Changes in the Physical Properties and Volatile Odor Characteristics of Shiitake Mushrooms (Lentinula edodes) in Far Infrared Radiation Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Drying Methods
2.2.1. Shade Drying (SD)
2.2.2. Far Infrared Radiation Drying (FID)
2.3. Drying Kinetics
2.4. Shrinkage Ratio
2.5. Rehydration Ratio
2.6. Microstructure
2.7. Color Measurements
2.8. Volatile Odor Profile Distinction
2.9. Volatile Compounds
2.10. Statistical Analysis
3. Results and Discussion
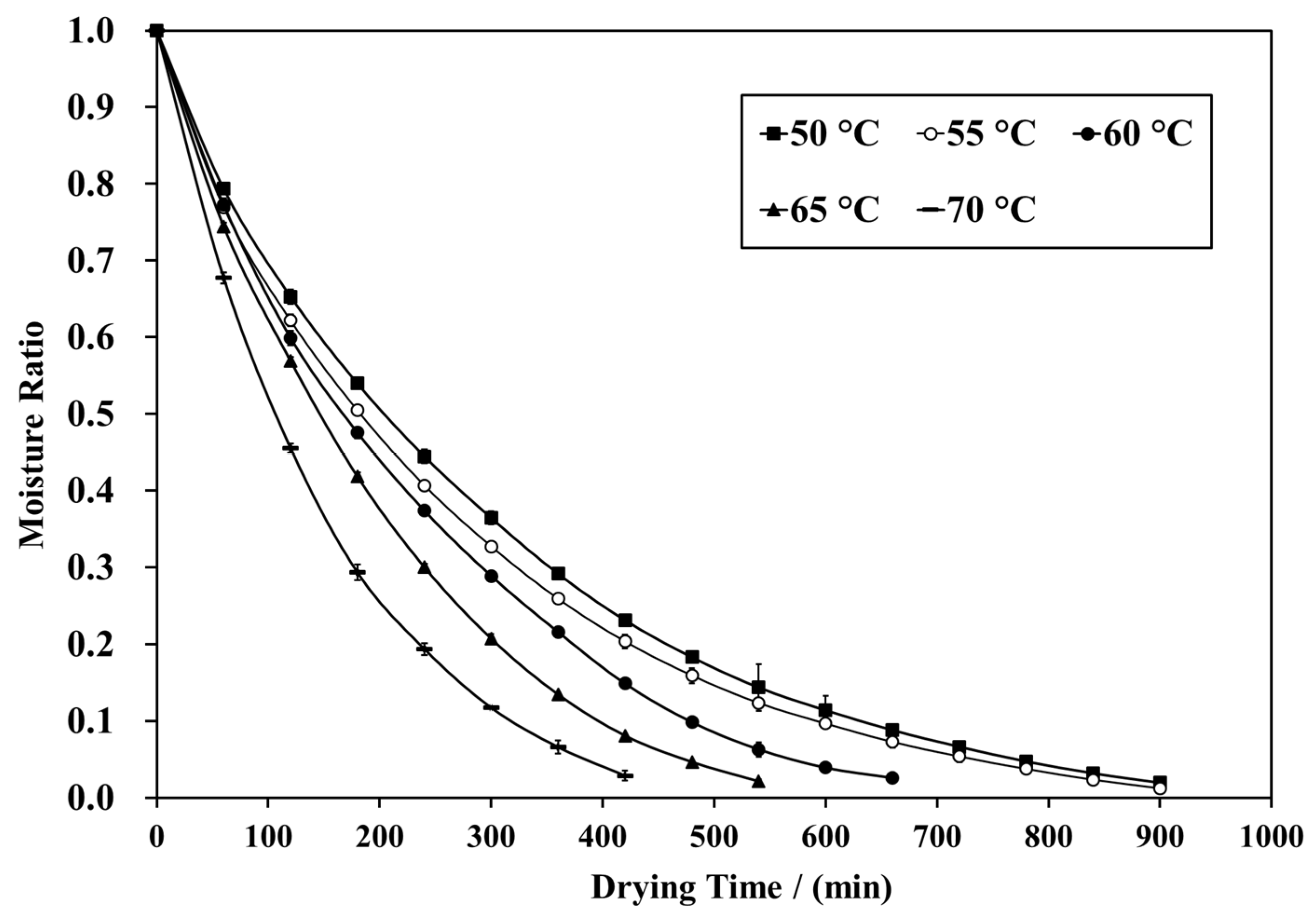
3.1. Moisture Ratio
3.2. Shrinkage Ratio and Rehydration Ratio
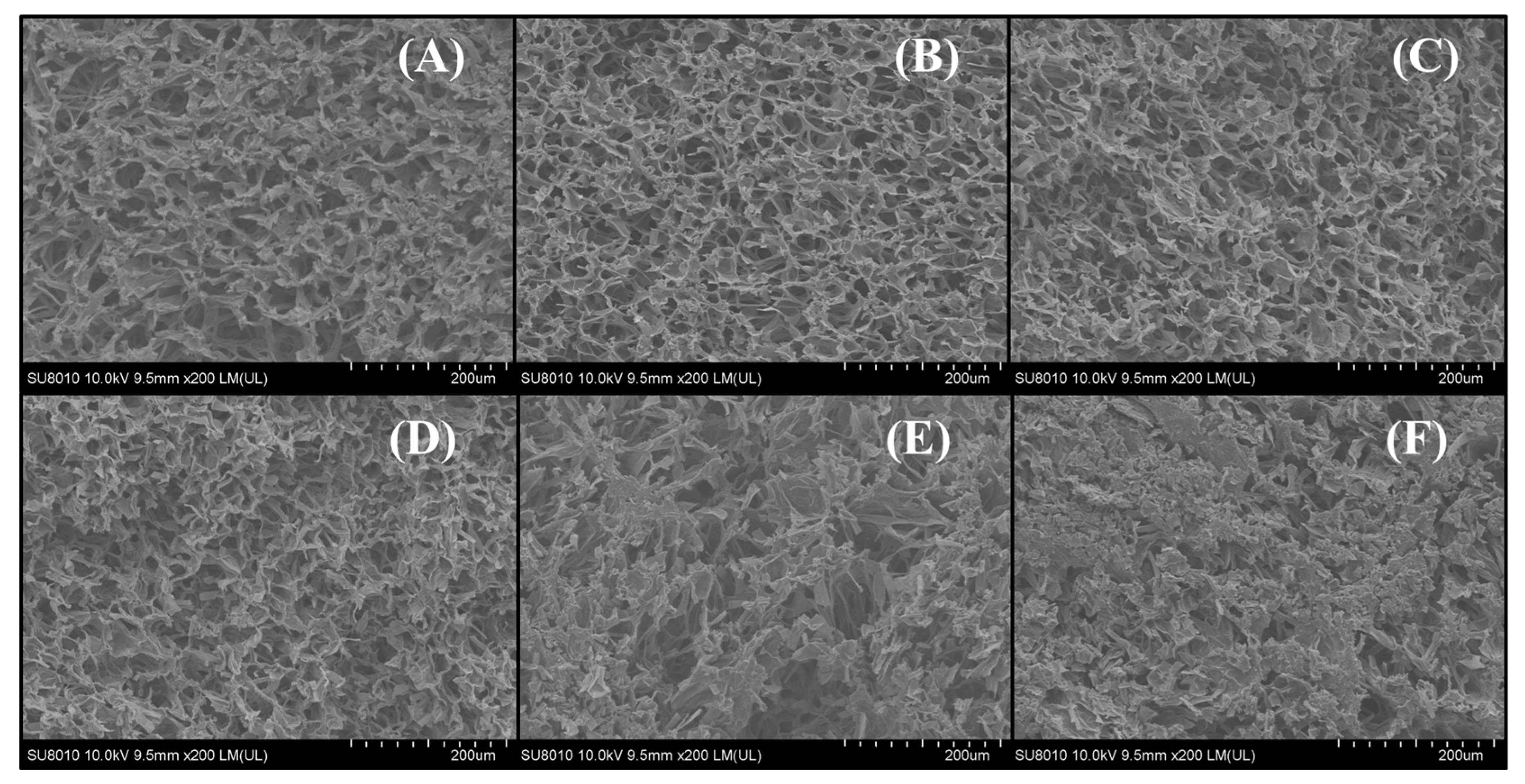
3.3. Microstructure
3.4. Surface Color
3.5. Volatile Odor Profile Distinction
3.6. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Li, R.; Chen, W.; Feng, J.; Wu, D.; Zhang, Z.; Zhang, J.; Yang, Y. The anabolism of sulphur aroma volatiles responds to enzymatic and non-enzymatic reactions during the drying process of shiitake mushrooms. Food Chem. 2022, 371, 131123. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Arif, M.; Xu, M.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Xiao, A.; Ding, C. Effect of electrohydrodynamic (EHD) on drying kinetics and quality characteristics of shiitake mushroom. Foods 2022, 11, 1303. [Google Scholar] [CrossRef]
- Singh, P.; Langowski, H.C.; Wani, A.A.; Sängerlaub, S. Recent advances in extending the shelf life of fresh Agaricus mushrooms: A review. J. Sci. Food Agric. 2010, 90, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Chaipoot, S.; Wiriyacharee, P.; Phongphisutthinant, R.; Buadoktoom, S.; Srisuwun, A.; Somjai, C.; Srinuanpan, S. Changes in physicochemical characteristics and antioxidant activities of dried shiitake mushroom in dry-moist-heat aging process. Foods 2023, 12, 2714. [Google Scholar] [CrossRef]
- García-Segovia, P.; Andrés-Bello, A.; Martínez-Monzó, J. Rehydration of air-dried Shiitake mushroom (Lentinus edodes) caps: Comparison of conventional and vacuum water immersion processes. LWT—Food Sci. Technol. 2011, 44, 480–488. [Google Scholar] [CrossRef]
- Xu, W.; Song, C.; Li, Z.; Song, F.; Hu, S.; Li, J.; Zhu, G.; Vijaya Raghavan, G.S. Temperature gradient control during microwave combined with hot air drying. Biosyst. Eng. 2018, 169, 175–187. [Google Scholar] [CrossRef]
- Alibas, I. Determination of drying parameters, ascorbic acid contents and color characteristics of nettle leaves during microwave-, air- and combined microwave-air-drying. J. Food Process Eng. 2010, 33, 213–233. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Kong, Y.; Zhao, J.; Sun, Y.; Huang, M. Comparative analysis of taste compounds in shiitake mushrooms processed by hot-air drying and freeze drying. Int. J. Food Prop. 2019, 22, 1100–1111. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, M.; Mujumdar, A.S. Studies on the Microwave Freeze Drying Technique and Sterilization Characteristics of Cabbage. Dry. Technol. 2007, 25, 1725–1731. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; Wang, Y.; Wan, F. Drying characteristics and quality of Stevia rebaudiana leaves by far-infrared radiation. LWT 2021, 140, 110638. [Google Scholar] [CrossRef]
