Lentinula edodes Sing Polysaccharide: Extraction, Characterization, Bioactivities, and Emulsifying Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Ultrasound-Assisted Extraction
2.3. Hot Water Extraction
2.4. Chemical Composition Analysis
2.5. Determination of Color Differences
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
2.7. Scanning Electron Microscope (SEM)
2.8. Emulsifying Properties
2.9. Congo Red Test and Iodine-Potassium Iodide Analysis
2.10. Antioxidant Activity
2.10.1. Scavenging Capacity of DPPH Free Radicals
2.10.2. Scavenging Capacity of ABTS Free Radicals
2.10.3. Ferric-Reducing Antioxidant Power (FRAP)
2.11. Assay for Hypoglycemic Activity
2.11.1. Inhibition of the α-Amylase Activity
2.11.2. Inhibition of the α-Glucoside Activity
2.11.3. Glucose Adsorption Capacity (GAC)
2.12. Effects of Polysaccharides on the Lipolysis In Vitro Digestion Model
2.12.1. Polysaccharide Stock Solutions Preparation
2.12.2. Stock Emulsion Preparation
2.12.3. Polysaccharide–Emulsion Mixture Preparation
2.12.4. Static In Vitro Digestion Model
2.13. Statistical Analysis
3. Results
3.1. Physicochemical Characterizations Analysis
3.2. Monosaccharide Composition Analysis
3.3. Determination of Appearance Difference
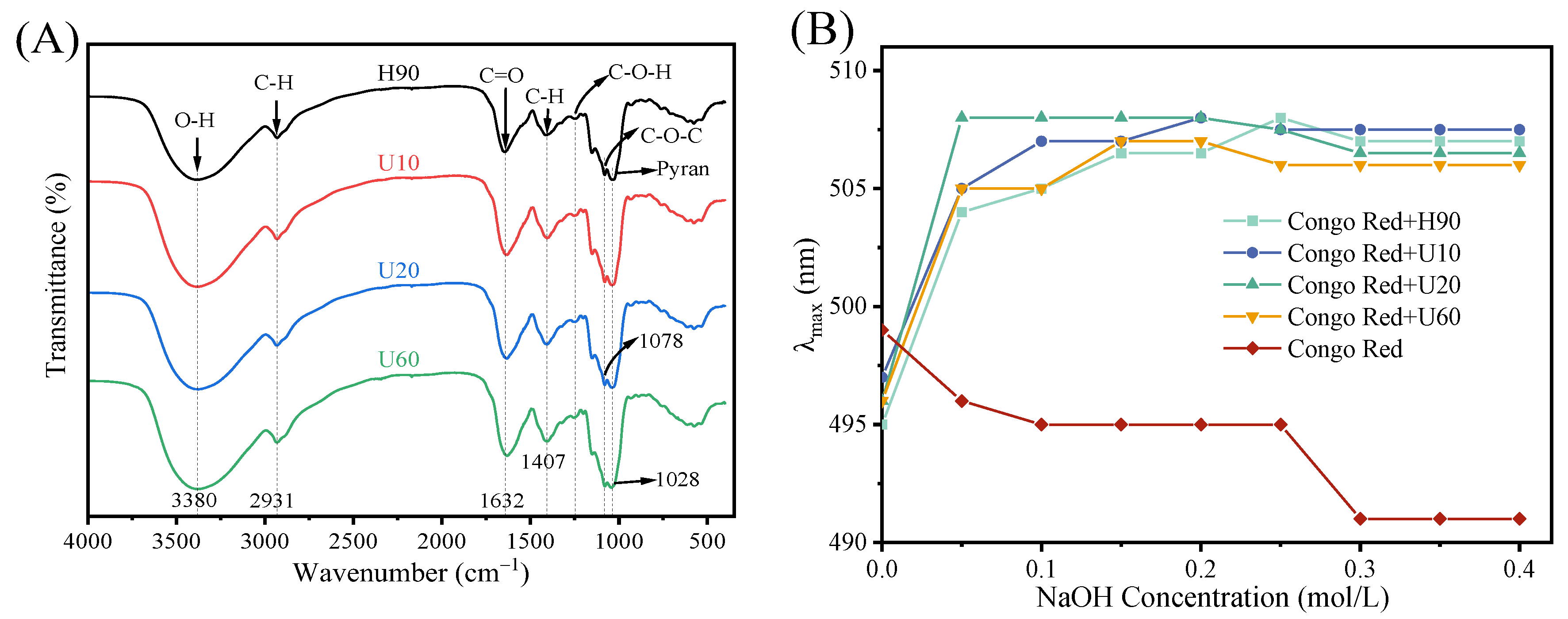
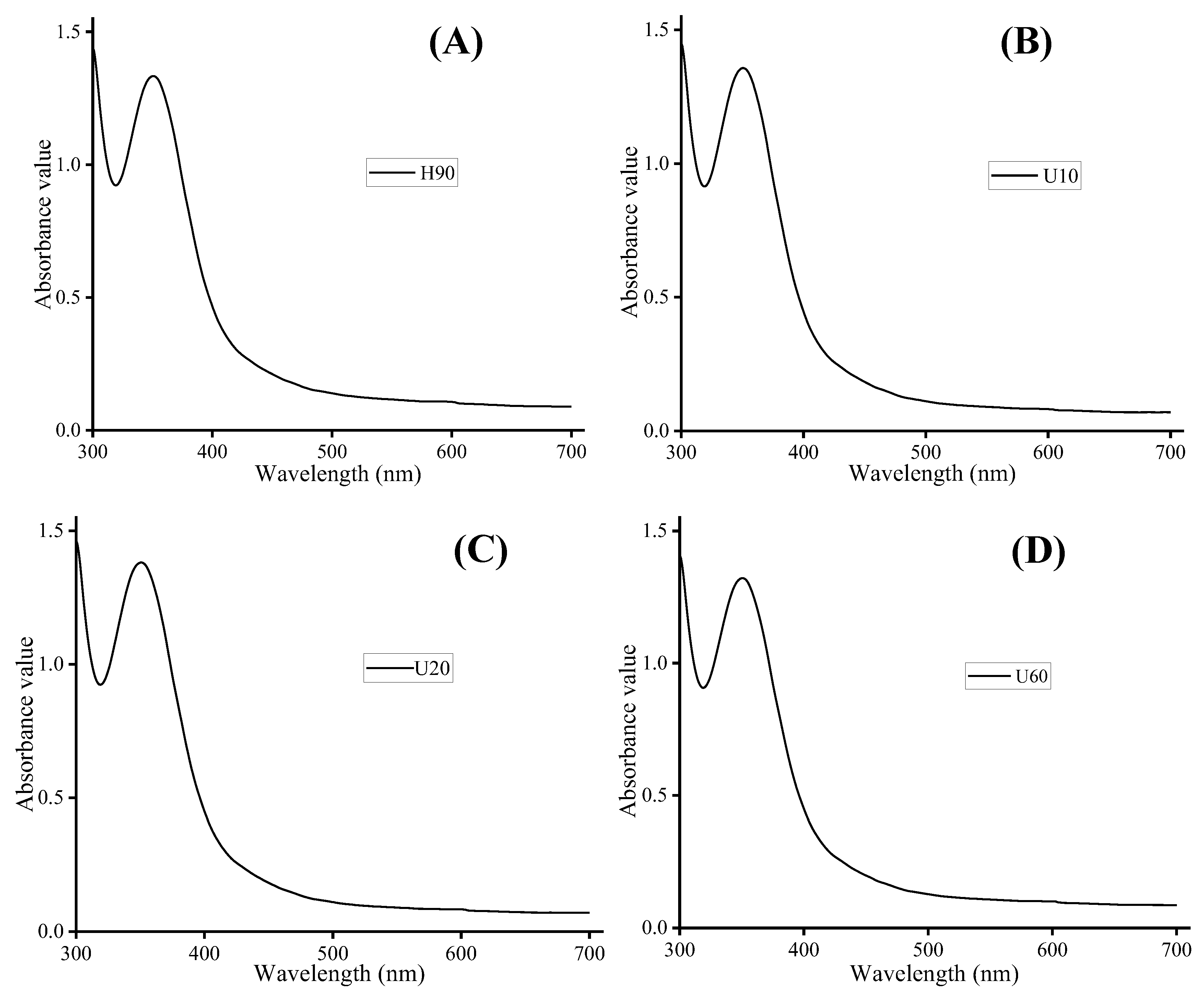
3.4. Analysis of Fourier Transform Infrared (FTIR) Spectroscopy, Congo Red Analysis, and Iodine-Potassium Iodide
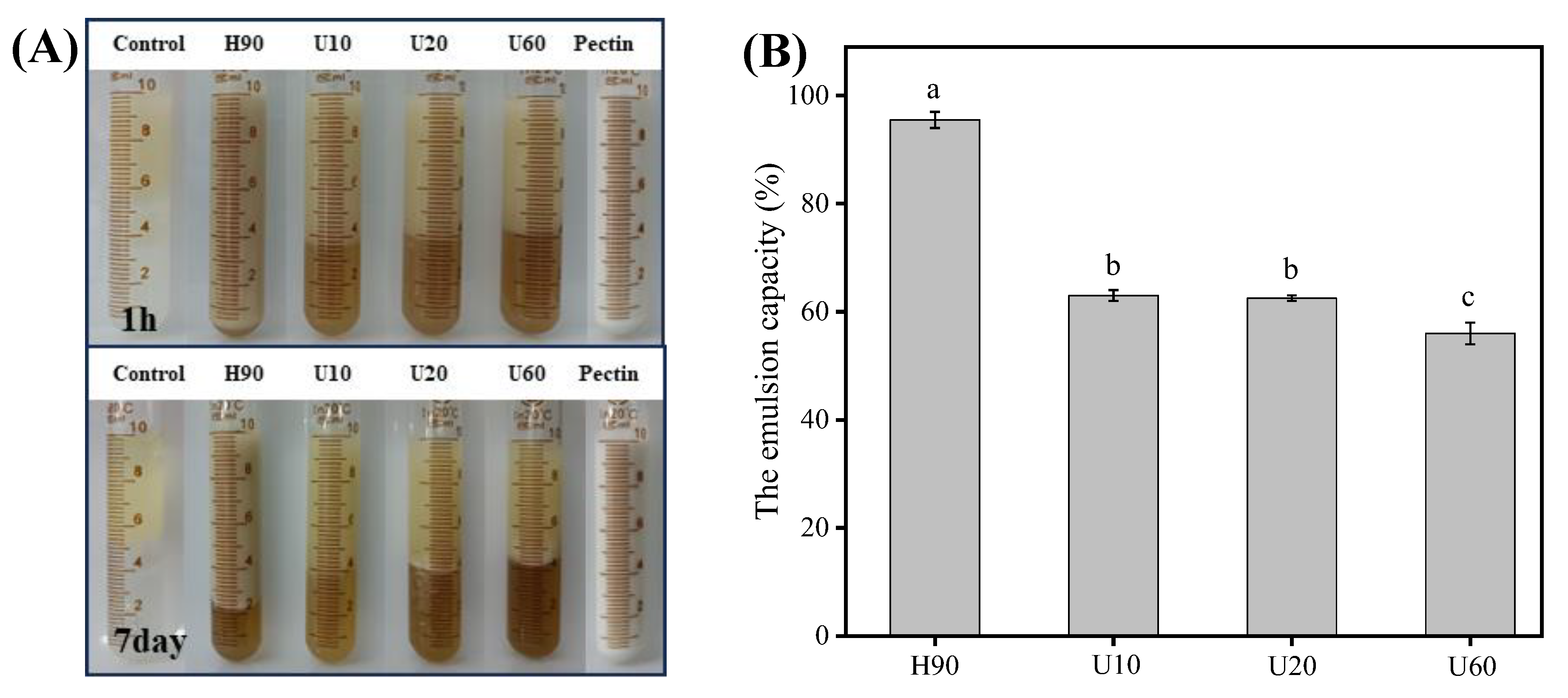
3.5. Emulsifying Properties
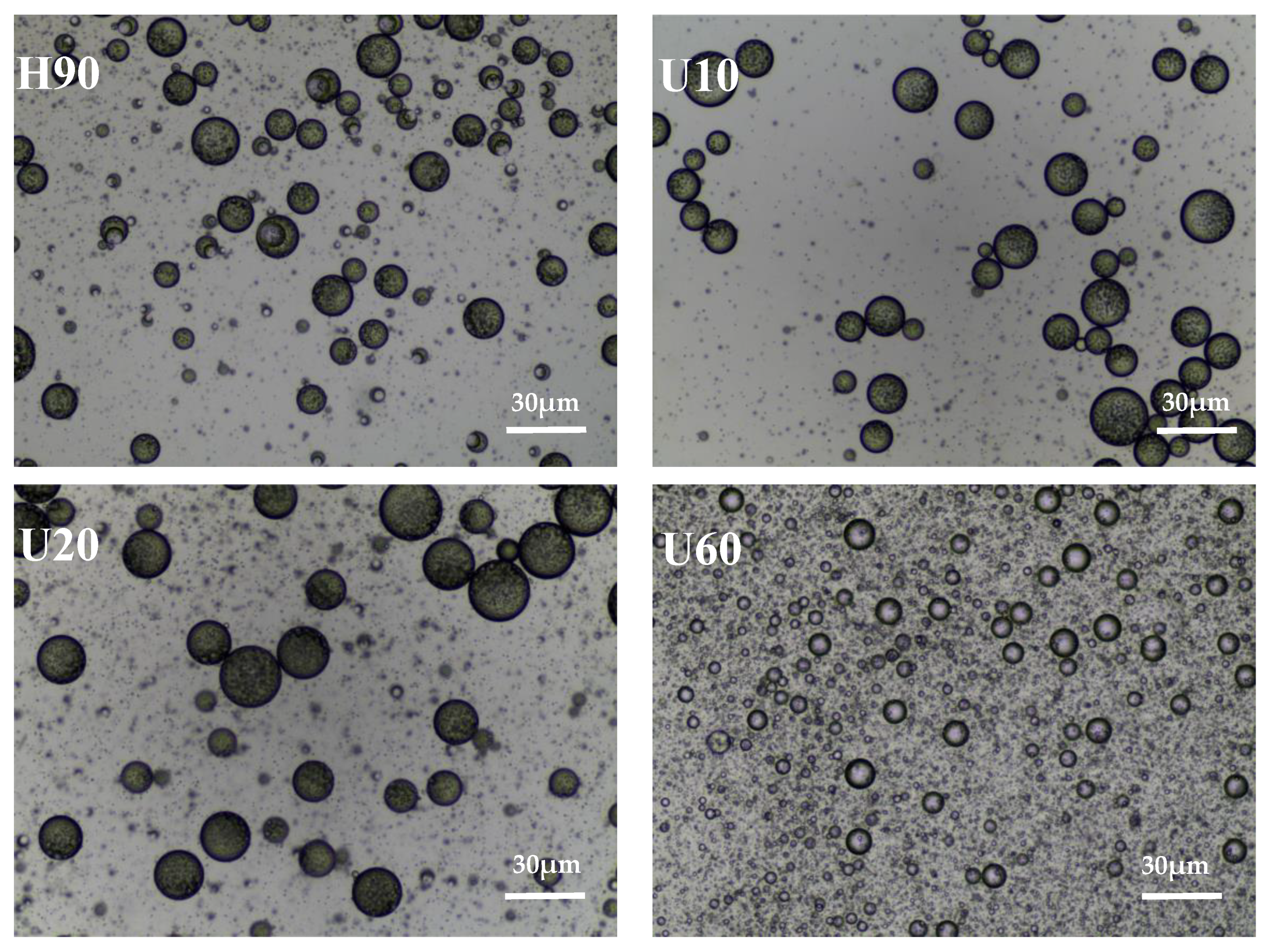
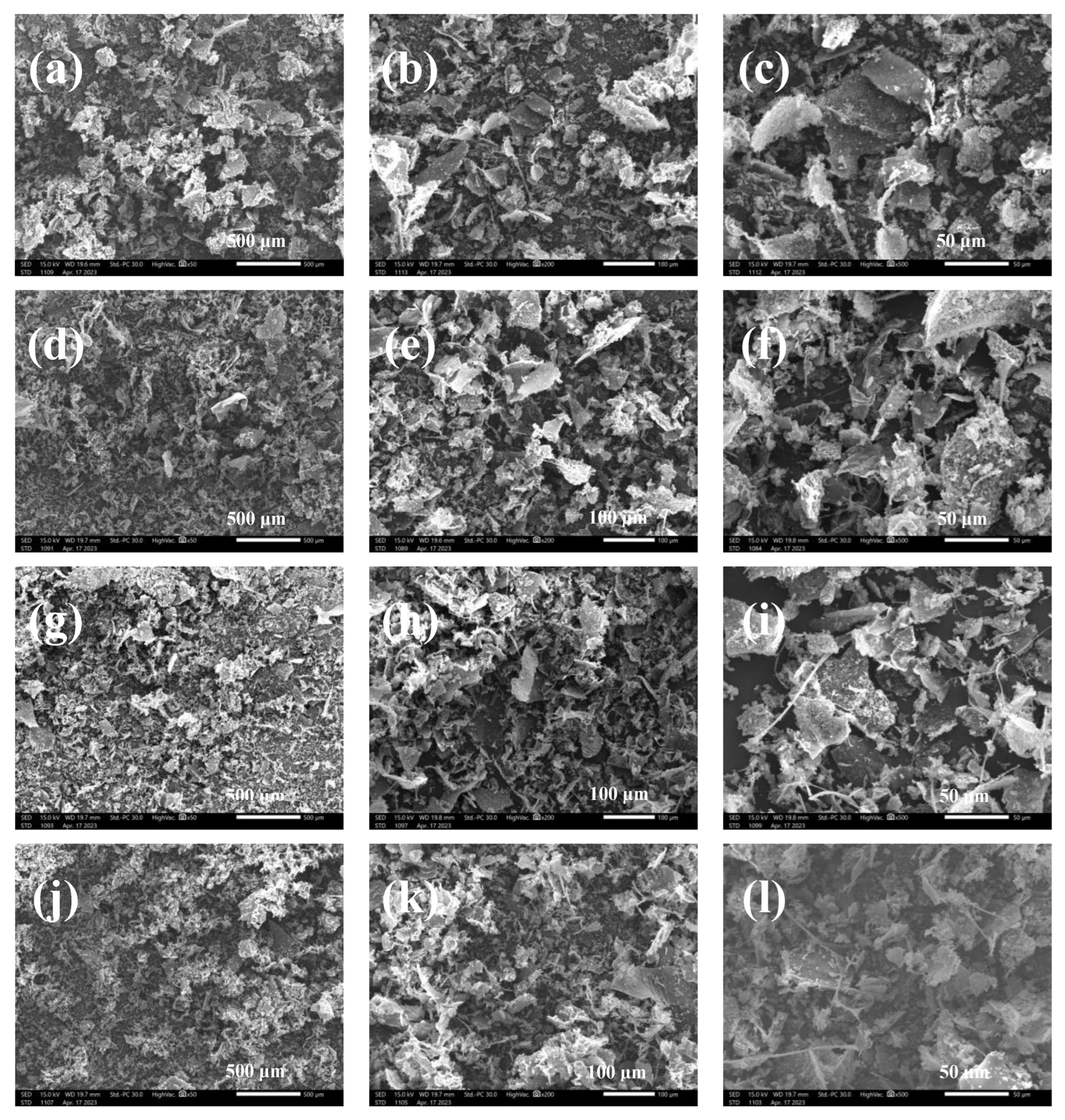
3.6. Scanning Electron Microscope (SEM)
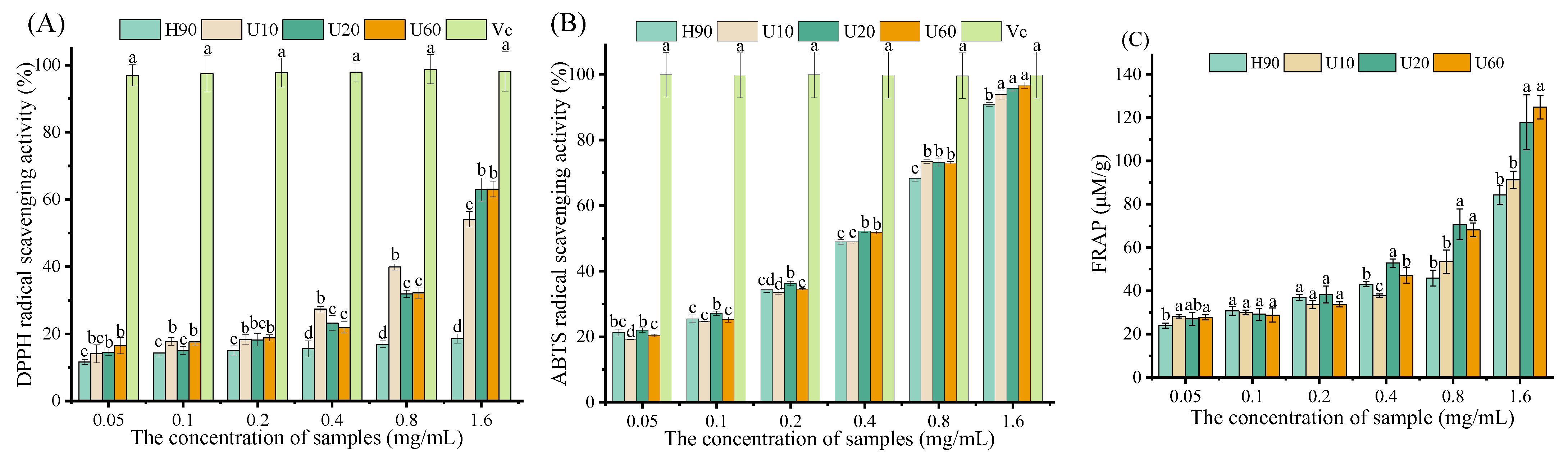
3.7. Antioxidant Activity In Vitro
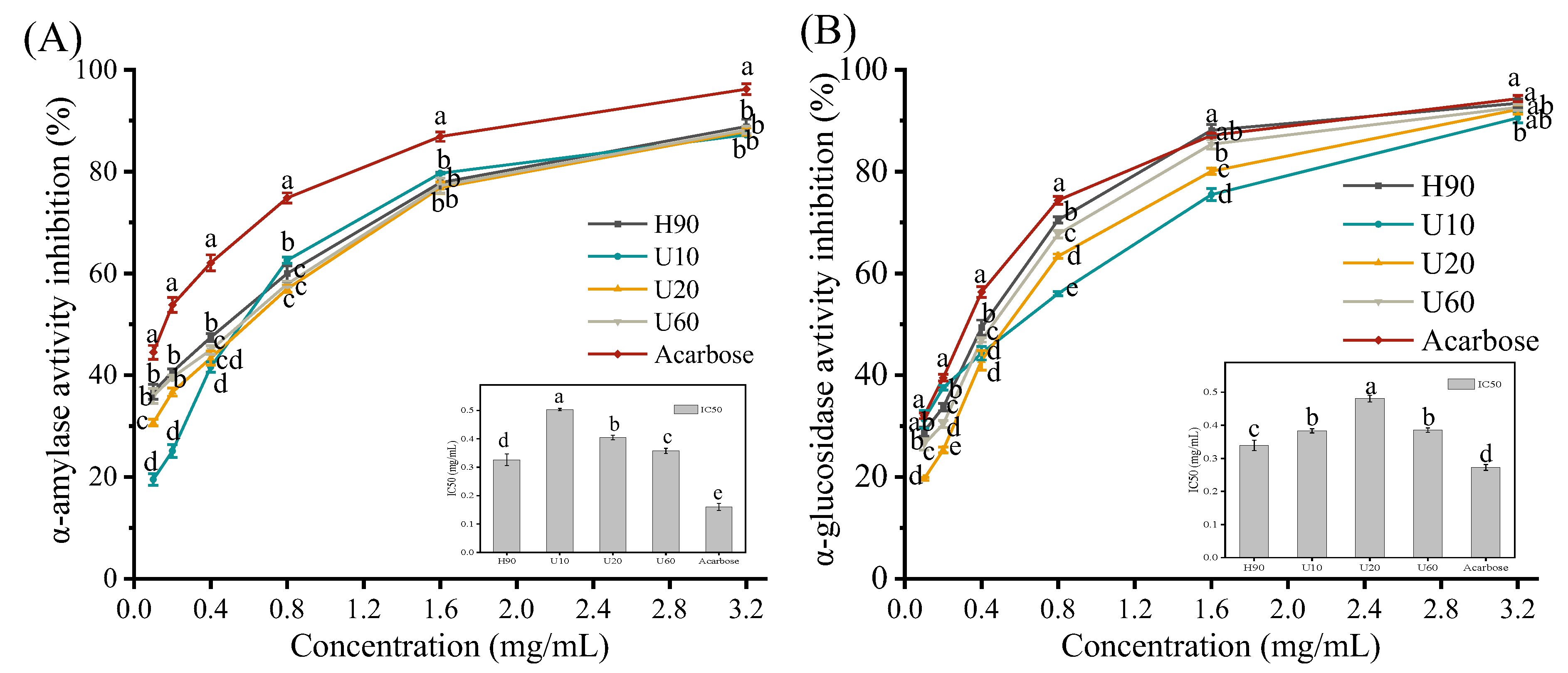
3.8. Inhibitory Effects of LES Polysaccharides on α-Amylase and α-Glucosidase Activities
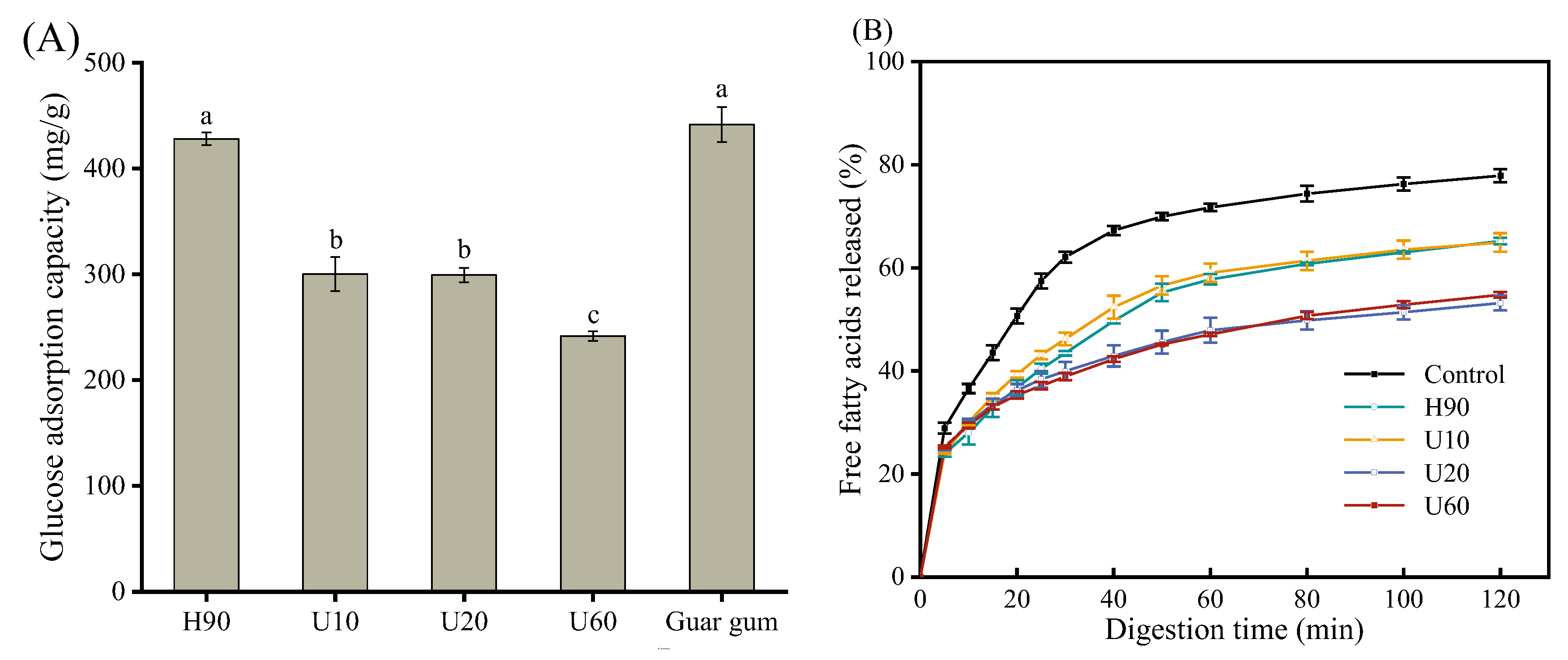
3.9. Effects of LES Polysaccharides on Glucose Adsorption Capacity
