Abstract
Oxalate is an antinutrient present in a wide range of foods, with plant products, especially green leafy vegetables, being the main sources of dietary oxalates. This compound has been largely associated with hyperoxaluria, kidney stone formation, and, in more severe cases, systematic oxalosis. Due to its impact on human health, it is extremely important to control the amount of oxalate present in foods, particularly for patients with kidney stone issues. In this review, a summary and discussion of the current knowledge on oxalate analysis, its extraction conditions, specific features of analytical methods, reported occurrence in foods, and its health implications are presented. In addition, a brief conclusion and further perspectives on whether high-oxalate foods are truly problematic and can be seen as health threats are shown.
Keywords:
oxalate; foods; extraction conditions; analytical methods; occurrence; health implications 1. Introduction
Oxalate is a chemical compound that can form soluble and insoluble salts in water. This substance is present in a wide range of foods, with plant products being the main sources of dietary oxalates [1]. In plants, it plays a relevant role in various functions such as calcium homeostasis; pH regulation; plant growth, development and protection; photosynthesis; and detoxification of heavy metals [2,3].
According to the literature, various methods have been employed for the determination of oxalate in foods, including enzymatic assays [4,5,6,7,8,9,10,11], spectrofluorimetry [12], spectrophotometry, amperometry [9,13,14], electrochemical [15,16], capillary electrophoresis [17,18], titration [19,20,21,22,23,24,25,26,27], gas chromatography (GC) [28], and high-performance liquid chromatography (HPLC). HPLC is the most recently referenced method used for the determination of oxalates because of its high sensitivity, accuracy, versatility, and reliability, despite being expensive to purchase, repair, and maintain [29]. Conversely, despite its lower sensitivity, spectrophotometry is an inexpensive, rapid, and simpler method, requiring only one main instrument [30,31]. Accurate measurement of oxalate in foods is extremely dependent on its extraction, the first step in oxalate analysis [1]. Despite being two completely different methods, HPLC and spectrophotometry extraction conditions of total and soluble oxalates in foods are similar. Regarding analytical conditions, they have different and specific features since they are completely distinct procedures.
Concerning oxalate occurrence in foods, green leafy vegetables are particularly relevant, and some are considered high-oxalate foods. For example, published oxalate values are 329.6–2350 mg total oxalates/100 g fresh weight (FW) for spinach [32,33,34,35,36,37,38,39], 1235 mg total oxalates/100 g FW for rhubarb [1], 874 [40] and 1458.1 [1] mg total oxalates/100 g FW for swiss chard, 1079 mg soluble oxalates/100 g FW for sorrel [41], and 300.2–721.9 mg total oxalates/100 g FW for taro leaves [34].
Considering human health, oxalates have been a concern for a long time due to their antinutritive effects and potential nephrotoxicity [42,43]. As antinutrients, oxalates restrict the bioavailability of some nutrients since they can bind to minerals, reducing their absorption and use [3,44]. Potentially toxic soluble oxalates are delivered to the kidneys and can form calcium oxalate crystals there, which can lead to hyperoxaluria and kidney stone formation, also known as nephrolithiasis or urolithiasis [3,45,46,47,48]. In more serious cases, systemic oxalosis has been reported, a phenomenon in which calcium oxalate crystals deposit in various organs, tissues, and bones, when renal function declines and excess oxalate exists in the bloodstream [49,50].
This review has the purpose of gathering a considerable amount of information about oxalate, focusing on its extraction and analytical conditions and content in various foods measured by HPLC and spectrophotometry. Optimization of these parameters for oxalate determination in foods is very relevant to achieve reliable and accurate results considering the impact that this antinutrient could have on human health, especially on patients with kidney stone problems.
2. Oxalates
Oxalate, or oxalic acid, is an antinutrient present, commonly in trace amounts, in fruits, nuts, cereals, fungi, vegetables, aromatic plants, and beverages, with plant-based products being the main sources of dietary oxalates [1]. However, some plants have high quantities of these compounds. In this matter, green leafy vegetables, such as spinach, Swiss chard, and rhubarb, are highlighted [3,32].
Oxalate can form soluble and insoluble salts in water. When binding with sodium, potassium, and ammonium ions, it forms soluble oxalates, whereas with calcium, iron, and magnesium it precipitates, forming insoluble compounds and making these minerals unavailable for absorption. Despite this fact, for example, zinc absorption and metabolism do not appear to be affected. In general, insoluble salts in water can be freely dissolved in acid [44,51,52]. Regarding health, one of the most important insoluble salts is calcium oxalate, having two hydration forms, monohydrates and dihydrates, which impact the shape of its crystals [1].
Depending on the pH of the cell sap, the liquid inside the vacuole of plants where oxalates are mostly found, they can present different chemical structures (Figure 1). On the one hand, when pH is 2, acid oxalate is the main oxalate. On the other hand, when pH is approximately 6, oxalate ion is the majority [51]. At the cytoplasmic pH of 7, oxalic acid also suffers deprotonation and exists as oxalate ion [2].