- Xie, L.; Mujumdar, A.S.; Fang, X.M.; Wang, J.; Dai, J.W.; Du, Z.L.; Xiao, H.W.; Liu, Y.H.; Gao, Z.J. Far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD) of wolfberry (Lycium barbarum L.): Effects on drying kinetics and quality attributes. Food Bioprod. Process. 2017, 102, 320–331. [Google Scholar] [CrossRef]
- Ratseewo, J.; Warren, F.J.; Meeso, N.; Siriamornpun, S. Effects of Far-Infrared Radiation Drying on Starch Digestibility and the Content of Bioactive Compounds in Differently Pigmented Rice Varieties. Foods 2022, 11, 4079. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fang, X.; Wu, W.; Chen, H.; Mu, H.; Gao, H. Effects of high-temperature pre-drying on the quality of air-dried shiitake mushrooms (Lentinula edodes). Food Chem. 2019, 285, 406–413. [Google Scholar] [CrossRef]
- Hu, L.; Bi, J.; Jin, X.; van der Sman, R. Microstructure evolution affecting the rehydration of dried mushrooms during instant controlled pressure drop combined hot air drying (DIC-HA). Innov. Food Sci. Emerg. Technol. 2022, 79, 103056. [Google Scholar] [CrossRef]
- Wang, H.-c.; Zhang, M.; Adhikari, B. Drying of shiitake mushroom by combining freeze-drying and mid-infrared radiation. Food Bioprod. Process. 2015, 94, 507–517. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, X.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of Drying Process on the Volatile and Non-Volatile Flavor Compounds of Lentinula edodes. Foods 2021, 10, 2836. [Google Scholar] [CrossRef]
- Stephenus, F.N.; Benjamin, M.A.Z.; Anuar, A.; Awang, M.A. Effect of Temperatures on Drying Kinetics, Extraction Yield, Phenolics, Flavonoids, and Antioxidant Activity of Phaleria macrocarpa (Mahkota Dewa) Fruits. Foods 2023, 12, 2859. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, Q.; Wan, F.; Ma, G.; Zang, Z.; Xu, Y.; Jiang, C.; Huang, X. Effects of different drying methods on the drying characteristics and quality of codonopsis pilosulae slices. Foods 2023, 12, 1323. [Google Scholar] [CrossRef]
- Sun, M.; Xu, Y.; Ding, Y.; Gu, Y.; Zhuang, Y.; Fan, X. Effect of Ultrasound Pretreatment on the Moisture Migration and Quality of Cantharellus cibarius Following Hot Air Drying. Foods 2023, 12, 2705. [Google Scholar] [CrossRef] [PubMed]
- Igual, M.; Flores-León, A.; Picó, B.; Martínez-Monzó, J.; García-Segovia, P. Physicochemical, structural, and functional properties of snake melon (Cucumis melo subsp. melo Var. flexuosus) microencapsulated with pea protein and pea fibre by freeze-drying. Foods 2023, 12, 2679. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Zhang, Q.; Wang, T.; Xu, Y.; Zang, Z.; Wan, F.; Yue, Y.; Huang, X. Effect of ultrasonic pretreatment on the far-infrared drying process and quality characteristics of Licorice. Foods 2023, 12, 2414. [Google Scholar] [CrossRef]