3.10. Effects of LES Polysaccharides on Lipolysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Yang, J.M.; Liu, Y.H.; Zhao, M.; Wang, J. Ultrasound assisted extraction of polysaccharides from Lentinus edodes and its anti-hepatitis B activity in vitro. Int. J. Biol. Macromol. 2018, 107 Pt. B, 2217–2223. [Google Scholar] [CrossRef]
- Wang, K.; Guo, J.; Cheng, J.; Zhao, X.; Ma, B.; Yang, X.; Shao, H. Ultrasound-assisted extraction of polysaccharide from spent Lentinus edodes substrate: Process optimization, precipitation, structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 1038–1045. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef]
- Morales, D.; Rutckeviski, R.; Villalva, M.; Abreu, H.; Soler-Rivas, C.; Santoyo, S.; Iacomini, M.; Smiderle, F.R. Isolation and comparison of alpha- and beta-D-glucans from shiitake mushrooms (Lentinula edodes) with different biological activities. Carbohydr. Polym. 2020, 229, 115521. [Google Scholar] [CrossRef]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Chen, Z.; Gao, X.; Chen, Y.; Xue, Z.; Guo, Q.; Ma, Q.; Chen, H. Physicochemical properties of polysaccharides from Lentinus edodes under high pressure cooking treatment and its enhanced anticancer effects. Int. J. Biol. Macromol. 2018, 115, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Peng, L.; Xiaofeng, L.; Zhun, X.; Chunying, D.; Jianwen, W. Identification and Biological Characteristics of Wild Lentinus edodes. North Hortic. 2021, 4, 118–123. [Google Scholar]
- Dong, Y.; Liao, X.; Jin, T.; Xie, Y. Nutrient Analysis and Safety Evaluation of Lentinus edodes (Beak.) Sing. Edible Fungi China 2021, 40, 91–96+102. [Google Scholar]
- Hanwu, L.; Gaochao, P.; Dongsheng, Q.; Fanglun, Z.; Yan, S. Analysis of Aroma Components of a Wild Sangria Mushroom in Guizhou Province. Edible Fungi China 2019, 38, 107–108. [Google Scholar]
- Xie, Y.; Li, P.; Lu, L.; Liao, X.; Zhang, L.; Yang, L.; Kang, C. A Kind of Cultivation Method of Masang Mushroom Returning to Nature. CN106665124A [P/OL], 17 May 2017. [Google Scholar]
