Quality of Edible Sesame Oil as Obtained by Green Solvents: In Silico versus Experimental Screening Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Plant Material
2.2. Computational Methods
2.2.1. Solute–Solvent Solubility Prediction by HSPs
2.2.2. COSMO-RS Prediction
2.3. Oil Extraction
2.4. Physicochemical Characteristics of Sesame Seed Oils
2.4.1. K232 and K270 Determination
2.4.2. Determination of Chlorophyll Content
2.4.3. Oxidative Stability
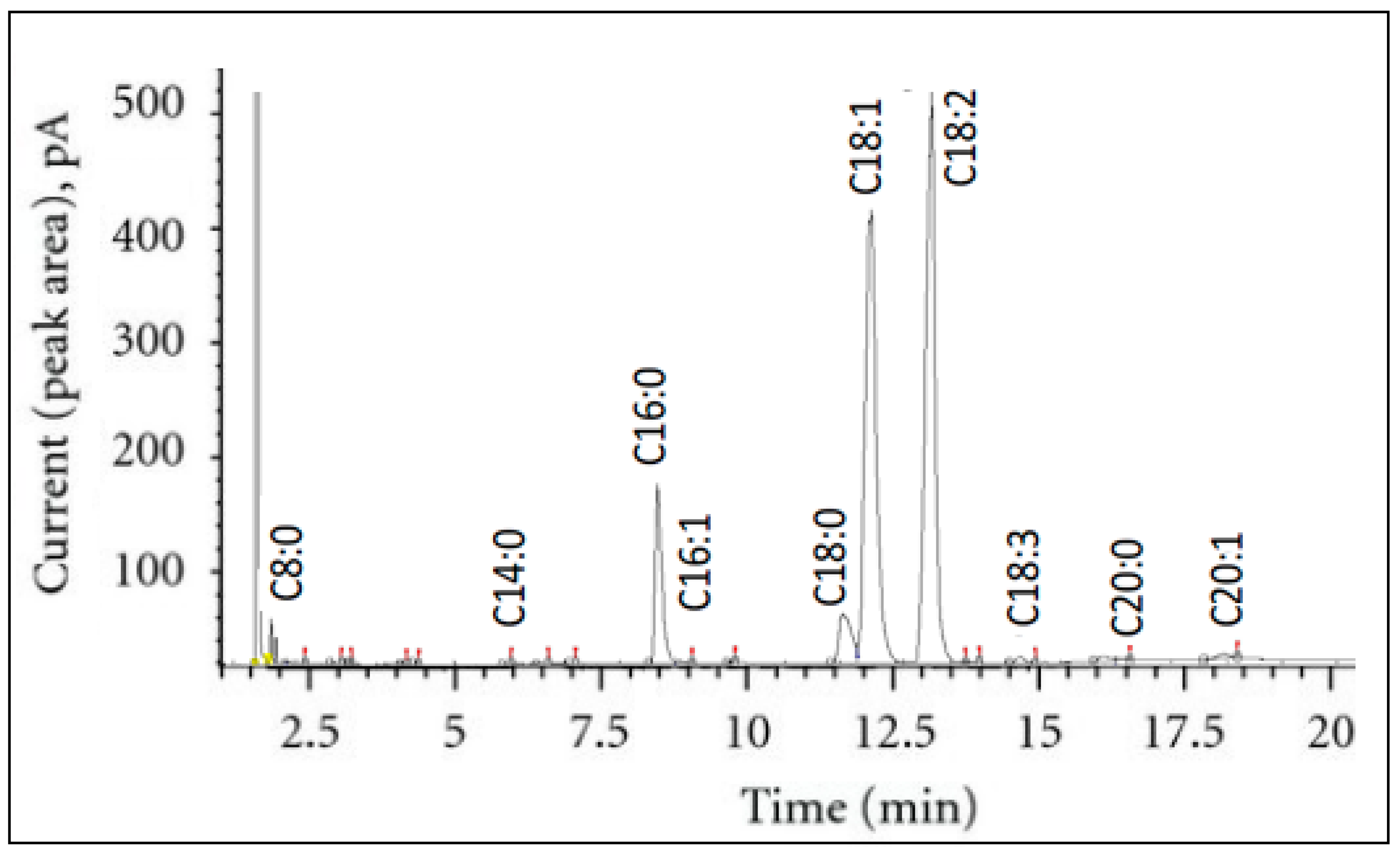
2.5. Fatty Acid Composition
2.6. Sterol Analysis
2.7. Tocopherol Contents
2.8. Total Phenolic Content
2.9. Antioxidant Potential Analyses
2.10. Anti-Inflammatory Activity
2.10.1. Cell Culture
2.10.2. Measurement of Nitrite Production
2.11. Statistical Analysis
3. Results and Discussion
3.1. Computational Methods
3.2. COSMO-RS Simulations
3.3. Physicochemical Properties
3.4. Experimental Solvent Screening
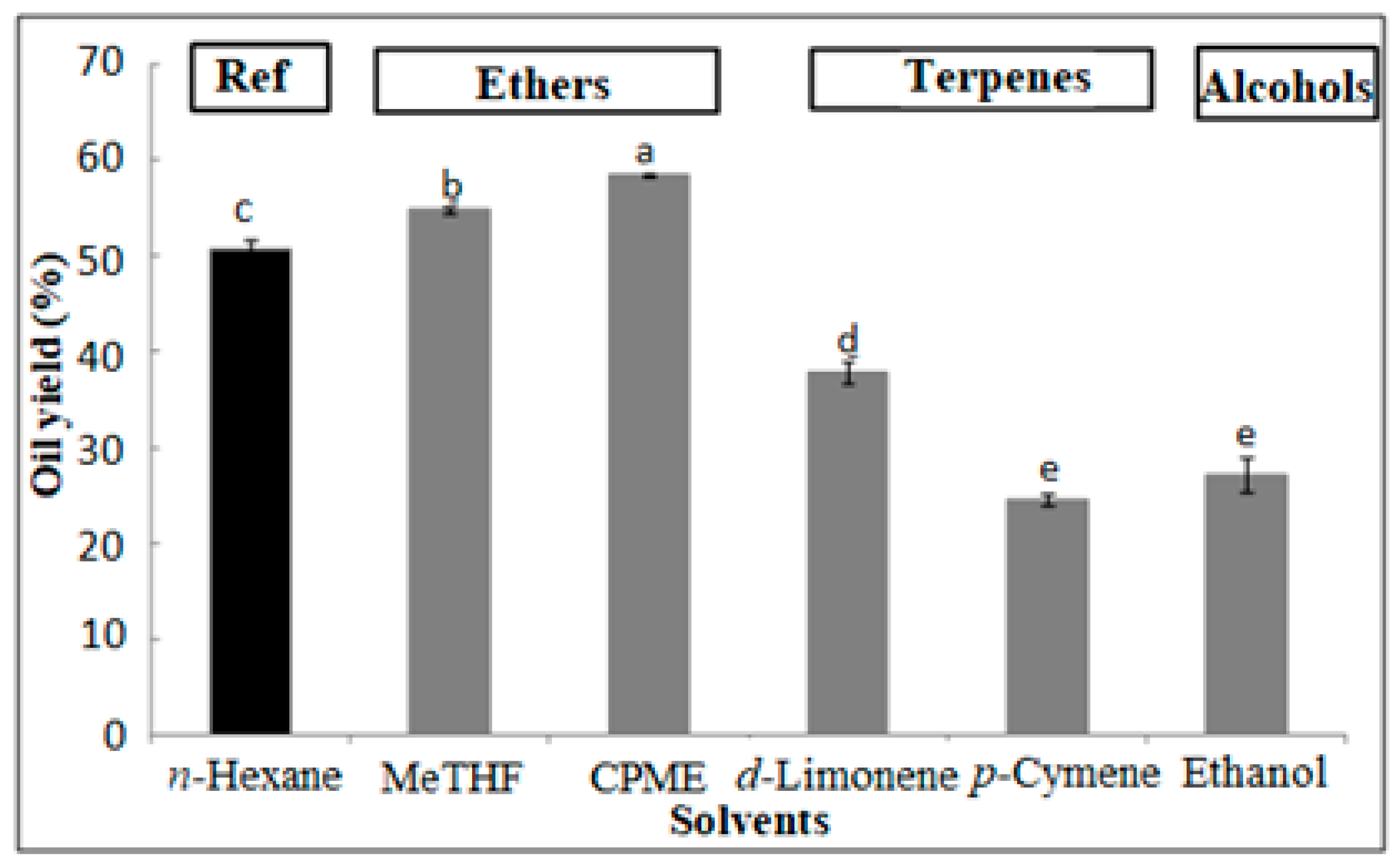
3.4.1. Oil Yield
3.4.2. Sterol Composition
3.4.3. Tocopherol Composition
3.4.4. Total Phenolic Content
3.4.5. Antioxidant Potential
3.5. In Silico Screening versus Experimental Screening
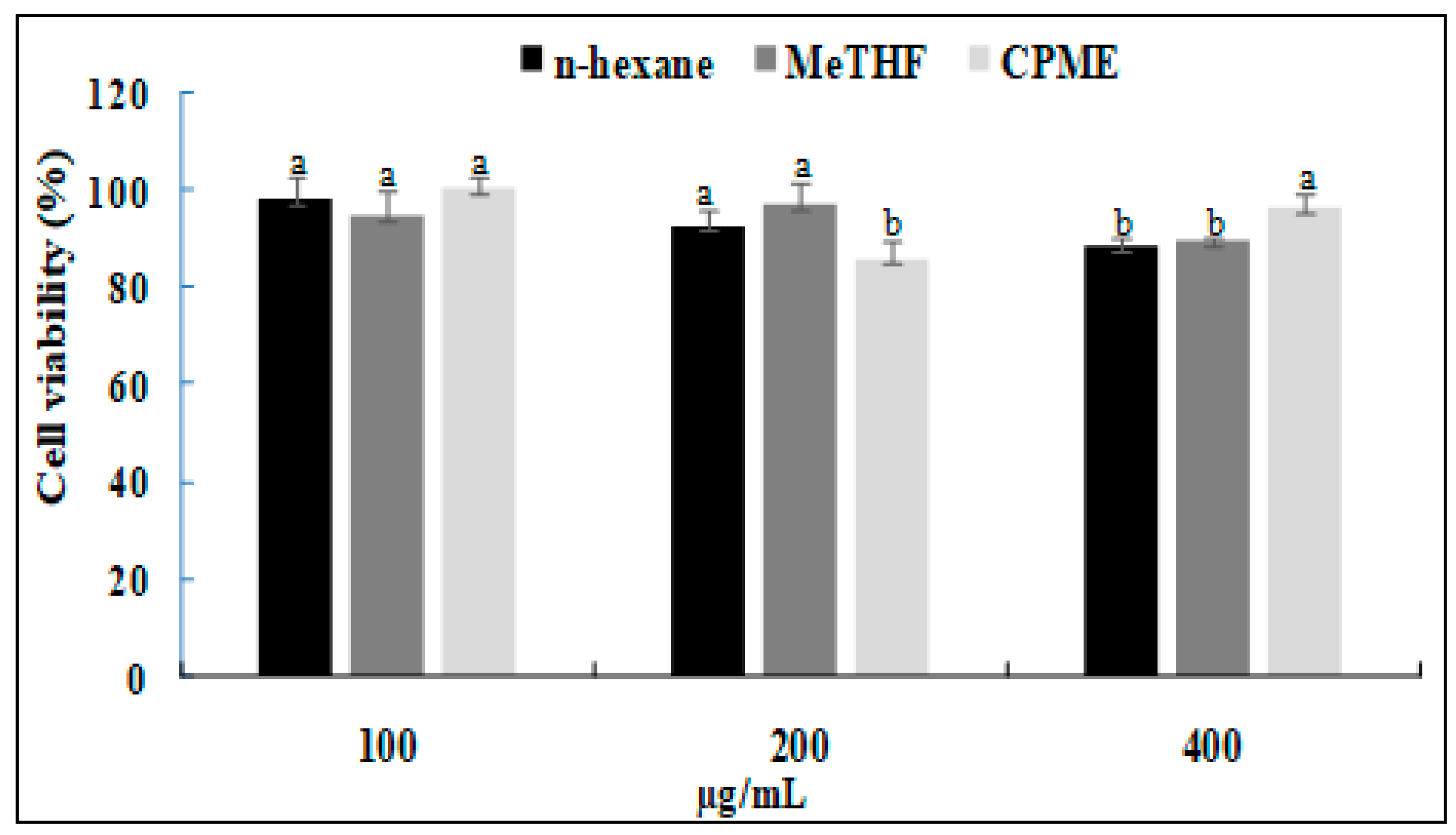
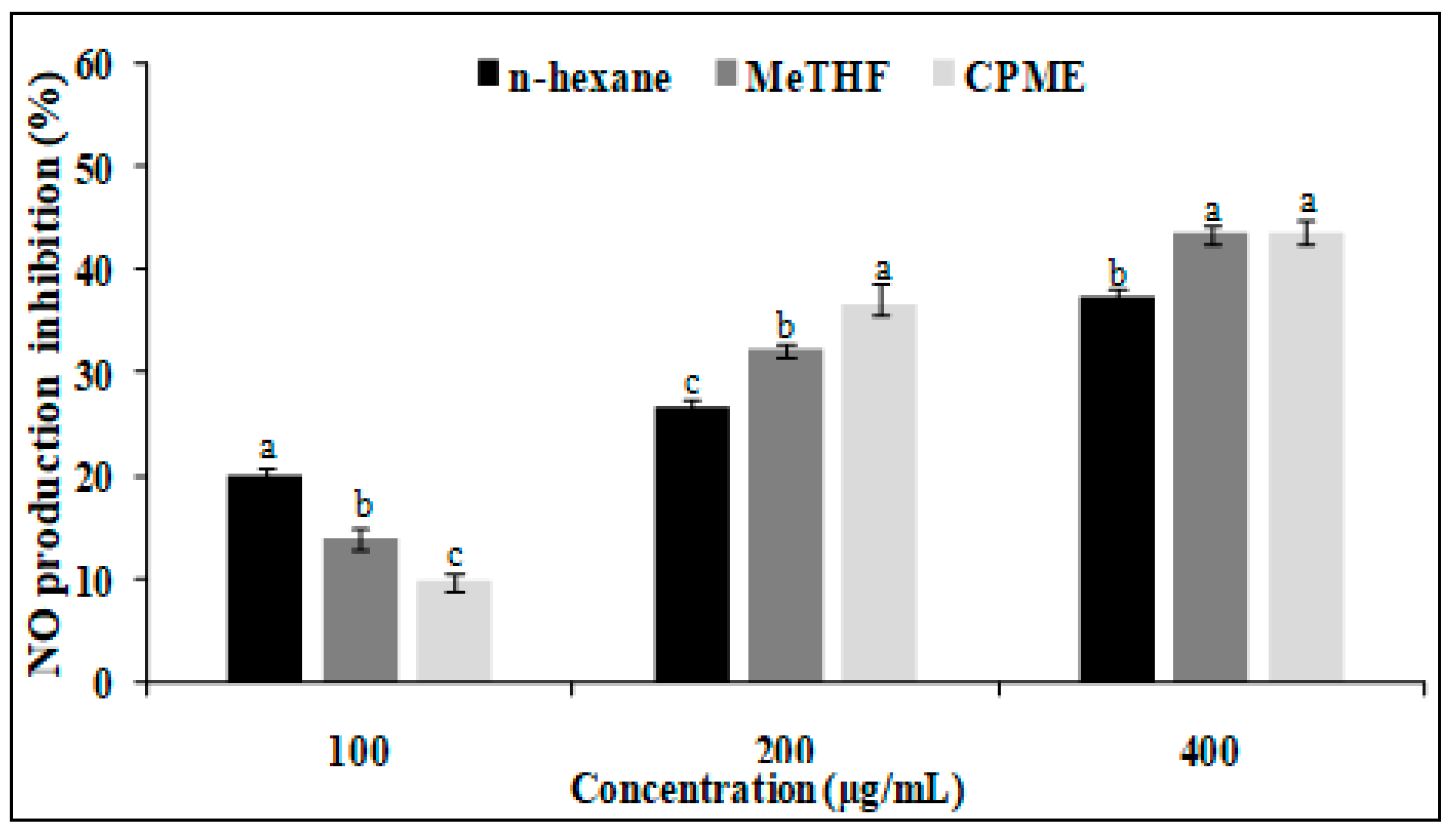
3.6. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buranachokpaisan, K.; Muangrat, R.; Chalermchat, Y. Supercritical CO2 extraction of residual oil from pressed sesame seed cake: Optimization and its physicochemical properties. J. Food Process. Preserv. 2021, 45, e15722. [Google Scholar] [CrossRef]
- Arab, R.; Casal, S.; Pinho, T.; Cruz, R.; Freidja, M.L.; Lorenzo, J.M.; Hano, C.; Madani, K.; Boulekbache-Makhlouf, L. Effects of seed roasting temperature on sesame oil fatty acid composition, lignan, sterol and tocopherol contents, oxidative stability and antioxidant potential for food applications. Molecules 2022, 27, 4508. [Google Scholar] [CrossRef]
- Tan, C.P.; Che Man, Y.B.; Selamat, J.; Yusoff, M.S.A. Comparative studies of oxidative stability of edible oils by DSC and oxidative stability index methods. Food Chem. 2002, 76, 385–389. [Google Scholar] [CrossRef]
- Abbas, S.; Sharif, M.K.; Sibt-e-Abbas, M.; Fikre Teferra, T.; Sultan, M.T.; Anwar, M.J. Nutritional and therapeuticpotential of sesame seeds. J. Food Qual. 2022, 2, 6163753. [Google Scholar]
- Sohouli, M.H.; Haghshenas, N.; Hernández-Ruiz, Á.; Shidfar, F. Consumption of sesame seeds and sesame products hasfavorable effects on blood glucose levels but not on insulin resistance: A systematic review and meta-analysis of controlled clinical trials. Phytother. Res. 2022, 36, 1126–1134. [Google Scholar] [CrossRef]