- Xu, N.; Zeng, X.; Wang, P.; Chen, X.; Xu, X.; Han, M. Investigation on taste characteristics and sensory perception of soft-boiled chicken during oral processing based on electronic tongue and electronic nose. Food Sci. Hum. Wellness 2024, 13, 313–326. [Google Scholar] [CrossRef]
- Chen, D.; Qin, L.; Geng, Y.; Kong, Q.; Wang, S.; Lin, S. The aroma fingerprints and discrimination analysis of shiitake mushrooms from three different drying conditions by GC-IMS, GC-MS and DSA. Foods 2021, 10, 2991. [Google Scholar] [CrossRef] [PubMed]
- Mayor, L.; Sereno, A.M. Modelling shrinkage during convective drying of food materials: A review. J. Food Eng. 2004, 61, 373–386. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Bai, J.W.; Wang, Y.C.; Cai, J.R.; Zhang, L.; Dai, Y.; Tian, X.Y.; Xiao, H.W. Three-dimensional appearance and physicochemical properties of pleurotus eryngii under different drying methods. Foods 2023, 12, 1999. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Zhao, Y.; Li, X.; Fan, J.; Wang, L. Effect of different drying methods on the quality and microstructure of fresh jujube crisp slices. J. Food Process. Preserv. 2021, 45, e15162. [Google Scholar] [CrossRef]
- Singh, P.; Chakraborty, S.; Talukdar, P. A Novel Non-Intrusive Imaging Technique to Quantify Shrinkage of Elephant Foot Yam During Convective Drying. J. Therm. Sci. Eng. Appl. 2023, 15, 050903. [Google Scholar] [CrossRef]
- Krokida, M.K.; Karathanos, V.T.; Maroulis, Z.B. Compression analysis of dehydrated agricultural products. Dry. Technol. 2000, 18, 395–408. [Google Scholar] [CrossRef]
- Ko, W.C.; Liu, W.C.; Tsang, Y.T.; Hsieh, C.W. Kinetics of winter mushrooms (Flammulina velutipes) microstructure and quality changes during thermal processing. J. Food Eng. 2007, 81, 587–598. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Y.; Zheng, Y.; Li, Z.; Zhang, Y.; Zheng, B.; Lo, Y.M.; Miao, S.; Tian, Y. Effects of microwave vacuum drying on macroscopic properties and microstructure of Lotus (Nelumbo nucifera Gaertn.) seeds. Int. J. Food Eng. 2018, 14, 20170313. [Google Scholar] [CrossRef]
- Xiao, H.W.; Bai, J.W.; Sun, D.W.; Gao, Z.J. The application of superheated steam impingement blanching (SSIB) in agricultural products processing—A review. J. Food Eng. 2014, 132, 39–47. [Google Scholar] [CrossRef]
- Somjai, C.; Siriwoharn, T.; Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Wiriyacharee, P. Utilization of Maillard reaction in moist-dry-heating system to enhance physicochemical and antioxidative properties of dried whole longan fruit. Heliyon 2021, 7, e07094. [Google Scholar] [CrossRef]