- Tang, W.; Liu, C.; Liu, J.; Hu, L.; Huang, Y.; Yuan, L.; Liu, F.; Pan, S.; Chen, S.; Bian, S.; et al. Purification of polysaccharide from Lentinus edodes water extract by membrane separation and its chemical composition and structure characterization. Food Hydrocoll. 2020, 105, 105851. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Wu, J.; Chen, R.; Tan, L.; Bai, H.; Tian, L.; Lu, J.; Gao, M.; Bai, C.; Sun, H.; Chi, Y. Ultrasonic disruption effects on the extraction efficiency, characterization, and bioactivities of polysaccharides from Panax notoginseng flower. Carbohydr. Polym. 2022, 291, 119535. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Muthukumaran, C.; Suriya, E.; Muppidathi Keerthana, R.; Kamatchi, M.; Kumar, N.M.; Anbarasan, T.; Jeyanthi, J. Ultrasound aided extraction of yellow pigment from Tecoma castanifolia floral petals: Optimization by response surface method and evaluation of the antioxidant activity. Ind. Crops Prod. 2019, 130, 467–477. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Tabarsa, M.; Anvari, M.; Joyner, H.S.; Behnam, S.; Tabarsa, A. Rheological behavior and antioxidant activity of a highly acidic gum from Althaea officinalis flower. Food Hydrocoll. 2017, 69, 432–439. [Google Scholar] [CrossRef]
- Guo, X.; Shang, X.; Zhou, X.; Zhao, B.; Zhang, J. Ultrasound-assisted extraction of polysaccharides from Rhododendron aganniphum: Antioxidant activity and rheological properties. Ultrason. Sonochem. 2017, 38, 246–255. [Google Scholar] [CrossRef]
- AlYammahi, J.; Hai, A.; Krishnamoorthy, R.; Arumugham, T.; Hasan, S.W.; Banat, F. Ultrasound-assisted extraction of highly nutritious date sugar from date palm (Phoenix dactylifera) fruit powder: Parametric optimization and kinetic modeling. Ultrason. Sonochem. 2022, 88, 106107. [Google Scholar] [CrossRef]
- Chen, S.; Qin, L.; Xie, L.; Yu, Q.; Chen, Y.; Chen, T.; Lu, H.; Xie, J. Physicochemical characterization, rheological and antioxidant properties of three alkali-extracted polysaccharides from mung bean skin. Food Hydrocoll. 2022, 132, 107867. [Google Scholar] [CrossRef]
