The Relationship between Mortality from Cardiovascular Diseases and Total Drinking Water Hardness: Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
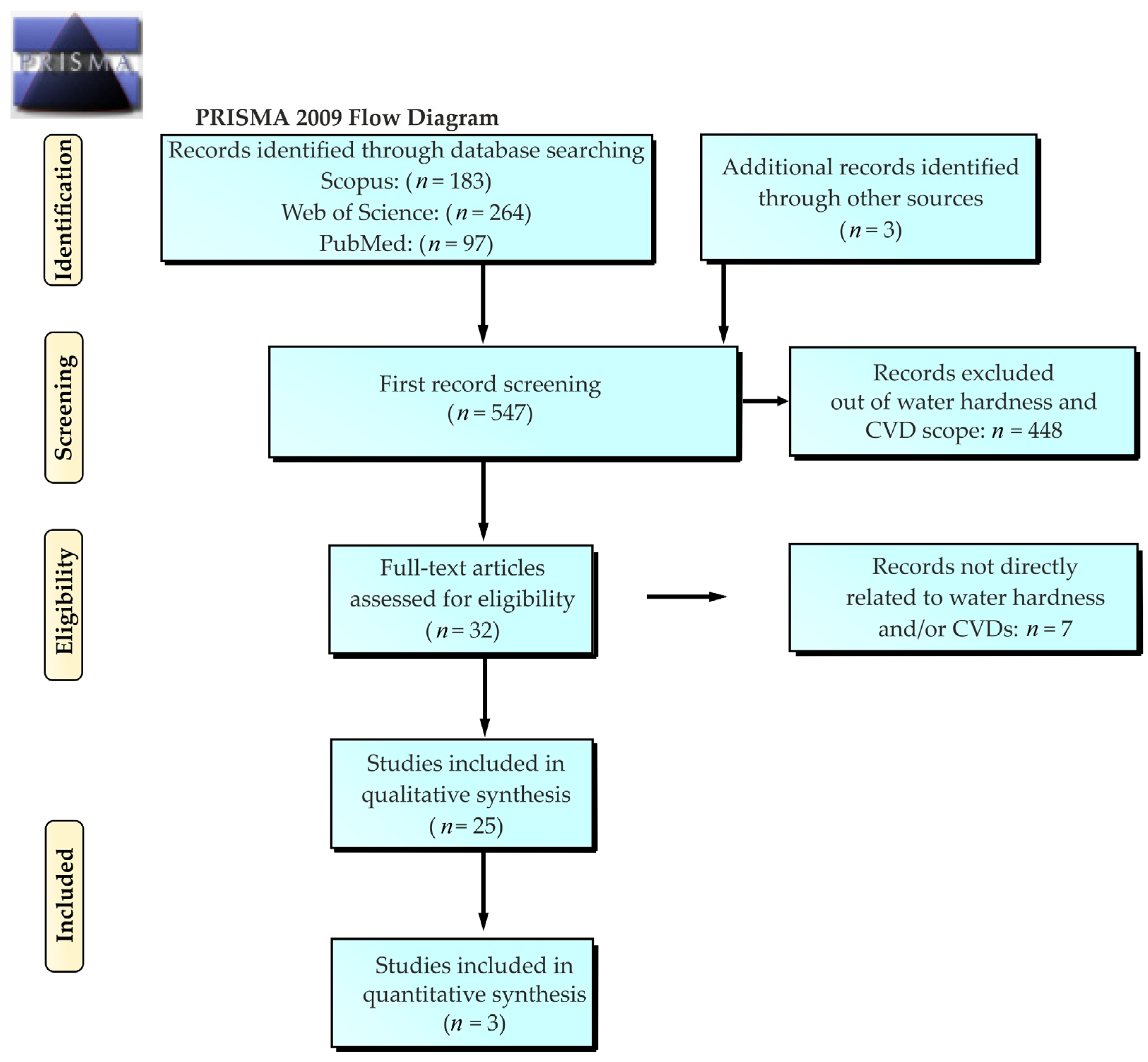
2.1. Study Selection Process
2.2. Inclusion and Exclusion Criteria
2.3. Statistics
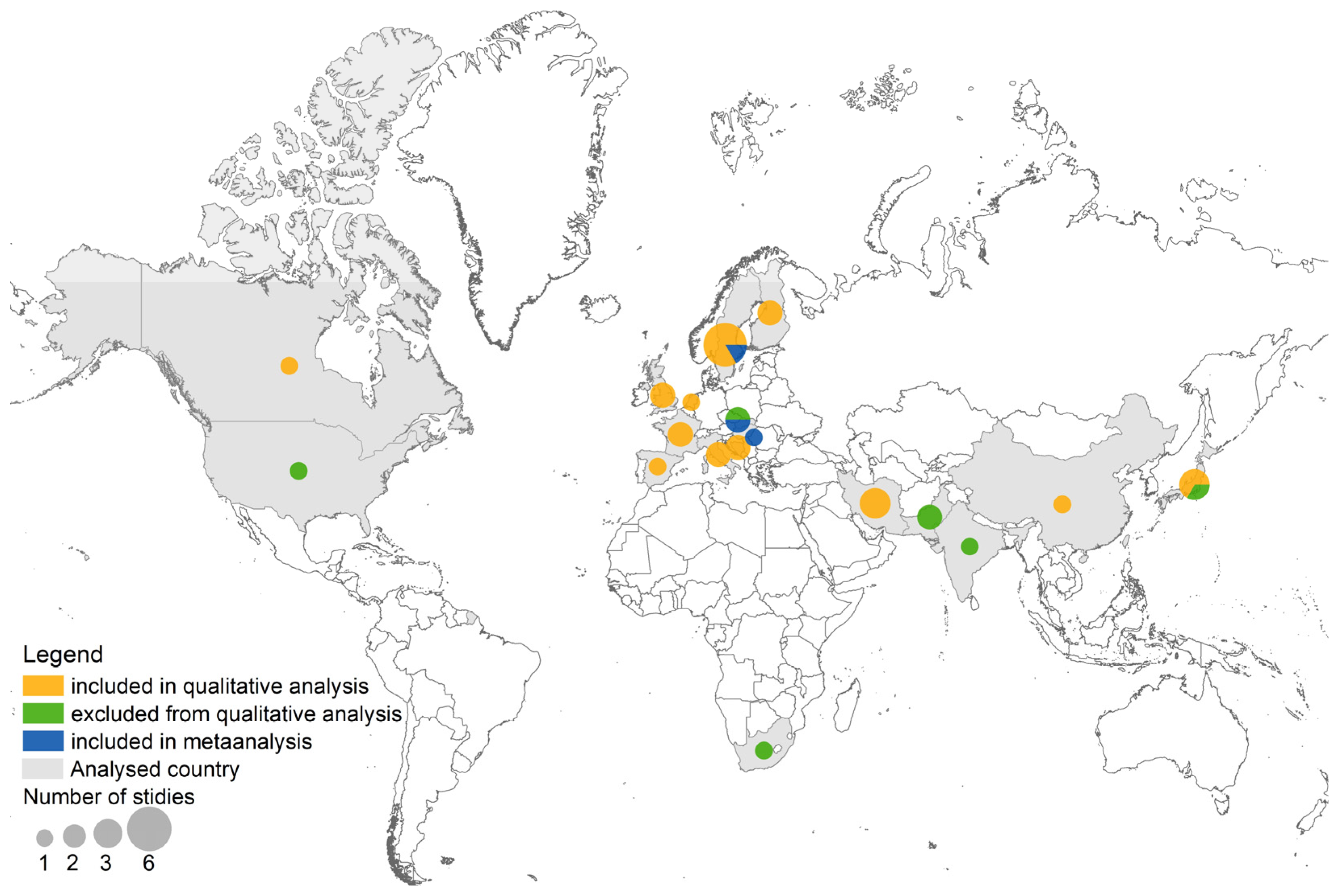
3. Results
4. Discussion
4.1. Possible Effects of Drinking Hard Water on CVD Mortality
4.2. Hard Water and Prevention from CVDs
4.3. Strengths and Limitations of the Review
4.4. Studies with No or Non-Significant Effect on CVDs
4.5. Corrosive Properties of Soft Water and CVDs Health Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Luvhimbi, N.; Tshitangano, T.G.; Mabunda, J.T.; Olaniyi, F.C.; Edokpayi, J.N. Water Quality Assessment and Evaluation of Human Health Risk of Drinking Water from Source to Point of Use at Thulamela Municipality, Limpopo Province. Sci. Rep. 2022, 12, 6059. [Google Scholar] [CrossRef] [PubMed]
- Babayan, G.; Sakoyan, A.; Sahakyan, G. Drinking Water Quality and Health Risk Analysis in the Mining Impact Zone, Armenia. Sustain. Water Resour. Manag. 2019, 5, 1877–1886. [Google Scholar] [CrossRef]
- Powers, J.E.; Mureithi, M.; Mboya, J.; Campolo, J.; Swarthout, J.M.; Pajka, J.; Null, C.; Pickering, A.J. Effects of High Temperature and Heavy Precipitation on Drinking Water Quality and Child Hand Contamination Levels in Rural Kenya. Environ. Sci. Technol. 2023, 57, 6975–6988. [Google Scholar] [CrossRef]
- Daghara, A.; Al-Khatib, I.A.; Al-Jabari, M. Quality of Drinking Water from Springs in Palestine: West Bank as a Case Study. J. Environ. Public Health 2019, 2019, 8631732. [Google Scholar] [CrossRef]
- Gandy, J. Water Intake: Validity of Population Assessment and Recommendations. Eur. J. Nutr. 2015, 54, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, Hydration, and Health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Jéquier, E.; Constant, F. Water as an Essential Nutrient: The Physiological Basis of Hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Hung, C.-F. Colon Cancer Mortality and Total Hardness Levels in Taiwan’s Drinking Water. Arch. Environ. Contam. Toxicol. 1998, 35, 148–151. [Google Scholar] [CrossRef]
- Gianfredi, V.; Bragazzi, N.L.; Nucci, D.; Villarini, M.; Moretti, M. Cardiovascular Diseases and Hard Drinking Waters: Implications from a Systematic Review with Meta-Analysis of Case-Control Studies. J. Water Health 2017, 15, 31–40. [Google Scholar] [CrossRef]
- Kanadhia, K.C.; Ramavataram, D.V.S.S.; Nilakhe, S.P.D.; Patel, S. A Study of Water Hardness and the Prevalence of Hypomagnesaemia and Hypocalcaemia in Healthy Subjects of Surat District (Gujarat). Magnes. Res. 2014, 27, 165–174. [Google Scholar] [CrossRef]