- Huang, X.H.; Qi, L.-B.; Fu, B.S.; Chen, Z.H.; Zhang, Y.Y.; Du, M.; Dong, X.P.; Zhu, B.W.; Qin, L. Flavor formation in different production steps during the processing of cold-smoked Spanish mackerel. Food Chem. 2019, 286, 241–249. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, J.; Chen, S.; Yu, H.; Chen, C.; Huang, J.; Yuan, H.; Lou, X. Rapid identification of adulteration in raw bovine milk with soymilk by electronic nose and headspace-gas chromatography ion-mobility spectrometry. Food Chem. X 2023, 18, 100696. [Google Scholar] [CrossRef]
- Song, F.; Xiang, H.; Li, Z.; Li, J.; Li, L.; Fang Song, C. Monitoring the baking quality of Tieguanyin via electronic nose combined with GC–MS. Food Res. Int. 2023, 165, 112513. [Google Scholar] [CrossRef]
- Guo, Q.; Adelina, N.M.; Hu, J.; Zhang, L.; Zhao, Y. Comparative analysis of volatile profiles in four pine-mushrooms using HS-SPME/GC-MS and E-nose. Food Control 2022, 134, 108711. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, D.J.; Kim, J.Y.; Lim, S.T. Volatile composition and sensory characteristics of onion powders prepared by convective drying. Food Chem. 2017, 231, 386–392. [Google Scholar] [CrossRef]
- Politowicz, J.; Lech, K.; Lipan, L.; Figiel, A.; Carbonell-Barrachina, Á.A. Volatile composition and sensory profile of shiitake mushrooms as affected by drying method. J. Sci. Food Agric. 2018, 98, 1511–1521. [Google Scholar] [CrossRef]
- Rajkumar, G.; Shanmugam, S.; Galvâo, M.d.S.; Dutra Sandes, R.D.; Leite Neta, M.T.S.; Narain, N.; Mujumdar, A.S. Comparative evaluation of physical properties and volatiles profile of cabbages subjected to hot air and freeze drying. LWT 2017, 80, 501–509. [Google Scholar] [CrossRef]
- Yang, W.; Yu, J.; Pei, F.; Mariga, A.M.; Ma, N.; Fang, Y.; Hu, Q. Effect of hot air drying on volatile compounds of Flammulina velutipes detected by HS-SPME-GC-MS and electronic nose. Food Chem. 2016, 196, 860–866. [Google Scholar] [CrossRef]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC–MS and electronic nose. LWT—Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Qin, L.; Gao, J.X.; Xue, J.; Chen, D.; Lin, S.Y.; Dong, X.P.; Zhu, B.W. Changes in aroma profile of shiitake mushroom (Lentinus edodes) during different stages of hot air drying. Foods 2020, 9, 444. [Google Scholar] [CrossRef]
- Hiraide, M.; Miyazaki, Y.; Shibata, Y. The smell and odorous components of dried shiitake mushroom, Lentinula edodes I: Relationship between sensory evaluations and amounts of odorous components. J. Wood Sci. 2004, 50, 358–364. [Google Scholar] [CrossRef]
- Chen, W.; Li, W.; Yang, Y.; Yu, H.; Zhou, S.; Feng, J.; Li, X.; Liu, Y. Analysis and evaluation of tasty components in the pileus and stipe of Lentinula edodes at different growth stages. J. Agric. Food Chem. 2015, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, Q.; Pei, F.; Mariga, A.M.; Yang, W. Influence of different storage conditions on physical and sensory properties of freeze-dried Agaricus bisporus slices. LWT 2018, 97, 164–171. [Google Scholar]