- Myint, D.; Gilani, S.A.; Kawase, M.; Watanabe, K.N. Sustainable Sesame (Sesamum indicum L.) Production through improved technology: An overview of production, challenges, and opportunities in Myanmar. Sustainability 2020, 12, 3515. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. Comparison of fatty acid methyl esters of palm and palmist oils determined by GCxGC–ToF–MS and GC–MS/FID. S. Afr. J. Bot. 2017, 112, 483–488. [Google Scholar] [CrossRef]
- Rapinel, V.; Claux, O.; Abert-Vian, M.; McAlinden, C.; Bartier, M.; Patouillard, N.; Jacques, L.; Chemat, F. 2-Methyloxolane (2-MeOx) as sustainable lipophilic solvent to substitute n-hexane for green extraction of natural products. Properties, applications, and perspectives. Molecules 2020, 25, 3417. [Google Scholar] [CrossRef]
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Rapinel, V.; Tomao, V.; Stathopoulos, P.; Skaltsounis, A.L.; Tabasso, S.; Jacques, L.; Chemat, F. Higher yield and polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent. Foods 2022, 11, 1357. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Bouzouba, Z.; Asdadi, A.; El Yadini, A.; Charrouf, Z. Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. J. Saudi Soci. Agric. Sci. 2017, 16, 105–111. [Google Scholar] [CrossRef]
- Chemat, F.; AbertVian, M.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Perrier, A.; Delsart, C.; Boussetta, N.; Grimia, N.; Citeau, M.; Vorobiev, E. Effect of ultrasound and green solvents addition on the oil extraction efficiency from rapeseed flakes. Ultrason. Sonochem. 2017, 39, 58–65. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M. (Eds.) Alternative Solvents for Natural Products Extraction, Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Cascant, M.M.; Breil, C.; Miguel de la Guardia, S.G.; Fabiano-Tixier, A.S.; Chemat, F. A green analytical chemistry approach for lipid extraction: Computation methods in the selection of green solvents as alternative to n-hexane. Anal. Bioanal. Chem. 2017, 409, 3527–3539. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen solubility parameters. In A User’s Handbook, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Klamt, A. The COSMO and COSMO-RS solvation models. Wiley Interdiscipl. Rev. Comput. Mol. Sci. 2011, 1, 699–709. [Google Scholar] [CrossRef]
- Bourgou, S.; BettaiebRebey, I.; Ben Kaab, S.; Hammami, M.; Dakhlaoui, S.; Sawsen, S.; Msaada, K.; Isoda, H.; Ksouri, R.; Fauconnier, M.-L. Green solvent to substitute n-hexane for bioactive lipids extraction from black cumin and basil seeds. Foods 2021, 10, 1493. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Bourgou, S.; Detry, P.; Wannes, W.A.; Kenny, T.; Ksouri, R.; Sellami, I.H.; Fauconnier, M.L. Green extraction of fennel and anise edible oils using bio-based solvent and supercritical fluid: Assessment of chemical composition, antioxidant property, and oxidative stability. Food Bioprocess Technol. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Chaabani, E.; Vian, M.; Dakhlaoui, S.; Bourgou, S.; Chemat, F.; Ksouri, R. Technological challenges in oilseedcrushing and refining/Défis technologiques de la trituration et du raffinage des oléagineux. OCL 2019, 25, 18. [Google Scholar]