- Rapant, S.; Letkovičová, A.; Jurkovičová, D.; Kosmovský, V.; Kožíšek, F.; Jurkovič, Ľ. Differences in Health Status of Slovak Municipalities Supplied with Drinking Water of Different Hardness Values. Environ. Geochem. Health 2020, 6, 2665–2677. [Google Scholar] [CrossRef] [PubMed]
- Marque, S.; Jacqmin-Gadda, H.; Dartigues, J.F.; Commenges, D. Cardiovascular Mortality and Calcium and Magnesium in Drinking Water: An Ecological Study in Elderly People. Eur. J. Epidemiol. 2003, 18, 305–309. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Karppanen, H.; Karppanen, P.; Mervaala, E. Why and How to Implement Sodium, Potassium, Calcium, and Magnesium Changes in Food Items and Diets? J. Hum. Hypertens. 2005, 19 (Suppl. S3), S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported Dietary Intake, Disparity between the Reported Consumption and the Level Needed for Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 168. [Google Scholar] [CrossRef]
- Wang, L.; Manson, J.E.; Sesso, H.D. Calcium Intake and Risk of Cardiovascular Disease. Am. J. Cardiovasc. Drugs 2012, 12, 105–116. [Google Scholar] [CrossRef]
- Rosique-Esteban, N.; Guasch-Ferré, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Dietary Magnesium and Cardiovascular Disease: A Review with Emphasis in Epidemiological Studies. Nutrients 2018, 10, 168. [Google Scholar] [CrossRef]
- Takahashi, Y.; Imaizumi, Y. Hardness in Drinking Water. Eisei Kagaku 1988, 34, 475–479. [Google Scholar] [CrossRef]
- Catling, L.A.; Abubakar, I.; Lake, I.R.; Swift, L.; Hunter, P.R. A Systematic Review of Analytical Observational Studies Investigating the Association between Cardiovascular Disease and Drinking Water Hardness. J. Water Health 2008, 6, 433–442. [Google Scholar] [CrossRef]
- Nawrocki, J.; Raczyk-Stanisławiak, U.; Swietlik, J.; Olejnik, A.; Sroka, M.J. Corrosion in a Distribution System: Steady Water and Its Composition. Water Res. 2010, 44, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Knezović, N.J.; Memić, M.; Mabić, M.; Huremović, J.; Mikulić, I. Correlation between Water Hardness and Cardiovascular Diseases in Mostar City, Bosnia and Herzegovina. J. Water Health 2014, 12, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Kozisek, F. Regulations for Calcium, Magnesium or Hardness in Drinking Water in the European Union Member States. Regul. Toxicol. Pharmacol. 2020, 112, 104589. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chiu, H.F.; Tsai, S.S.; Cheng, M.F.; Lin, M.C.; Sung, F.C. Calcium and magnesium in drinking water and risk of death from prostate cancer. J. Toxicol. Environ. Health A 2000, 60, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Najafi Saleh, H.; Yaseri, M.; Jalilzadeh, M.; Mohammadi, A.A. Association of Consumption of Excess Hard Water, Body Mass Index and Waist Circumference with Risk of Hypertension in Individuals Living in Hard and Soft Water Areas. Environ. Geochem. Health 2019, 41, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, Y.; Okada, J.; Yamada, E.; Okada, K.; Okada, S.; Yamada, M. Association between Percentage of Obese People and Water Hardness in Water Purification Plants. Obes. Med. 2020, 19, 100244. [Google Scholar] [CrossRef]