- Tan, H.R.; Lau, H.; Liu, S.Q.; Tan, L.P.; Sakumoto, S.; Lassabliere, B.; Leong, K.-C.; Sun, J.; Yu, B. Characterisation of key odourants in Japanese green tea using gas chromatography-olfactometry and gas chromatography-mass spectrometry. LWT 2019, 108, 221–232. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Huang, A. A metabolomics-based approach investigates volatile flavor formation and characteristic compounds of the Dahe black pig dry-cured ham. Meat Sci. 2019, 158, 107904. [Google Scholar] [CrossRef]




| No. | Compounds | Formula | Areas (%) | Odorant Description | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | SD | FID 50 °C | FID 55 °C | FID 60 °C | FID 65 °C | FID 70 °C | ||||
| Alcohols | 63.69% | 15.62% | 5.45% | 1.44% | 8.50% | 4.14% | 2.82% | |||
| 1 | 1-Butanol, 3-methyl- | C5H12O | nd | nd | 0.88% | nd | nd | nd | nd | Malty, bitter |
| 2 | 1-Decanol | C10H22O | nd | 0.19% | nd | nd | 0.25% | nd | nd | * |
| 3 | 1-Dodecanol | C12H26O | nd | nd | nd | 0.27% | nd | nd | nd | * |
| 4 | 1-Octen-3-ol | C8H16O | 62.33% | 9.75% | 2.21% | nd | 0.82% | 1.04% | 1.42% | Mushroom, grass a |
| 5 | 1-Undecanol | C11H24O | nd | 2.53% | nd | nd | 0.42% | 0.58% | 0.54% | * |
| 6 | 2-Octen-1-ol, (Z)- | C8H16O | 1.36% | nd | nd | nd | nd | nd | nd | * |
| 7 | Phenylethyl Alcohol | C8H10O | nd | 3.16% | 2.36% | 1.17% | 7.01% | 2.52% | 0.86% | Sweet a |
| Ketones | 4.70% | 4.91% | 1.32% | 1.82% | 1.70% | 1.27% | 1.32% | |||
| 8 | 1-Octen-3-one | C8H14O | 4.08% | nd | nd | 0.50% | nd | nd | nd | Mushroom |
| 9 | 2,3-Pentanedione | C5H8O2 | nd | nd | nd | nd | nd | nd | 0.28% | Almond, butter, pungent a |
| 10 | 2-Undecanone | C11H22O | nd | 1.50% | 1.32% | nd | nd | 0.96% | 1.04% | Dusty, ketone, tallow a |
| 11 | 3-Octanone | C8H16O | 0.62% | 3.11% | nd | 1.32% | 0.65% | nd | nd | Earthy, mushroom a |
| 12 | 3-Octen-2-one | C8H14O | nd | nd | nd | nd | nd | 0.31% | nd | * |
| 13 | Acetophenone | C8H8O | nd | 0.31% | nd | nd | nd | nd | nd | Glue, musty a |
| 14 | Furaneol | C6H8O3 | nd | nd | nd | nd | 1.04% | nd | nd | * |
| Hydrocarbons | 0.00% | 28.81% | 38.80% | 14.10% | 16.44% | 15.38% | 16.53% | |||
| 15 | 1-Tetradecene | C14H28 | nd | nd | nd | 0.76% | 0.71% | nd | nd | * |
| 16 | Bicyclo [4.1.0]heptane, 3,7,7-trimethyl-, (1.alpha., 3.alpha., 6.alpha.) | C10H18 | nd | nd | 1.50% | nd | nd | nd | nd | * |
| 17 | Butane | C4H10 | nd | nd | 0.98% | nd | nd | nd | 0.37% | * |
| 18 | Cetene | C16H32 | nd | nd | 1.26% | nd | nd | nd | nd | * |
| 19 | Cyclohexene,3-(1-methylpropyl)- | C10H18 | nd | 1.85% | 3.49% | 0.37% | nd | nd | 0.28% | * |
| 20 | Cyclopentane, 1-methyl-1-(2-methyl-2-propenyl)- | C10H18 | nd | 0.90% | nd | nd | nd | nd | nd | * |
| 21 | Cyclopentane, nonyl- | C14H28 | nd | nd | 1.05% | nd | nd | nd | nd | * |
| 22 | Cyclopropane, propyl- | C6H12 | nd | 0.57% | nd | nd | nd | nd | nd | * |
| 23 | Cyclotetradecane | C14H28 | nd | 1.12% | nd | nd | nd | nd | nd | * |
| 24 | Cyclotridecane | C13H26 | nd | nd | 1.99% | nd | nd | nd | nd | * |
| 25 | Dodecane | C12H26 | nd | 8.07% | 8.46% | 4.32% | 5.53% | 5.44% | 4.85% | Alkane a |