- Alzorqi, I.; Sudheer, S.; Lu, T.-J.; Manickam, S. Ultrasonically extracted beta-D-glucan from artificially cultivated mushroom, characteristic properties and antioxidant activity. Ultrason. Sonochem. 2017, 35, 531–540. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, H.; Wang, K.; Lin, H.; Li, P.; Huang, Y.; Zhou, S.; Zhang, W.; Chen, C.; Fan, H. Microwave-assisted aqueous two-phase extraction of diverse polysaccharides from Lentinus edodes: Process optimization, structure characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 136, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.H.; Song, A.X.; Li, L.Q.; Yang, Y.; Yao, Z.P.; Wu, J.Y. A high-molecular weight exopolysaccharide from the Cs-HK1 fungus: Ultrasonic degradation, characterization and in vitro fecal fermentation. Carbohydr. Polym. 2020, 246, 116636. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.; Shahbaz, H.M.; Kim, G.R.; Farooq, U.; Kwon, J.H. Improved Extraction and Quality Characterization of Water-Soluble Polysaccharide from Gamma-Irradiated Lentinus edodes. J. Food Sci. 2017, 82, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bach, F.; Ferreira Zielinski, A.A.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Avila, S.; Isidoro Haminiuk, C.W. Bio compounds of edible mushrooms: In vitro antioxidant and antimicrobial activities. LWT Food Sci. Technol. 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Tao, L.; Wang, J.; Wei, Y.; Xiong, B.; Zhang, J.; Lan, W.; Yu, L. Efficient decolorization of oligosaccharides in ginseng (Panax ginseng) residue using ultrasound-assisted macroporous resin. Food Chem. 2023, 419, 136098. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S.; Mariatti, F.; Cravotto, G. Subcritical water extraction as an efficient technique to isolate biologically-active fucoidans from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 128, 244–253. [Google Scholar] [CrossRef]
- Xu, K.; Martinez, M.M.; Yang, B.; Guo, M. Fine structure, physicochemical and antioxidant properties of LM-pectins from okra pods dried under different techniques. Carbohydr. Polym. 2020, 241, 116272. [Google Scholar] [CrossRef]