- Rosborg, I. Drinking Water Minerals and Mineral Balance; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Bellizzi, V.; DeNicola, L.; Minutolo, R.; Russo, D.; Cianciaruso, B.; Andreucci, M.; Conte, G.; Andreucci, V.E. Effects of Water Hardness on Urinary Risk Factors for Kidney Stones in Patients with Idiopathic Nephrolithiasis. Nephron 1998, 81, 66–70. [Google Scholar] [CrossRef]
- Lopez, D.J.; Singh, A.; Waidyatillake, N.T.; Su, J.C.; Bui, D.S.; Dharmage, S.C.; Lodge, C.J.; Lowe, A.J. The Association between Domestic Hard Water and Eczema in Adults from the UK Biobank Cohort Study. Br. J. Dermatol. 2022, 187, 704–712. [Google Scholar] [CrossRef]
- Willis, S.; Goldfarb, D.S.; Thomas, K.; Bultitude, M. Water to Prevent Kidney Stones: Tap vs. Bottled; Soft vs. Hard—Does It Matter? BJU Int. 2019, 124, 905–906. [Google Scholar] [CrossRef]
- Siener, R.; Jahnen, A.; Hesse, A. Influence of a Mineral Water Rich in Calcium, Magnesium and Bicarbonate on Urine Composition and the Risk of Calcium Oxalate Crystallization. Eur. J. Clin. Nutr. 2004, 58, 270–276. [Google Scholar] [CrossRef]
- Smith, W.C.; Crombie, I.K. Coronary Heart Disease and Water Hardness in Scotland--Is There a Relationship? J. Epidemiol. Community Health 1987, 41, 227–228. [Google Scholar] [CrossRef]
- Lake, I.R.; Swift, L.; Catling, L.A.; Abubakar, I.; Sabel, C.E.; Hunter, P.R. Effect of Water Hardness on Cardiovascular Mortality: An Ecological Time Series Approach. J. Public Health 2010, 32, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.W.; Walker, M.; Lennon, L.T.; Shaper, A.G.; Whincup, P.H. Hard Drinking Water Does Not Protect against Cardiovascular Disease: New Evidence from the British Regional Heart Study. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 185–189. [Google Scholar] [CrossRef]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Vohl, M.-C. The Metabolic Signature Associated with the Western Dietary Pattern: A cross-sectional study. Nutr. J. 2013, 158, 213. [Google Scholar] [CrossRef]
- WHO. Hardness in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Nagy, J.; Sipka, S.; Sipka, S.; Kocsis, J.; Horváth, Z. The Hardness of Drinking Water Negatively While Socio-Economic Deprivation Positively Correlate with the Age-Adjusted Mortality Rates Due to Cardiovascular Diseases in Hungarian Wine Regions. Int. J. Environ. Res. Public Health 2019, 16, 3437. [Google Scholar] [CrossRef]