Figure 1.
Chemical structure of (a) oxalic acid; (b) oxalate ion; (c) acid oxalate ion; and (d) oxalate salt, being M2+ a metallic cation.
Chemically, oxalic acid is characterized as a dicarboxylic organic acid with low molecular weight, high acidity (pKa1 = 1.25, pKa2 = 4.27), and chelating and reducing abilities. Therefore, in plants, it plays a relevant role in many biological processes such as calcium homeostasis; pH regulation; plant growth, development, and protection; photosynthesis; and detoxification of heavy metals [2,3]. However, when in excess in plants because of a metabolic disorder, this will promote impairment of its functions and, thus, reduction of crop quality [2]. Many factors can influence oxalate accumulation in plants, such as growth, ripeness, variety, season, time of harvest, and cultivation conditions (e.g., use of nitrate fertilizer or soil conditions) [36,39,47,51].
The biosynthesis of oxalate in plants can result from different mechanisms, with glyoxylate, ascorbic acid, and oxaloacetate being the precursors of oxalate, an end product of their metabolisms. Therefore, there are three most-studied pathways: the glycolic acid/glyoxalic acid pathway, the ascorbic acid pathway, and the oxaloacetic acid pathway [2,51].
In addition to photosynthetic organisms, mammals can also produce oxalates in small amounts. In mammals, oxalate produced endogenously is a metabolite of ascorbate, hydroxyproline, glyoxylate, and glycine [3].
3. Analysis of Oxalates in Foods
According to the literature, various methods have been employed for the determination of oxalate in foods, including enzymatic assays, spectrofluorimetry, spectrophotometry, amperometry, electrochemistry, capillary electrophoresis, titration, GC, and HPLC [53]. All of these methods have advantages and disadvantages like their high sensitivity and specificity but also high costs and time-consuming and complex sample handling [54]. For example, samples require an additional step (esterification) for GC analysis [28,55]. Even though there are many others, this manuscript will only focus on HPLC and UV–Vis spectrophotometry, specifically on their extraction and analytical conditions. HPLC is the most recently referenced method used for the determination of oxalates because of its high sensitivity, accuracy, versatility, and reliability, despite requiring equipment that is expensive to purchase, repair, and maintain [29]. Conversely, despite its lower sensitivity, spectrophotometry is an inexpensive, simple, rapid, and accurate technique, using only a spectrophotometer as the main instrument [30,31].
3.1. Extraction Conditions
Accurate measurement of oxalate in foods is extremely dependent on its extraction, the first step in oxalate analysis. However, it seems to be difficult because of its extraction from plant tissue or its generation due to the oxidation of ascorbic acid during extraction [1].
Commonly, total oxalates (which include soluble and insoluble) are extracted with hydrochloric acid (HCl), whereas for soluble oxalates water is used [51]. However, a few papers describe different solutions, e.g., metaphosphoric acid [56], potassium phosphate buffer (pH 2.4) [57], or HCl with drops of octanol [58], for the extraction of total oxalates, and carbonate and sodium bicarbonate solution for the extraction of soluble oxalates [59,60]. Altunay et al. [30] also used HCl for the extraction of total oxalates and water for soluble oxalates; however, both extractions were conducted under ultrasonic power (300 W, 50 Hz).
The volume of the added extraction solution depends on the sample quantity, presenting a wide range of different values. Regarding other parameters, for HPLC analysis the hot extraction of total and soluble oxalates at 80 °C is more common, whereas for spectrophotometry 100 °C is more recurrent. For both methods, the most frequent time for extraction is 15 min. However, for HPLC, extraction times from 1–180 min (Table 1) are reported, whereas, for spectrophotometry, 15–1200 min is indicated (Table 2).

Table 1.
Extraction conditions for quantification of oxalates in foods by high-performance liquid chromatography.