- Mutaillifu, P.; Bobakulov, K.; Abuduwaili, A.; Huojiaaihemaiti, H.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020, 144, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, D.; Yan, T.; Jiang, W.; Han, X.; Yan, J.; Guo, Y. Nanostructures assembly and the property of polysaccharide extracted from Tremella Fuciformis fruiting body. Int. J. Biol. Macromol. 2019, 137, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.; Zhang, X.; Wang, Y.; Qiang, Y.; Wang, B.; Zou, J.; Niu, J.; Wang, Z. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohydr. Polym. 2022, 291, 119524. [Google Scholar] [CrossRef]
- Niu, G.; You, G.; Zhou, X.; Fan, H.; Liu, X. Physicochemical properties and in vitro hypoglycemic activities of hsian-tsao polysaccharide fractions by gradient ethanol precipitation method. Int. J. Biol. Macromol. 2023, 231, 123274. [Google Scholar] [CrossRef]
- Tang, Y.; He, X.; Liu, G.; Wei, Z.; Sheng, J.; Sun, J.; Li, C.; Xin, M.; Li, L.; Yi, P. Effects of different extraction methods on the structural, antioxidant and hypoglycemic properties of red pitaya stem polysaccharide. Food Chem. 2023, 405 Pt. A, 134804. [Google Scholar] [CrossRef]
- Lin, D.; Long, X.; Xiao, L.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Xing, B. Study on the functional properties and structural characteristics of soybean soluble polysaccharides by mixed bacteria fermentation and microwave treatment. Int. J. Biol. Macromol. 2020, 157, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Xu, Q.; Du, H.; Muinde Kimatu, B.; Su, A.; Yang, W.; Hu, Q.; Xiao, H. Characterization of polysaccharide from Pleurotus eryngii during simulated gastrointestinal digestion and fermentation. Food Chem. 2022, 370, 131303. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Espinal-Ruiz, M.; Parada-Alfonso, F.; Restrepo-Sanchez, L.P.; Narvaez-Cuenca, C.E.; McClements, D.J. Impact of dietary fibers [methyl cellulose, chitosan, and pectin] on digestion of lipids under simulated gastrointestinal conditions. Food Funct. 