- Nerbrand, C.; Agréus, L.; Lenner, R.A.; Nyberg, P.; Svärdsudd, K. The Influence of Calcium and Magnesium in Drinking Water and Diet on Cardiovascular Risk Factors in Individuals Living in Hard and Soft Water Areas with Differences in Cardiovascular Mortality. BMC Public Health 2003, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kmet, L.M.; Lee, R.C.; Cook, L.S. HTA Initiative # 13. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; Alberta Heritage Foundation for Medical Research (AHFMR): Edmonton, AB, Canada, 2004; ISBN 1-896956-71-XX. [Google Scholar]
- Hossienifar, F.; Entezari, M.; Hosseini, S. Water Hardness Zoning of Isfahan Province, Iran, and Its Relationship with Cardiovascular Mortality, 2013–2015. ARYA Atheroscler. 2019, 15, 275–280. [Google Scholar] [CrossRef]
- Momeni, M.; Gharedaghi, Z.; Amin, M.M.; Poursafa, P.; Mansourian, M. Does Water Hardness Have Preventive Effect on Cardiovascular Disease? Int. J. Prev. Med. 2014, 5, 159–163. [Google Scholar] [PubMed]
- Miyake, Y.; Iki, M. Ecologic Study of Water Hardness and Cerebrovascular Mortality in Japan. Arch. Environ. Health Int. J. 2003, 58, 163–166. [Google Scholar] [CrossRef]
- Miyake, Y.; Iki, M. Lack of Association between Water Hardness and Coronary Heart Disease Mortality in Japan. Int. J. Cardiol. 2004, 96, 25–28. [Google Scholar] [CrossRef] [PubMed]
- McLeod, L.; Bharadwaj, L.; Epp, T.Y.; Waldner, C.L. Ecological Analysis of Associations between Groundwater Quality and Hypertension and Cardiovascular Disease in Rural Saskatchewan, Canada Using Bayesian Hierarchical Models and Administrative Health Data. Environ. Res. 2018, 167, 329–340. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chiu, J.F.; Chiu, H.F.; Wang, T.N.; Lee, C.H.; Ko, Y.C. Relationship between Water Hardness and Coronary Mortality in Taiwan. J. Toxicol. Environ. Health 1996, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Derry, C.W.; Bourne, D.E.; Sayed, A.R. The Relationship between the Hardness of Treated Water and Cardiovascular Disease Mortality in South African Urban Areas. S. Afr. Med. J. 1990, 77, 522–524. [Google Scholar] [PubMed]
- Jamil, H.; Bilal, M.; Khan, M.R.; Arshad, A. An Incidence of Hypertension Among People and Its Association to Consumption of Hard Water: A Cross-Sectional Research. Indo Am. J. Pharm. Sci. 2018, 5, 3118–3123. [Google Scholar]
- Sindh, B.; Sindh, G.K. Hypertension in People Consuming Hard Water in Haji Samoa Goth Keti. ASH & KMDC 2015, 20, 17. [Google Scholar]
- Leurs, L.J.; Schouten, L.J.; Mons, M.N.; Goldbohm, R.A.; Van Den Brandt, P.A. Relationship between Tap Water Hardness, Magnesium, and Calcium Concentration and Mortality Due to Ischemic Heart Disease or Stroke in the Netherlands. Environ. Health Perspect. 2010, 118, 414–420. [Google Scholar] [CrossRef]
- Rosenlund, M.; Berglind, N.; Hallqvist, J.; Bellander, T.; Bluhm, G. Daily Intake of Magnesium and Calcium from Drinking Water in Relation to Myocardial Infarction. Epidemiology 2005, 16, 570–576. [Google Scholar] [CrossRef][Green Version]