- Sicaire, A.G.; Abert Vian, M.; Fine, F.; Carré, P.; Tostain, S.; Chemat, F. Experimental approach versus COSMO-RS assisted solvent screening for predicting the solubility of rapeseed oil. OCL 2015, 22, D404. [Google Scholar] [CrossRef]
- Ben Youssef, S.; Fakhfakh, J.; Breil, C.; Abert-Vian, M.; Chemat, F.; Allouche, N. Green extraction procedures of lipids from Tunisian date palm seeds. Ind. Crop. Prod. 2017, 108, 520–525. [Google Scholar] [CrossRef]
- Ribeiro, S.A.O.; Nicacio, A.E.; Zanqui, A.B.; Biondo, P.B.F.; de Abreu-Filho, B.A.; Visentainer, J.V.; Sandra Gomes, T.M.; Matsushita, M. Improvements in the quality of sesame oil obtained by a green extraction method using enzymes. LWT Food Sci. Technol. 2016, 65, 464–470. [Google Scholar] [CrossRef]
- Angles, E.; Jaouen, P.; Pruvost, J.; Marchal, L. Wet lipid extraction from the microalga Nannochloropsis sp.: Disruption, physiological effects and solvent screening. Algal Res. 2017, 21, 27–34. [Google Scholar] [CrossRef]
- Bundeesomchok, K.; Filly, A.; Rakotomanomana, N.; Panichayupakaranant, P.; Chemat, F. Extraction of a-mangostin from Garcinia mangostana L. using alternative solvents: Computational predictive and experimental studies. LWT-Food Sci. Technol. 2016, 65, 297–303. [Google Scholar] [CrossRef]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-based solvents for green extraction of lipids from oleaginous yeast biomass for sustainable aviation biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef]
- Claux, O.; Rapinel, V.; Goupy, P.; Patouillard, N.; Vian, M.A.; Jacques, L.; Chemat, F. Dry and Aqueous 2-Methyloxolane as Green Solvents for Simultaneous Production of Soybean Oil and Defatted Meal. ACS Sustain. Chem. Eng. 2021, 9, 7211–7223. [Google Scholar] [CrossRef]
- Firestone, D. Official Methods and Recommended Practices of the AOCS; AOCS: Urbana, IL, USA, 2009. [Google Scholar]
- Tanouti, K.; Serghini-Caid, H.; Chaieb, E.; Benali, A.; Harkous, M.; Elamrani, A. Amelioration qualitative d’huiles d’olive produites dans le maroc oriental. Technol. Lab. 2011, 6, 1–12. [Google Scholar]
- Chtourou, M.; Gargouri, B.; Jaber, H.; Abdelhedi, R.; Bouaziz, M. Comparative study of olive oil quality from Chemlali Sfax versus Arbequina cultivated in Tunisia. Eur. J. Lipid Sci. Technol. 2013, 115, 631–640. [Google Scholar] [CrossRef]
- Tabee, E.; Azadmard-Damirchi, S.; Jägerstad, M.; Dutta, P.C. Lipids and phytosterol oxidation in commercial French fries commonly consumed in Sweden. J. Food Compos. Anal. 2008, 21, 169–177. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Yurawecz, M.P.; Parry, J.; Su, L.; Luther, M.; Whittaker, P.; Yu, L. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005, 53, 566–573. [Google Scholar] [CrossRef]
