem. 2019, 57, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Mulas, G.; Anedda, R.; Longo, D.; Roggio, T.; Uzzau, S. An MRI Method for Monitoring the Ripening of Grana Padano Cheese. Int. Dairy J. 2016, 52, 19–25. [Google Scholar] [CrossRef]
- Noronha, N.; Duggan, E.; Ziegler, G.; O’Riordan, E.; O’Sullivan, M. Inclusion of Starch in Imitation Cheese: Its Influence on Water Mobility and Cheese Functionality. Food Hydrocoll. 2008, 22, 1612–1621. [Google Scholar] [CrossRef]
- Kerjean, J.-R.; Condon, S.; Lodi, R.; Kalantzopoulos, G.; Chamba, J.-F.; Suomalainen, T.; Cogan, T.; Moreau, D. Improving the Quality of European Hard-Cheeses by Controlling of Interactions between Lactic Acid Bacteria and Propionibacteria. Food Res. Int. 2000, 33, 281–287. [Google Scholar] [CrossRef]
- Auer, J.; Reiter, M.; Senck, S.; Reiter, A.; Kastner, J.; Mathmann, K. Investigation of Opening Eye Defects and Effects of Different Ripening Profiles on Eye Structure in Semi-Hard Cheese Using X-ray Micro-Computed Tomography. Food Struct. 2021, 28, 100190. [Google Scholar] [CrossRef]
- O’Sullivan, D.J.; McSweeney, P.L.; Cotter, P.D.; Giblin, L.; Sheehan, J.J. Compromised Lactobacillus Helveticus Starter Activity in the Presence of Facultative Heterofermentative Lactobacillus Casei DPC6987 Results in Atypical Eye Formation in Swiss-Type Cheese. J. Dairy Sci. 2016, 99, 2625–2640. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Defective Cheese (n = 3) | ||
|---|---|---|---|
| Mean | St. Dev. | ||
| Opening area (mm2) | Min. | 0.39 | 0.03 |
| D25 | 0.71 | 0.13 | |
| D50 | 1.31 | 0.25 | |
| D75 | 3.48 | 1.83 | |
| Max. | 36.45 | 12.37 | |
| Mean | 3.92 | 1.10 | |
| Porosity (%) | 0.26 | 0.10 | |
| Cheese | αs1-I/αs1 | αs(f1–23)I/αs(1+0) | αs1-PL/αs1 | ɣ/β |
|---|---|---|---|---|
| NDC | 0.38 ± 0.04 | 0.19 ± 0.05 | 0.68 ± 0.20 | 4.38 ± 1.45 |
| DC | 0.29 ± 0.12 | 0.13 ± 0.01 | 0.56 ± 0.06 | 3.55 ± 0.58 |
| Sign. | n.s. | n.s. | n.s. | n.s. |
| Cheese | Pop. A (%) | Pop. B (%) | Pop. C (%) | Pop. D (%) | T2A (ms) | T2B (ms) | T2C (ms) | T2D (ms) |
|---|---|---|---|---|---|---|---|---|
| NDC | 5.44 ± <0.01 | 55.06 ± 0.08 | 23.58 ± 0.05 | 15.92 ± 0.04 | 1.55 ± 0.12 | 10.78 ± 0.05 | 51.10 ± 1.37 | 170.79 ± 5.68 |
| DC | 5.32 ± 0.08 | 54.55 ± 1.01 | 23.71 ± 0.79 | 16.42 ± 0.48 | 1.62 ± 0.19 | 11.13 ± 0.19 | 54.07 ± 2.16 | 180.54 ± 1.79 |
| Sign. (* p < 0.05) | n.s. (p = 0.065) | n.s. | n.s. | n.s. | n.s. | * | n.s. | * |
| Cheese | Lactobacilli | Total Mesophilic | Yeasts & Moulds | Propionibacteria |
|---|---|---|---|---|
| NDC | 4.36 ± 0.3 | 4.86 ± 0.14 | 3.8 ± 0.73 | 4.19 ± 0.33 |
| DC | 3.52 ± 0.48 | 3.87 ± 0.41 | 4.1 ± 0.1 | 4 ± 0.97 |
| Sign. (* p < 0.05) | n.s (p = 0.063) | * | n.s | n.s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettera, L.; Alinovi, M.; D’Incecco, P.; Gatti, M.; Carini, E.; Pellegrino, L.; Bancalari, E. Investigating Structural Defects in Extra Hard Cheese Produced from Low-Temperature Centrifugation of Milk. Foods 2023, 12, 3302. https://doi.org/10.3390/foods12173302
Bettera L, Alinovi M, D’Incecco P, Gatti M, Carini E, Pellegrino L, Bancalari E. Investigating Structural Defects in Extra Hard Cheese Produced from Low-Temperature Centrifugation of Milk. Foods. 2023; 12(17):3302. https://doi.org/10.3390/foods12173302
Chicago/Turabian StyleBettera, Luca, Marcello Alinovi, Paolo D’Incecco, Monica Gatti, Eleonora Carini, Luisa Pellegrino, and Elena Bancalari. 2023. "Investigating Structural Defects in Extra Hard Cheese Produced from Low-Temperature Centrifugation of Milk" Foods 12, no. 17: 3302. https://doi.org/10.3390/foods12173302
APA StyleBettera, L., Alinovi, M., D’Incecco, P., Gatti, M., Carini, E., Pellegrino, L., & Bancalari, E. (2023). Investigating Structural Defects in Extra Hard Cheese Produced from Low-Temperature Centrifugation of Milk. Foods, 12(17), 3302. https://doi.org/10.3390/foods12173302