2014, 5, 3083–3095. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Gao, H. Optimization of enzymes-microwave-ultrasound assisted extraction of Lentinus edodes polysaccharides and determination of its antioxidant activity. Int. J. Biol. Macromol. 2018, 111, 446–454. [Google Scholar] [CrossRef]
- Baeva, E.; Bleha, R.; Lavrova, E.; Sushytskyi, L.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Polysaccharides from Basidiocarps of Cultivating Mushroom Pleurotus ostreatus: Isolation and Structural Characterization. Molecules 2019, 24, 2740. [Google Scholar] [CrossRef]
- Guo, X.; Kang, J.; Xu, Z.; Guo, Q.; Zhang, L.; Ning, H.; Cui, S.W. Triple-helix polysaccharides: Formation mechanisms and analytical methods. Carbohydr. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, G. Improving method, properties and application of polysaccharide as emulsifier. Food Chem. 2021, 376, 131937. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, L.; Liu, C.; McClements, D.J. Utilization of anionic polysaccharides to improve the stability of rice glutelin emulsions: Impact of polysaccharide type, pH, salt, and temperature. Food Hydrocoll. 2017, 64, 112–122. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, C.; Shi, H.; Li, X. Structural studies of a mannoglucan from Cremastra appendiculata (Orchidaceae) by chemical and enzymatic methods. Carbohydr. Polym. 2021, 272, 118524. [Google Scholar] [CrossRef] [PubMed]
- Goff, H.D.; Repin, N.; Fabek, H.; El Khoury, D.; Gidley, M.J. Dietary fibre for glycaemia control: Towards a mechanistic understanding. Bioact. Carbohydr. Diet. Fibre 2018, 14, 39–53. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Zhang, X.; Zhang, Y.; Zheng, Q.; Lan, M.; Li, B. Structural characteristics and antioxidant and hypoglycemic activities of a heteropolysaccharide from Anemarrhena asphodeloides Bunge. Int. J. Biol. Macromol. 2023, 236, 123843. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, H.; Zhang, J.; Li, F.; Wei, K.; Wei, X.; Wang, Y. Valorization of Polysaccharides Obtained from Dark Tea: Preparation, Physicochemical, Antioxidant, and Hypoglycemic Properties. Foods 2021, 10, 2276. [Google Scholar] [CrossRef]