- Ganesan, P.; Kumar, C.S.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008, 99, 2717–2723. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Aboura, I.; Nani, A.; Belarbib, M.; Murtaza, B.; Fluckiger, A.; Dumont, A.; Benammar, C.; Saidani Tounsi, M.; Ghiringhelli, F.; Rialland, M.; et al. Protective effects of polyphenol-rich infusions from carob (Ceratonia siliqua) leaves and cladodes of Opuntia ficus-indica against inflammation associated with diet-induced obesity and DSS-induced colitis in Swiss mice. Biomed. Pharmacother. 2017, 96, 1022–1035. [Google Scholar] [CrossRef]
- Olaleye, O.O.; Kukwa, R.E.; Eke, M.O.; Aondo, T.O. Extraction, physicochemical and phytochemical characterization of oil from sesame seed. Asian Food Sci. J. 2018, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.D. Extraction and characterization of oil from sesame seed. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 752. [Google Scholar]
- Zhang, W.; Li, K.; Zheng, H.; Liu, L.; Ge, S.; Zhang, H. Quality analysis of Phyllanthus emblic L. seed oil extracted by different solvents. Sci. Technol. Food Ind. 2017, 38, 261–265. [Google Scholar]
- Farhan, S.; Aiza, Q.; Muhammad, T.N.; Rabia, S.A.; Muhammad, S.A.; Muhammad, A. Nutritional composition and fatty acid profile of some promising sesame cultivars. Pak. J. Food Sci. 2015, 25, 98–103. [Google Scholar]
- Sabah EL Khier, M.K.; Ishag, K.E.A.; Yagoub, A.E.A. Chemical composition and characteristics of sesame seed cultivars grown in Sudan. Res. J. Agric. Biol. Sci. 2009, 4, 761–766. [Google Scholar]
- Nzikou, J.M.; Mvoula-tsiéri, M.; Ndangui, C.B.; Pambou-Tobi, N.P.G.; Kimbonguila, A.; Loumouamou, B.; Silou, T.; Desobry, S. Characterization of seeds and oil of sesame (Sesamum indicum L.) and the kinetics of degradation of the oil during heating. Res. J. Appl. Sci. Engin. Technol. 2010, 2, 227–232. [Google Scholar]
- Li, X.J.; Shen, Y.B.; Wu, G.C.; Qi, X.G.; Zhang, H.; Wang, L.; Qian, H.F. Determination of key active components in different edible oils affecting lipid accumulation and reactive oxygen species production in HepG2 cells. J. Agric. Food Chem. 2018, 66, 11943–11956. [Google Scholar] [CrossRef]
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Attia, H. Quality characteristics of sesame seeds and by products. Food Chem. 2007, 103, 641–650. [Google Scholar] [CrossRef]
- Wei, P.; Zhao, F.; Wang, Z.; Wang, Q.; Chai, X.; Hou, G.; Meng, Q. Sesame (Sesamum indicum L.): A Comprehensive review of nutritional value, phytochemical composition, health benefits, development of food, and industrial applications. Nutrients 2022, 14, 4079. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Standard for Named Vegetable Oils: Codex Stan 210 (Amended 2003, 2005); Codex Alimentarius: Rome, Italy, 2005; p. 13. [Google Scholar]