| 26 | Heptylcyclohexane | C13H26 | nd | 0.59% | 0.58% | nd | 0.22% | nd | 0.28% | * |
| 27 | n-Nonylcyclohexane | C15H30 | nd | 0.63% | 0.74% | nd | 0.22% | 0.29% | 0.42% | * |
| 28 | Nonane, 5-(2-methylpropyl)- | C13H28 | nd | 0.99% | nd | nd | nd | nd | nd | Linseed oil, oily, sweaty a |
| 29 | Oxirane, trimethyl- | C5H10O | nd | 0.55% | nd | nd | nd | nd | nd | * |
| 30 | Styrene | C8H8 | nd | nd | 2.18% | 0.99% | 0.87% | 0.74% | 1.36% | * |
| 31 | Tetradecane | C14H30 | nd | nd | 1.93% | 0.78% | 0.91% | 0.81% | 0.95% | * |
| 32 | Tetradecane, 3-methyl- | C15H32 | nd | 0.37% | nd | nd | nd | nd | nd | * |
| 33 | Tridecane | C13H28 | nd | 12.04% | 13.61% | 6.19% | 7.05% | 7.28% | 7.38% | * |
| 34 | Undecane | C11H24 | nd | 1.13% | 1.05% | 0.68% | 0.93% | 0.82% | 0.65% | * |
| S-compounds | 28.28% | 15.33% | 17.72% | 67.47% | 48.21% | 51.18% | 41.42% | |||
| 35 | 1,2,4,5-Tetrathiane | C2H4S4 | 8.53% | 3.03% | 3.27% | 7.79% | 4.45% | 2.87% | nd | * |
| 36 | 1,2,4,6-Tetrathiepane | C3H6S4 | nd | 0.86% | 1.12% | 2.21% | 2.98% | 2.27% | 2.27% | * |
| 37 | 1,2,4-Trithiolane | C2H4S3 | 3.37% | 8.70% | 9.43% | 14.66% | 17.06% | 25.68% | 24.62% | Garlic a |
| 38 | 2,4,5-trithiahexane 2,2-dioxide | C3H8O2S3 | 0.59% | nd | nd | nd | nd | nd | nd | * |
| 39 | Butane, 1-isothiocyanato-3-methyl- | C6H11NS | nd | nd | nd | 1.58% | 1.76% | 1.84% | nd | * |
| 40 | Carbon disulfide | CS2 | 6.57% | 1.46% | 1.85% | 11.68% | 7.04% | 8.49% | 4.52% | * |
| 41 | Dimethyl sulfone | C2H6O2S | nd | nd | nd | nd | 1.28% | 3.23% | 6.55% | * |
| 42 | Dimethyl trisulfide | C2H6S3 | nd | nd | nd | 8.99% | 2.46% | nd | 1.46% | Alliaceous, meaty a |
| 43 | Lenthionine | C2H4S5 | 9.22% | 1.28% | 2.04% | 16.53% | 10.18% | 6.81% | 2.00% | Mushroom a |
| 44 | Tetrasulfide, dimethyl | C2H6S4 | nd | nd | nd | 4.03% | 0.99% | nd | nd | * |
| Acids | 0.00% | 8.90% | 10.20% | 5.56% | 15.01% | 13.55% | 14.96% | |||
| 45 | Acetic acid | C2H4O2 | nd | 8.40% | 8.81% | 5.17% | 15.01% | 12.94% | 14.12% | Vinegar-like a |
| 46 | Butanoic acid | C4H8O2 | nd | 0.50% | nd | nd | nd | nd | nd | * |
| 47 | Butanoic acid, 2-methyl- | C5H10O2 | nd | nd | 0.21% | 0.39% | nd | nd | nd | * |
| 48 | Butanoic acid, 3-methyl- | C5H10O2 | nd | nd | 0.24% | nd | nd | 0.61% | 0.84% | * |
| 49 | Pentanoic acid | C5H10O2 | nd | nd | 0.93% | nd | nd | nd | nd | * |
| Aldehydes | 0.72% | 2.79% | 7.02% | 3.86% | 1.51% | 6.01% | 6.17% | |||
| 50 | 2-Furancarboxaldehyde, 5-methyl- | C6H6O2 | nd | nd | nd | nd | nd | nd | 0.51% | * |
| 51 | 2-Isopropyl-5-methylhex-2-enal | C10H18O | nd | nd | nd | nd | nd | 0.46% | 0.33% | * |
| 52 | 2-Phenylpropenal | C9H8O | 0.72% | nd | nd | nd | nd | nd | nd | * |
| 53 | Benzaldehyde | C7H6O | nd | 2.79% | 2.97% | 2.80% | 0.76% | 1.52% | 1.55% | Almond, bitter almond a |
| 54 | Benzeneacetaldehyde, .alpha.-ethylidene- | C10H10O | nd | nd | nd | nd | nd | 0.57% | 0.42% | * |
| 55 | Butanal, 2-methyl- | C5H10O | nd | nd | 0.90% | nd | 0.20% | 1.33% | 1.24% | * |