- Rubenowitz, E.; Axelsson, G.; Rylander, R. Magnesium and Calcium in Drinking Water and Death from Acute Myocardial Infarction in Women. Epidemiology 1999, 10, 31–36. [Google Scholar] [CrossRef]
- Rylander, R.; Bonevik, H.; Rubenowitz, E. Magnesium and Calcium in Drinking Water and Cardiovascular Mortality. Scand. J. Work Environ. Health 1991, 17, 91–94. [Google Scholar] [CrossRef]
- Helte, E.; Säve-Söderbergh, M.; Larsson, S.C.; Åkesson, A. Calcium and Magnesium in Drinking Water and Risk of Myocardial Infarction and Stroke—A Population-Based Cohort Study. Am. J. Clin. Nutr. 2022, 116, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Rubenowitz, E.; Axelsson, G.; Rylander, R. Magnesium in Drinking Water and Death from Acute Myocardial Infarction. Am. J. Epidemiol. 1996, 143, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, J.; Abellán, J.J.; Gómez-Rubio, V.; López-Quílez, A.; Sanmartín, P.; Abellán, C.; Martínez-Beneito, M.A.; Melchor, I.; Vanaclocha, H.; Zurriaga, Ó.; et al. Spatial Analysis of the Relationship between Mortality from Cardiovascular and Cerebrovascular Disease and Drinking Water Hardness. Environ. Health Perspect. 2004, 112, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Kousa, A.; Moltchanova, E.; Viik-Kajander, M.; Rytkönen, M.; Tuomilehto, J.; Tarvainen, T.; Karvonen, M. Geochemistry of Ground Water and the Incidence of Acute Myocardial Infarction in Finland. J. Epidemiol. Community Health 2004, 58, 136–139. [Google Scholar] [CrossRef]
- Piispanen, R. Water Hardness and Cardiovascular Mortality in Finland. Environ. Geochem. Health 1993, 15, 201–208. [Google Scholar] [CrossRef]
- Sauvant, M.P.; Pepin, D. Geopraphic Variation of the Mortality from Cardiovascular Disease and Drinking Water in a French Small Area (Puy de Dome). Environ. Res. 2000, 84, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.; Dini, F.L.; Azzarelli, A.; Giaconi, A.; Volterrani, C.; Lunardi, M. Sudden Cardiac Death Rate in an Area Characterized by High Incidence of Coronary Artery Disease and Low Hardness of Drinking Water. Angiology 1995, 46, 145–149. [Google Scholar] [CrossRef]
- Dore, M.P.; Parodi, G.; Portoghese, M.; Errigo, A.; Pes, G.M. Water Quality and Mortality from Coronary Artery Disease in Sardinia: A Geospatial Analysis. Nutrients 2021, 13, 2858. [Google Scholar] [CrossRef]
- Stevanovic, S.; Nikolic, M.; Ilic, M.D. Calcium and Magnesium in Drinking Water as Risk Factors for Ischemic Heart Disease. Pol. J. Environ. Stud. 2017, 26, 1673–1680. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chang, C.-C.; Tsai, S.-S.; Chiu, H.-F. Calcium and Magnesium in Drinking Water and Risk of Death from Acute Myocardial Infarction in Taiwan. Environ. Res. 2006, 101, 407–411. [Google Scholar] [CrossRef]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef]