- Lawrence, G.D. The Fats of Life: Essential Fatty Acids in Health and Disease; Rutgers University Press: Piscataway, NJ, USA, 2010. [Google Scholar]
- Hijab, M.; Saleem, J.; Parthasarathy, P.; Mackey, H.R.; McKay, G. Two-stage optimisation for malachite green removal using activated date pits. Biomass Convers. Biorefinery 2021, 11, 727–740. [Google Scholar] [CrossRef]
- Villaseñor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on β-sitosterol and its glucoside. Phytother. Res. 2002, 16, 417–421. [Google Scholar] [CrossRef]
- Borges, T.H.; Pereira, J.A.; Cabrera–Vique, C.; Seiquer, I. Study of the antioxidant potential of Arbequina extra virgin olive oils from Brazil and Spain applying combined models of simulated digestion and cell culture markers. J. Funct. Foods. 2017, 37, 209–218. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, Z.; Wang, Y.; Ying, L. The effect of alternative solvents to n-hexane on the green extraction of Litsea cubeba kernel oils as new oil sources. Ind. Crop. Prod. 2018, 126, 340–346. [Google Scholar] [CrossRef]
- Bopitiya, D.; Madhujith, T. Antioxidant activity and total phenolic content of sesame (Sesamum indicum L.) seed oil. Trop. Agricult. Res. 2013, 24, 296–302. [Google Scholar] [CrossRef]
- Sumara, A.; Stachniuk, A.; Olech, M.; Nowak, R.; Montowska, M.; Fornal, E. Identification of sunflower, rapeseed, flaxseed and sesame seed oil metabolomic markers as a potential tool for oil authentication and detecting adulterations. PLoS ONE 2023, 18, e0284599. [Google Scholar] [CrossRef] [PubMed]
- Sani, I.; Ajuma Sule, F.; Warra, A.A.; Bello, F.; Fakai, I.M.; Abdulhamid, A. Phytochemicals and mineral elements composition of white Sesamum indicum L. seed oil. Int. J. Trad. Nat. Med. 2013, 2, 118–130. [Google Scholar]
- Oliveira, R.M.A.; Henriques, J.D.O.; Sartoratto, A.; Maciel, M.R.W.; Martinez, P.F.M. Evaluation of Limonene in sugarcane wax extraction. Sustain. Chem. Pharm. 2022, 27, 100657. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Inomata, S.; Hirokawa, J. Oligomerization reaction of the criegee intermediate leads to secondary organic aerosol formation in ethylene ozonolysis. J. Phys. Chem. A 2013, 117, 12912–12921. [Google Scholar] [CrossRef]
- Clark, M.; Steger-Hartmann, T. A big data approach to the concordance of the toxicity of pharmaceuticals in animals and humans. Regul. Toxicol. Pharmacol. 2018, 96, 94–105. [Google Scholar] [CrossRef]
- Scott, C.; Lundgren, H.; Thompson, P. Guide to Supply Chain Management; Springer: Berlin/Heidelberg, Germany, 2011; 189p. [Google Scholar]