| 56 | Butanal, 3-methyl- | C5H10O | nd | nd | 1.26% | 0.86% | 0.56% | 1.59% | 1.34% | * |
| 57 | Hexanal | C6H12O | nd | nd | 0.92% | 0.21% | nd | 0.53% | 0.78% | Aldehyde, leaves, vinous a |
| 58 | Propanal, 2-methyl- | C4H8O | nd | nd | 0.97% | nd | nd | nd | nd | * |
| N-compounds | 0.00% | 3.23% | 3.93% | 2.77% | 3.37% | 6.00% | 12.59% | |||
| 59 | 1-Butanamine, 3-methyl-N-(3-methylbutylidene)- | C10H21N | nd | nd | nd | 0.64% | nd | nd | nd | * |
| 60 | 1-Butanamine, N-(2-furanylmethylene)-3-methyl- | C10H15NO | nd | nd | nd | 0.97% | 1.82% | 0.98% | 0.63% | * |
| 61 | 1H-Isoindole, 3-methoxy-4,7-dimethyl- | C11H13NO | nd | nd | 2.21% | nd | nd | nd | nd | * |
| 62 | 1H-Pyrrole-2-carboxaldehyde, 1-ethyl- | C7H9NO | nd | 0.31% | nd | nd | nd | nd | nd | * |
| 63 | 2-(3-Methylbutyl)-3,5-dimethylpyrazine | C11H18N2 | nd | nd | nd | nd | nd | nd | 0.40% | * |
| 64 | 2-Isoamyl-6-methylpyrazine | C10H16N2 | nd | nd | nd | nd | nd | nd | 0.30% | * |
| 65 | 3-Acetyl-1H-pyrroline | C6H7NO | nd | 0.33% | nd | nd | nd | nd | nd | * |
| 66 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | nd | nd | nd | nd | nd | 0.64% | 1.44% | * |
| 67 | Aziridine, 1-ethenyl- | C4H7N | nd | 0.62% | nd | nd | nd | nd | nd | * |
| 68 | Ethanone, 1-(1H-pyrrol-2-yl)- | C6H7NO | nd | nd | nd | 0.39% | 0.68% | nd | nd | * |
| 69 | Formamide, N,N-dimethyl- | C3H7NO | nd | 0.24% | 0.64% | nd | 0.26% | nd | nd | * |
| 70 | Pyrazine, 2,3-dimethyl- | C6H8N2 | nd | nd | nd | nd | nd | nd | 0.38% | * |
| 71 | Pyrazine, 2,5-dimethyl- | C6H8N2 | nd | nd | nd | nd | nd | nd | 1.74% | * |
| 72 | Pyrazine, 2,6-diethyl- | C8H12N2 | nd | nd | nd | nd | nd | 1.90% | nd | * |
| 73 | Pyrazine, 2-ethyl-6-methyl- | C7H10N2 | nd | 0.80% | 0.89% | 0.29% | 0.60% | 1.87% | 2.22% | * |
| 74 | Pyrazine, 3,5-diethyl-2-methyl- | C9H14N2 | nd | nd | nd | nd | nd | nd | 0.40% | * |
| 75 | Pyrazine, 3-ethyl-2,5-dimethyl- | C8H12N2 | nd | nd | nd | 0.48% | nd | nd | 3.48% | * |
| 76 | Pyrazine, methyl- | C5H6N2 | nd | 0.26% | 0.19% | nd | nd | nd | 0.34% | * |
| 77 | Pyrazine, trimethyl- | C7H10N2 | nd | nd | nd | nd | nd | 0.61% | 1.27% | * |
| 78 | Triethylamine | C6H15N | nd | 0.67% | nd | nd | nd | nd | nd | * |
| Esters | 2.61% | 10.32% | 6.95% | 0.32% | 2.24% | 0.00% | 0.00% | * | ||
| 79 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | nd | nd | 0.47% | nd | nd | nd | nd | * |
| 80 | 2(3H)-Furanone, dihydro-5-methyl- | C5H8O2 | nd | 2.09% | 5.66% | nd | 0.78% | nd | nd | * |
| 81 | 2(3H)-Furanone, dihydro-5-pentyl- | C9H16O2 | nd | 0.72% | nd | nd | nd | nd | nd | * |
| 82 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | C16H30O4 | nd | nd | nd | 0.32% | 0.40% | nd | nd | * |
| 83 | Butyrolactone | C4H6O2 | nd | 6.70% | nd | nd | nd | nd | nd | * |
| 84 | Cyclohexene,3-(1-methylpropyl)- | C10H18 | nd | nd | nd | nd | 0.35% | nd | nd | * |
| 85 | Dibutyl phthalate | C16H22O4 | nd | 0.45% | nd | nd | nd | nd | nd | * |