- Reid, I.R.; Birstow, S.M.; Bolland, M.J. Calcium and Cardiovascular Disease. Endocrinol. Metab. 2017, 32, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Wang, L.; Manson, J.E.; Sesso, H.D. The Role of Calcium in the Prevention of Cardiovascular Disease—A Review of Observational Studies and Randomized Clinical Trials. Curr. Atheroscler. Rep. 2013, 15, 362. [Google Scholar] [CrossRef]
- Alomaim, H.; Griffin, P.; Swist, E.; Plouffe, L.J.; Vandeloo, M.; Demonty, I.; Kumar, A.; Bertinato, J. Dietary Calcium Affects Body Composition and Lipid Metabolism in Rats. PLoS ONE 2019, 14, e0210760. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, S.; Bouziotas, D. Water Hardness in the Eye of the Beholder: Exploring Links between Central Softening, Customer Perception and Behavior, and Citizen Science. Citiz. Sci. 2022, 7, 1–14. [Google Scholar] [CrossRef]
- Mosca, L.; Barrett-Connor, E.; Kass Wenger, N. Sex/Gender Differences in Cardiovascular Disease Prevention: What a Difference a Decade Makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Oguzturk, O. Differences in Quality of Life in Rural and Urban Populations. Clin. Investig. Med. 2008, 31, 346–351. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wilson, P.W.F.; Kannel, W.B. Beyond Established and Novel Risk Factors Lifestyle Risk Factors for Cardiovascular Disease. Circulation 2008, 117, 3031–3038. [Google Scholar] [CrossRef]
- Jabbar-Lopez, Z.K.; Craven, J.; Logan, K.; Greenblatt, D.; Marrs, T.; Radulovic, S.; McLean, W.H.I.; Lack, G.; Strachan, D.P.; Perkin, M.R.; et al. Longitudinal Analysis of the Effect of Water Hardness on Atopic Eczema: Evidence for Gene–Environment Interaction. Br. J. Dermatol. 2020, 183, 285–293. [Google Scholar] [CrossRef]
- Environmental Protection Agency, Aquatic Life Ambient Freshwater Quality Criteria—1984, Revised 2007, Federal Register, 22 Feb. 2007. Available online: https://www.federalregister.gov/documents/2007/02/22/E7-3007/aquatic-life-ambient-freshwater-quality-criteria-copper-2007-revision (accessed on 12 July 2023).
- Vazdirvanidis, A.; Papadopoulou, S.; Papaefthymiou, S.; Pantazopoulos, G.; Skarmoutsos, D. Copper Tubing Failure due to Ant-Nest Corrosion. In Proceedings of the 5th International Conference of Engineering against Failure (ICEAF-V 2018), Chios, Grece, 20–22 June 2018; Volume 188. [Google Scholar] [CrossRef][Green Version]
- Zaribaf, F.; Entezari, M.H.; Hassanzadeh, A.; Mirzaian, S. Association between Dietary Iron, Iron Stores, and Serum Lipid Profile in Reproductive Age Women. J. Educ. Health Promot. 2014, 3, 15. [Google Scholar] [CrossRef]
| Study | Reason for Exclusion | Outcome |
|---|---|---|
| Miyake et al., 2003 [43] (Japan) | Ecological study | No relation between water hardness and mortality from cerebrovascular diseases. |