- Probst, K.V.; Wales, M.D.; Rezac, M.E.; Vadlani, P.V. Evaluation of green solvents: Oil extraction from oleaginous yeast Lipomyces starkeyi using cyclopentyl methyl ether (CPME). Biotechnol. Prog. 2017, 33, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; BettaiebRebey, I.; Dakhlaoui, S.; Msaada, K.; SaidaniTounsi, M.; Ksouri, R.; Fauconnier, M.; Hamrouni-Sellami, I. Green extraction of oil from Carum carvi seeds using bio-based solvent and supercritical fluid: Evaluation of its antioxidant and anti-inflammatory activities. Phytochem. Anal. 2019, 31, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, K.; Narasimhulu, C.A.; Bapputty, R.; Parthasarath, S. Anti-inflammatory and antioxidant activities of then on lipid (aqueous) components of sesame oil: Potential use in atherosclerosis. J. Med. Food 2015, 18, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Van, Q.; Nayak, B.N.; Reimer, M.; Jones, P.J.H.; Fulcher, R.G.; Rempel, C.B. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L. and Viburnum trilobum in a cell screening assay. J. Ethnopharmacol. 2009, 125, 487–493. [Google Scholar] [CrossRef]
- Ren, J.; Chung, S.H. Anti-inflammatory effect of α-linolenic acid and its mode of action through the inhibition of nitric oxide productionand inducible nitric oxide synthase gene expression via NF-κBandmitogen-activated protein kinase pathways. J. Agric. Food Chem. 2007, 55, 5073–5080. [Google Scholar] [CrossRef]
- Chavali, S.; Zhong, W.; Forse, R. Dietary α-linolenic acid increases TNF-α, and decreases IL-6, IL-10 in response to LPS: Effects of sesaminon the Δ-5 desaturation of ω6 and ω3 fatty acids in mice. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 185–191. [Google Scholar] [CrossRef]
| Solvent Properties | ||||||
|---|---|---|---|---|---|---|
| Bp (°C) | Log P | Mw (g/mol) | Viscosity, (25 °C Cp) | Resource | CMR * | |
| n-Hexane | 68.5 | 3.94 | 86.2 | 0.31 | Petroleum | 2 |
| MeTHF | 80 | 0.82 | 86.1 | 0.6 | Cereal crop | No |
| CPME | 105.3 | 1.41 | 100.2 | 0.55 | Chemical synthesis | No |
| d-limonene | 175.4 | 4.45 | 136.2 | 0.923 | Cereal crop | No |
| p-cymene | 173.9 | 4.02 | 134.2 | 0.79 | Wood | No |
| Ethanol | 72.6 | −0.19 | 46.1 | 1.095 | Cereal crop | No |
| HSPs | RED | |||||||
|---|---|---|---|---|---|---|---|---|
| δd | δp | δh | C16:0 | C18:1 | C18:2 | γ-Tocopherol | β-Sitosterol | |
| n-Hexane | 13.9 | 0.1 | 0.1 | 1.77 | 1.77 | 1.95 | 2.03 | 2.00 |
| MeTHF | 15 | 4.7 | 3.9 | 0.57 | 0.65 | 0.75 | 1.41 | 1.24 |
| CPME | 14.5 | 3.4 | 2.8 | 0.87 | 0.94 | 1.17 | 1.97 | 1.89 |
| d-Limonene | 13.9 | 1.8 | 2.1 | 0.73 | 0.68 | 0.82 | 0.31 | 0.30 |
| p-Cymene | 14.6 | 2.2 | 1.3 | 1.26 | 1.12 | 1.23 | 1.96 | 1.87 |
| Ethanol | 14 | 9.1 | 15.2 | 2.94 | 3.03 | 2.98 | 5.80 | 5.90 |
 Reference or equivalent
Reference or equivalent  Better than reference
Better than reference  Lesser than reference.