| 86 | Dimethyl phthalate | C10H10O4 | nd | nd | 0.50% | nd | 0.39% | nd | nd | * |
| 87 | Formic acid, octyl ester | C9H18O2 | 2.61% | nd | nd | nd | nd | nd | nd | * |
| 88 | Phthalic acid, butyl hept-4-yl ester | C19H28O4 | nd | nd | 0.33% | nd | nd | nd | nd | * |
| 89 | Phthalic acid, hept-4-yl isobutyl ester | C19H28O4 | nd | 0.36% | nd | nd | nd | nd | nd | * |
| 90 | Propanoic acid, 2-methyl-, 2-phenylethyl ester | C12H16O2 | nd | nd | nd | nd | 0.32% | nd | nd | * |
| Others | 0.00% | 10.08% | 8.61% | 2.65% | 3.01% | 2.47% | 4.18% | |||
| 91 | (3aS,8aS)-6,8a-Dimethyl-3-(propan-2-ylidene)-1,2,3,3a,4,5,8,8a-octahydroazulene | C15H24 | nd | nd | 1.14% | 0.00% | 0.33% | nd | nd | * |
| 92 | 1H-Indene, 1-ethylidene- | C11H10 | nd | nd | nd | 0.29% | nd | nd | nd | * |
| 93 | Benzene, 1,2-dichloro- | C6H4Cl2 | nd | nd | 4.58% | 1.32% | 1.94% | 1.38% | 2.15% | * |
| 94 | Benzene, 1,4-dichloro- | C6H4Cl2 | nd | 8.42% | nd | nd | nd | nd | nd | * |
| 95 | Diisobutyl cellosolve | C10H22O2 | nd | nd | nd | 0.00% | nd | nd | nd | * |
| 96 | Dimethyl ether | C2H6O | nd | nd | 0.76% | 0.20% | nd | nd | nd | * |
| 97 | Ethylbenzene | C8H10 | nd | nd | 0.31% | nd | nd | nd | nd | * |
| 98 | Furan, 2-pentyl- | C9H14O | nd | nd | nd | 0.85% | nd | 1.09% | 1.51% | * |
| 99 | Naphthalene, 1,4-dimethyl- | C12H12 | nd | nd | 0.70% | nd | nd | nd | nd | * |
| 100 | Naphthalene, 1,6-dimethyl- | C12H12 | nd | nd | 0.21% | nd | nd | nd | nd | * |
| 101 | Naphthalene, 2-methyl- | C11H10 | nd | nd | 0.91% | nd | 0.36% | nd | nd | * |
| 102 | Phthalic acid, 4-fluoro-2-nitrophenyl methyl ester | C15H10FNO6 | nd | nd | nd | 0.00% | nd | nd | nd | * |
| 103 | p-Xylene | C8H10 | nd | 1.66% | nd | nd | 0.38% | nd | 0.52% | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Jiang, Y.-S.; Wang, Y.-B.; Xiao, H.-W.; Liu, W.; Ma, Y.; Zhao, X.-Y. Changes in the Physical Properties and Volatile Odor Characteristics of Shiitake Mushrooms (Lentinula edodes) in Far Infrared Radiation Drying. Foods 2023, 12, 3213. https://doi.org/10.3390/foods12173213
Xie L, Jiang Y-S, Wang Y-B, Xiao H-W, Liu W, Ma Y, Zhao X-Y. Changes in the Physical Properties and Volatile Odor Characteristics of Shiitake Mushrooms (Lentinula edodes) in Far Infrared Radiation Drying. Foods. 2023; 12(17):3213. https://doi.org/10.3390/foods12173213
Chicago/Turabian StyleXie, Long, Yu-Si Jiang, Yu-Bin Wang, Hong-Wei Xiao, Wei Liu, Yue Ma, and Xiao-Yan Zhao. 2023. "Changes in the Physical Properties and Volatile Odor Characteristics of Shiitake Mushrooms (Lentinula edodes) in Far Infrared Radiation Drying" Foods 12, no. 17: 3213. https://doi.org/10.3390/foods12173213
APA StyleXie, L., Jiang, Y.-S., Wang, Y.-B., Xiao, H.-W., Liu, W., Ma, Y., & Zhao, X.-Y. (2023). Changes in the Physical Properties and Volatile Odor Characteristics of Shiitake Mushrooms (Lentinula edodes) in Far Infrared Radiation Drying. Foods, 12(17), 3213. https://doi.org/10.3390/foods12173213