| Miyake et al., 2004 [44] (Japan) | Ecological study | No relation between water hardness and mortality from coronary heart disease. |
| Ferrandiz et al., 2004 [56] (Spain) | Ecological study | A non-significant inverse relationship between water hardness and mortality from CVDs. |
| Kousa et al., 2004 [57] (Finland) | Ecological study | An inverse relationship between water hardness and coronary heart disease. |
| Lake et al., 2010 [33] (United Kingdom) | Ecological study | No relation between water hardness from coronary heart disease mortality. |
| McLeod et al., 2018 [45] (Canada) | Ecological study | An inverse relationship between water hardness and magnesium levels and CVDs. |
| Hossienifar et al., 2019 [41] (Iran) | Ecological study | A non-significant inverse relationship between the water hardness and CVDs. |
| Dore et al., 2021 [61] (Italy) | Ecological study | An inverse relationship between water hardness and mortality from coronary artery disease. |
| Rosenlund et al. [51] 2005 (Sweden) | Prevention of CVDs | An inverse relationship between water calcium and acute myocardial infarction. |
| Momeni et al., 2014 [42] (Iran) | Prevention of CVDs | An inverse relationship between water hardness and magnesium levels and CVDs. |
| Knezovic et al., 2014 [22] (Bosnia and Herzegovina) | Prevention of CVDs | An inverse relationship between water hardness and CVDs. |
| Stevanovic et al., 2017 [62] (Serbia) | Prevention of CVDs | An inverse relationship between Ca and Mg from drinking water and risk factors for ischemic heart disease. |
| Yousefi et al., 2019 [25] (Iran) | Prevention of CVDs | An inverse relationship between water hardness and hypertension. |
| Helte et al., 2022 [54] (Sweden) | Prevention of CVDs | An inverse relationship between water magnesium and calcium content and risk of stroke in postmenopausal women. |
| Rylander et al., 1991 [53] (Sweden) | Data missing | An inverse relationship between water hardness and CVDs mortality. |
| Piispanen 1993 [58] (Finland) | Data missing | An inverse relationship between water hardness and CVDs mortality between the highest and lowest region but a small relationship nationwide. |
| Bernardi et al., 1995 [60] (Italy) | Data missing | An inverse relationship between water hardness and ischemic heart disease mortality. |
| Rubenowitz et al., 1996 [55] (Sweden) | Data missing | An inverse relation between calcium and magnesium in drinking water and mortality from acute myocardial infarction in men while comparing it with oncology patients. |
| Rubenowitz et al., 1999 [52] (Sweden) | Data missing | An inverse relation between calcium and magnesium in drinking water and mortality from acute myocardial infarction in women while comparing it with oncology patients. |