Lesser than reference.| C16:0 | C18:1 | C18:2 | γ-Tocopherol | β-Sitosterol | |
|---|---|---|---|---|---|
| n-Hexane | −1.27 | −1.34 | −1.31 | −0.2974 | −0.8017 |
| MeTHF | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CPME | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| d-Limonene | −0.96 | −0.98 | −0.88 | −0.1324 | −0.6992 |
| p-Cymene | −1.03 | −1.03 | −0.9 | −0.2018 | −0.7829 |
| Ethanol | −0.001 | −0.05 | 0.0 | −0.8650 | −0.5797 |
 Reference or equivalent
Reference or equivalent  Better than reference
Better than reference  Lesser than reference.
Lesser than reference.| Quality Parameters | n-Hexane | MeTHF | CPME | d-Limonene | p-Cymene | Ethanol |
|---|---|---|---|---|---|---|
| AV (mgKOH/g oil) | 1.69 ± 0.04 a | 1.05 ± 0.01 a | 1.73 ± 0.02 a | 0.92 ± 0.01 a | 0.95 ± 0.00 a | 1.07 ± 0.04 a |
| PV (mEq O2/kg) | 3.42 ± 0.34 b | 2.67 ± 0.84 b | 3.95 ± 0.37 a | 4.83 ± 0.94 a | 4.36 ± 0.04 a | 4.01 ± 0.02 a |
| IV (g of I2/100 g) | 111 ± 2.83 a | 123 ± 6.10 a | 105 ± 1.89 a | 104 ± 0.93 a | 93 ± 0.71 ab | 96 ± 1.22 ab |
| RI (20 °C) | 1.42 ± 0.04 a | 1.45 ± 0.02 a | 1.47 ± 0.00 a | 1.45 ± 0.01 a | 1.44 ± 0.01 a | 1.47 ± 0.00 a |
| K232 | 0.94 ± 0.00 a | 1.33 ± 0.22 a | 1.05 ± 0.03 a | 1.18 ± 0.05 a | 1.52 ± 0.00 a | 0.82 ± 0.01 a |
| K270 | 0.24 ± 0.01 b | 0.38 ± 0.03 a | 0.31 ± 0.03 a | 0.41 ± 0.00 a | 0.47 ± 0.01 a | 0.23 ± 0.01 b |
| Chlorophyll (mg/kg) | 2.05 ± 0.45 a | 1.89 ± 0.17 a | 1.92 ± 0.06 a | 2.34 ± 0.04 a | 2.67 ± 0.00 a | 1.73 ± 0.09 a |
| Oxidative stability (h) | 27.83 ± 0.64 a | 28.34 ± 0.25 a | 28.56 ± 0.03 a | 28.05 ± 0.01 a | 27.30 ± 0.66 a | 27.04 ± 0.75 b |
| Fatty acids (%) | ||||||
| C8:0 | 0.02 ± 0.01 c | 0.43 ± 0.07 a | 0.05 ± 0.1 b | 0.48 ± 0.1 a | 0.45 ± 0.39 a | 0.05 ± 0.02 c |
| C14:0 | 0.01 ± 0.01 c | 0.05 ± 0.02 c | 0.08 ± 0.03 c | 0.54 ± 0.05 a | 0.11 ± 0.02 b | 0.01 ± 0.01 c |
| C16:0 | 10.71 ± 0.11 b | 8.94 ± 0.1 b | 8.14 ± 0.01 b | 9.57 ± 0.26 b | 10.5 ± 0.3 a | 9.86 ± 0.17 b |
| C16:1 | 0.05 ± 0.03 b | 0.32 ± 0.15 a | 0.31 ± 0.02 a | 0.33 ± 0.04 a | 0.33 ± 0.28 a | 0.06 ± 0.04 b |
| C18:0 | 3.74 ± 0.31 c | 5.22 ± 0.04 b | 4.15 ± 0.16 bc | 5.07 ± 0.64 b | 6.67 ± 2.12 a | 6.01 ± 0.1 a |
| C18:1 | 42.77 ± 0.52 a | 39.27 ± 0.27 c | 42.08 ± 0.38 a | 44.35 ± 2.83 a | 40.46 ± 1.51 b | 40.79 ± 0 b |
| C18:2 | 43.99 ± 0.25 a | 42.17 ± 0.26 a | 43.41 ± 0.01 a | 39.38 ± 1.97 b | 38.88 ± 1.65 b | 43.18 ± 0.05 a |
| C18:3 | 0.33 ± 0.04 b | 0.39 ± 0.03 b | 0.17 ± 0.1 c | 0.14 ± 0.2 c | 0.71 ± 0.61 a | 0.39 ± 0.01 b |
| C20:0 | 0.09 ± 0.02 c | 1.07 ± 0.75 a | 0.17 ± 0.04 bc | 0.10 ± 0.07 c | 0.37 ± 0.06 b | 0.27 ± 0.13 b |
| C20:1 | 0.12 ± 0.03 b | 0.23 ± 0.01 a | 0.03 ± 0.01 c | 0.08 ± 0.02 c | 0.20 ± 0.05 a | 0.12 ± 0.03 b |
| ∑SFA | 14.57 c | 15.71 b | 12.59 b | 15.66 b | 18.10 a | 16.20 b |
| ∑MUFA | 42.94 a | 39.82 b | 42.42 a | 44.76 a | 40.99 b | 40.97 b |
| ∑PUFA | 44.32 a | 42.56 a | 43.58 a | 39.52 b | 39.59 b | 43.57 a |
| PUFA/SFA | 3.04 a | 2.70 a | 3.46 a | 2.52 a | 2.18 ab | 2.68 a |
| n-Hexane | MeTHF | CPME | d-Limonene | p-Cymene | Ethanol | |
|---|---|---|---|---|---|---|
| Sterol contents (mg/100 g oil) | ||||||
| Cholesterol | 1.13 ± 0.01 c | 1.47 ± 0.16 c | 0.46 ± 0.00 d | 4.83 ± 0.02 b | 5.15 ± 0.04 a | 6.64 ± 0.11 a |