- Zheng, Q.; Jia, R.B.; Ou, Z.R.; Li, Z.R.; Zhao, M.; Luo, D.; Lin, L. Comparative study on the structural characterization and alpha-glucosidase inhibitory activity of polysaccharide fractions extracted from Sargassum fusiforme at different pH conditions. Int. J. Biol. Macromol. 2022, 194, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Yu, Y.B.; Wang, C.; Cai, W.D.; Wu, L.X.; Yang, Y.; Zhang, H.N. Production, physicochemical characteristics, and in vitro biological activities of polysaccharides obtained from fresh bitter gourd (Momordica charantia L.) via room temperature extraction techniques. Food Chem. 2021, 337, 127798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Li, C.; Huang, Q.; You, L.J.; Chen, C.; Fu, X.; Liu, R.H. Comparative study on the physicochemical properties and bioactivities of polysaccharide fractions extracted from Fructus Mori at different temperatures. Food Funct. 2019, 10, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, M.; Wang, Z.; Huang, X.; Hu, X.; Wang, Q.; Nie, S. A novel soluble powder containing high Dendrobium huoshanense polysaccharide and its in vitro hypoglycemic activities evaluation. Bioact. Carbohydr. Diet. Fibre 2023, 30, 100362. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; You, L.J.; Fu, X. Chemical property and impacts of different polysaccharide fractions from Fructus Mori. on lipolysis with digestion model in vitro. Carbohydr. Polym. 2017, 178, 360–367. [Google Scholar]
| Sample | Reducing Sugar (%) | Polyphenol (%) | Protein (%) | Uronic Acid (%) | Yield (%) |
|---|---|---|---|---|---|
| H90 | 46.08 ± 0.22 a | 1.08 ± 0.01 b | 5.32 ± 0.18 | 4.31 ± 0.09 | 6.24 ± 0.10 d |
| U10 | 45.08 ± 0.28 ab | 1.11 ± 0.04 b | 5.35 ± 0.25 | 4.71 ± 0.08 | 7.83 ± 0.17 c |
| U20 | 44.26 ± 0.15 b | 1.17 ± 0.02 b | 5.76 ± 0.17 | 4.78 ± 0.10 | 8.48 ± 0.15 b |