| Sauvant et al., 2000 [59] (France) | Data missing | An inverse relation between calcium in drinking water and mortality from CVDs. |
| Marque et al., 2003 [13] (France) | Data missing | An inverse relation between calcium in drinking water and mortality from CVDs. |
| Yang et al., 2006 [63] (Taiwan) | Data missing | An inverse relation between calcium in water and mortality from acute myocardial infarction. |
| Morris et al., 2008 [34] (United Kingdom) | Data missing | No relation between water hardness from CVDs mortality. |
| Leurs et al., 2010 [50] (Netherlands) | Data missing | No relationship between water hardness and ischemic heart disease mortality. |
| Study | Years | Area | Water Hardness (CaCO3 mg/L) | Adjustments |
|---|---|---|---|---|
| Nagy et al. (2019) [38] | 2000–2010 | Hungary; 5 wine regions Szekszárd-Villány, Eger, Balaton, Tokaj, Hódmezővásárhely | Szekszárd-Villány: 294.2, Eger: 194.9, Balaton: 249.2, Tokaj: 138.6 CaO mg/L, Hódmezővásárhely: 81.90 CaO mg/L | Age-adjusted death rates, Socio-Economic Deprivation Index correlated with CVD |
| Nerbrandt et al. (2003) [39] | 1989–1998 | Sweden, Osthammar, Tocksfors | High: 167 CaCO3 mg/L Low: 45 CaCO3 mg/L (Calculated from medians of Mg2+ and Ca2+) | Asked for filtered water, mortality CVD/1000 inh |
| Rapant et al. (2020) [12] | 1994–2008 | Slovakia; Soft water region (34 municipalities) Hard water region (21 municipalities) | Hard water: 283.5 CaCO3 mg/L Soft water: 76.6 CaCO3 mg/L (Calculated from Mg2+ and Ca2+) | 30 health indicators |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bykowska-Derda, A.; Spychala, M.; Czlapka-Matyasik, M.; Sojka, M.; Bykowski, J.; Ptak, M. The Relationship between Mortality from Cardiovascular Diseases and Total Drinking Water Hardness: Systematic Review with Meta-Analysis. Foods 2023, 12, 3255. https://doi.org/10.3390/foods12173255
Bykowska-Derda A, Spychala M, Czlapka-Matyasik M, Sojka M, Bykowski J, Ptak M. The Relationship between Mortality from Cardiovascular Diseases and Total Drinking Water Hardness: Systematic Review with Meta-Analysis. Foods. 2023; 12(17):3255. https://doi.org/10.3390/foods12173255
Chicago/Turabian StyleBykowska-Derda, Aleksandra, Marcin Spychala, Magdalena Czlapka-Matyasik, Mariusz Sojka, Jerzy Bykowski, and Mariusz Ptak. 2023. "The Relationship between Mortality from Cardiovascular Diseases and Total Drinking Water Hardness: Systematic Review with Meta-Analysis" Foods 12, no. 17: 3255. https://doi.org/10.3390/foods12173255
APA StyleBykowska-Derda, A., Spychala, M., Czlapka-Matyasik, M., Sojka, M., Bykowski, J., & Ptak, M. (2023). The Relationship between Mortality from Cardiovascular Diseases and Total Drinking Water Hardness: Systematic Review with Meta-Analysis. Foods, 12(17), 3255. https://doi.org/10.3390/foods12173255