| Campesterol | 99.22 ± 1.72 bc | 123.20 ± 1.29 b | 164.53 ± 0.83 a | 101.00 ± 0.92 b | 86.93 ± 0.35 c | 66.79 ± 2.64 d |
| Stigmasterol | 33.39 ± 1.86 b | 25.89 ± 0.02 c | 16.64 ± 0.90 d | 29.61 ± 0.73 c | 38.11 ± 1.94 a | 37.92 ± 1.05 a |
| β-Sitosterol | 373.63 ± 2.34 c | 437.80 ± 3.94 b | 555.93 ± 2.11 a | 400.78 ± 1.22 c | 364.19 ± 2.03 c | 387.96 ± 4.26 c |
| D-5 Avenasterol | 40.21 ± 2.84 b | 37.37 ± 1.34 bc | 39.48 ± 0.71 b | 41.89 ± 0.66 b | 44.03 ± 0.03 a | 35.22 ± 0.05 c |
| D-7 Avenasterol | 6.13 ± 0.03 d | 13.90 ± 0.33 a | 8.87 ± 0.21 c | 11.83 ± 0.54 b | 4.01 ± 0.03 e | 5.64 ± 0.04 d |
| Total | 568 ± 8.34 c | 641 ± 12.03 b | 785 ± 10.92 a | 590 ± 23.17 c | 543 ± 19.12 c | 588 ± 10.08 c |
| Tocopherol contents (mg/100 g of oil) | ||||||
| α-Tocopherol | 1.56 ± 0.13 c | 1.61 ± 0.26 a | 62.7 ± 0.82 d | 1.75 ± 0.91 c | 0.83 ± 0.31 cd | 1.35 ± 0.73 b |
| γ-Tocopherol | 43.44 ± 3.11 ab | 46.65 ± 2.45 a | 48.74 ± 2.30 a | 39.51 ± 1.69 b | 39.19 ± 3.04 b | 36.37 ± 2.47 c |
| δ-Tocopherol | 3.50 ± 0.23 b | 2.17 ± 0.04 d | 2.92 ± 0.09 c | 4.21 ± 0.02 a | 3.67 ± 0.02 b | 2.06 ± 0.09 d |
| Total | 48 ± 0.56 b | 50.6 ± 0.33 a | 52.3 ± 1.43 a | 44.8 ± 0.92 b | 43.7 ± 0.26 b | 39.8 ± 0.09 c |
| TPC (mg GAE/g) | TAC (mg GAE/g) | DPPH Assay (IC50 µg/mL) | Reducing Power (EC50 µg/mL) | |
|---|---|---|---|---|
| n-Hexane | 8 ± 0.08 c | 1.94 ± 0.72 b | 134.64 ± 2.40 d | 823.26 ± 4.92 d |
| MeTHF | 22.53 ± 1.18 b | 3.14 ± 0.40 ab | 90.02 ± 1.39 b | 670.15 ± 2.18 c |
| CPME | 23.51 ± 0.09 a | 3.92 ± 0.09 a | 30.71 ± 0.46 a | 435.93 ± 3.84 a |
| d-Limonene | 22.35 ± 0.70 c | 2.05 ± 0.22 b | 101.45 ± 2.45 c | 580.62 ± 3.91 b |
| p-Cymene | 11.35 ± 0.22 e | 1.92 ± 0.54 b | 107.23 ± 1.82 bc | 1045 ± 2.01 e |
| Ethanol | 21.54 ± 0.82 d | 0.34 ± 0.03 c | 197.56 ± 2.64 e | 807 ± 1.70 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trad, S.; Chaabani, E.; Aidi Wannes, W.; Dakhlaoui, S.; Nait Mohamed, S.; Khammessi, S.; Hammami, M.; Bourgou, S.; Saidani Tounsi, M.; Fabiano-Tixier, A.-S.; et al. Quality of Edible Sesame Oil as Obtained by Green Solvents: In Silico versus Experimental Screening Approaches. Foods 2023, 12, 3263. https://doi.org/10.3390/foods12173263
Trad S, Chaabani E, Aidi Wannes W, Dakhlaoui S, Nait Mohamed S, Khammessi S, Hammami M, Bourgou S, Saidani Tounsi M, Fabiano-Tixier A-S, et al. Quality of Edible Sesame Oil as Obtained by Green Solvents: In Silico versus Experimental Screening Approaches. Foods. 2023; 12(17):3263. https://doi.org/10.3390/foods12173263
Chicago/Turabian StyleTrad, Sinda, Emna Chaabani, Wissem Aidi Wannes, Sarra Dakhlaoui, Salma Nait Mohamed, Saber Khammessi, Majdi Hammami, Soumaya Bourgou, Moufida Saidani Tounsi, Anne-Sylvie Fabiano-Tixier, and et al. 2023. "Quality of Edible Sesame Oil as Obtained by Green Solvents: In Silico versus Experimental Screening Approaches" Foods 12, no. 17: 3263. https://doi.org/10.3390/foods12173263
APA StyleTrad, S., Chaabani, E., Aidi Wannes, W., Dakhlaoui, S., Nait Mohamed, S., Khammessi, S., Hammami, M., Bourgou, S., Saidani Tounsi, M., Fabiano-Tixier, A.-S., & Bettaieb Rebey, I. (2023). Quality of Edible Sesame Oil as Obtained by Green Solvents: In Silico versus Experimental Screening Approaches. Foods, 12(17), 3263. https://doi.org/10.3390/foods12173263