| U60 | 45.13 ± 0.19 ab | 1.38 ± 0.05 a | 6.22 ± 0.61 | 5.09 ± 0.26 | 9.43 ± 0.19 a |
| Sample | Fucose | Rhamnose | Arabinose | Galactose | Glucose | Xylose | Mannose | Glucuronic Acid |
|---|---|---|---|---|---|---|---|---|
| H90 | 2.11% | 0.28% | 0.19% | 8.58% | 80.06% | 1.04% | 6.92% | 0.84% |
| U10 | 2.26% | 0.14% | 0.10% | 8.94% | 80.38% | 0.51% | 6.84% | 0.82% |
| U20 | 2.33% | 0.14% | 0.09% | 9.07% | 80.15% | 0.51% | 6.87% | 0.85% |
| U60 | 2.32% | 0.19% | 0.10% | 9.14% | 79.79% | 0.44% | 6.51% | 1.50% |
| Sample | L* | a* | b* | WI | ΔE* |
|---|---|---|---|---|---|
| H90 | 83.78 ± 0.14 b | 3.58 ± 0.17 c | 5.50 ± 0.34 c | 82.50 ± 0.21 b | 13.08 ± 0.21 b |
| U10 | 86.02 ± 0.18 a | 4.18 ± 0.06 b | 8.94 ± 0.12 b | 82.88 ± 0.17 ab | 12.83 ± 0.17 b |
| U20 | 85.88 ± 0.24 a | 4.06 ± 0.14 b | 8.62 ± 0.30 b | 82.96 ± 0.37 a | 12.72 ± 0.38 b |
| U60 | 83.32 ± 0.26 c | 4.84 ± 0.04 a | 10.11 ± 0.12 a | 79.91 ± 0.25 c | 15.77 ± 0.25 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Wang, L.; Chen, X.; Song, A.; He, L.; Wang, L.; Huang, D. Lentinula edodes Sing Polysaccharide: Extraction, Characterization, Bioactivities, and Emulsifying Applications. Foods 2023, 12, 3289. https://doi.org/10.3390/foods12173289
Dai Y, Wang L, Chen X, Song A, He L, Wang L, Huang D. Lentinula edodes Sing Polysaccharide: Extraction, Characterization, Bioactivities, and Emulsifying Applications. Foods. 2023; 12(17):3289. https://doi.org/10.3390/foods12173289
Chicago/Turabian StyleDai, Yan, Lei Wang, Xingyi Chen, Angxin Song, Laping He, Lingyuan Wang, and Diandian Huang. 2023. "Lentinula edodes Sing Polysaccharide: Extraction, Characterization, Bioactivities, and Emulsifying Applications" Foods 12, no. 17: 3289. https://doi.org/10.3390/foods12173289
APA StyleDai, Y., Wang, L., Chen, X., Song, A., He, L., Wang, L., & Huang, D. (2023). Lentinula edodes Sing Polysaccharide: Extraction, Characterization, Bioactivities, and Emulsifying Applications. Foods, 12(17), 3289. https://doi.org/10.3390/foods12173289