Table 2.
Extraction conditions for quantification of oxalates in foods by spectrophotometry.
Additionally, some studies have shown the influence of modifying these extraction conditions. Hönow et al. [1] concluded that increasing extraction time from 30 to 180 min for the extraction of total oxalates resulted in oxalate generation and the increase was higher when treated at 100 °C (reflux) than at 80 °C (water bath). For soluble oxalates, results increased significantly after extraction at 80 °C compared to extraction at 21 °C and there were no significant differences between extractions of 15 or 30 min, proposing that soluble oxalate should be extracted with distilled water for 15 min at room temperature. In contrast, other authors suggested that room temperature might not be enough for the complete extraction of oxalates, leading to an underestimation [5]. This theory is also supported by Kusuma et al. [65] who considered that extraction temperatures above 65 °C are required for efficient extraction of total oxalates and pH should be at least 1. Therefore, the ideal temperature of oxalate extraction remains a controversial question because it can lead to oxalate generation due to in vitro conversion from precursors or failure to dissolve all pre-existing insoluble oxalates [1,5]. Concerning time, 15 min was considered to be the minimum for extraction and increasing it has not been associated with any advantage [65].
Frequently, further procedures after extraction mainly consist of filtration and centrifugation. However, some authors utilize more specific techniques. For example, prior to HPLC analysis, there have been reports of the use of solid-phase extraction (SPE) [35,55,69], purification with ion exchange [35], concentration [35,69], and, specifically for soluble oxalates, acidification with HCl to stabilize ascorbic acid which can be present and cause oxaloneogenesis at pH above 5, resulting in an overestimation [1,63,64,72]. For spectrophotometric methods, evaporation and subsequent redissolution for total oxalate analysis have been mentioned [58] and, once again, adjustment of pH, which is relevant for soluble oxalates [41,76,78].
Insoluble oxalates are also extracted during treatment with HCl, so their content is always calculated by the difference between total and soluble oxalates as Holloway et al. [83] suggested.
3.2. Methods’ Conditions
Despite having similar extraction conditions, chromatographic and spectrophotometric methods have different and specific features (Table 3 and Table 4).

Table 3.
High-performance liquid chromatography conditions for the analysis of oxalates in foods.

Table 4.
Spectrophotometric conditions for the analysis of oxalates in foods.
3.2.1. HPLC Conditions
Regarding HPLC conditions for the determination of oxalates (Table 3), ion exchange, ion exclusion, and reversed-phase columns are used. Considering ion exchange columns, one of the most referenced columns is IonPac AS4A [1,59,60,62,63,64,72]. Other authors have used different ones: propylamine anion exchange column (25 cm × 4.6 mm; 6 μm particle size), diethylaminoethyl (DEAE) anion exchange column (250 × 4.6 mm; 5 μm particle size), and Hamilton HA-X8.00 column with strong anion exchange resin in the sulfate form (255 × 5 mm; 7–10 μm particle size) [84]; Alltech All-Sep anion exchange column (100 × 4.6 mm; 7 µm particle size) [17] and Shodex IC SI-90 anion exchange column (250 × 4 mm; 9 μm particle size) filled with KanK-ASt (120 × 5 mm, 14 μm particle size) [71]. For ion exclusion, it seems that an ion exclusion column (300 × 7.8 mm) is frequently the selected one, either from Bio-Rad [32,34,40,61,70] or Rezex [65,66,67,68] brands. Regarding reversed-phase columns, the frequent use of 250 × 4.6 mm columns with different particle sizes has been reported [35,36,39,55,56,58,73,84], with 5 μm being the most common. Additionally, a radial compression column with C-18 or C-8 functionality (100 mm; 10 μm particle size) [84] has also been used. Some consumables listed in Table 3 may no longer be available in the market or have been replaced by columns with enhanced characteristics that contribute to the improvement of the analysis.
These columns are chosen considering different separation modes of HPLC. Ion chromatography is an effective method for the determination of oxalate ions because oxalic acid is a strong acid, giving away its protons and becoming negatively charged [71].
When using ion exclusion chromatography, the dissociated functional groups of the ion exchange resin present in the stationary phase have the same charge signal as the oxalate ion and, thus, it is repulsed and eluted [85]. In contrast, ion exchange chromatography is based on the exchange of oxalate ions with the counter-ions, which are anions of the ionic groups attached to the solid support being strongly retained [29]. Reversed-phase chromatography uses a non-polar stationary phase (e.g., C-18) with a polar mobile phase, being the most popular HPLC mode. The separation mechanism is based on polarity and hydrophobic/hydrophilic interactions between oxalates and these two phases. Furthermore, the use of a guard column, a smaller column applied before the analytical column to protect it from impurities present in samples and enhancing the lifetime of the main column, is commonly observed [29]. For the determination of oxalates, C-18 [55], amino [35], IonPac AG4A [59,60], and cation-H [32,34,40,65,66,67,68] guard columns are mentioned.
The main used type of elution for oxalate determination is undoubtedly isocratic. In a study using different elution programs for the separation of various organic acids, including oxalic acid, a gradient of (NH4)2SO4 and MgSO4 resulted in a rise in the baseline of the chromatograms [84]. Thus, using an isocratic elution promotes better results.
For the measurement of oxalates, there is a wide variety of mobile phases. For ion exchange columns, a carbonate and sodium bicarbonate solution for a conductivity detector [17,59,60,71] and an aqueous EDTA solution for amperometric detection are frequently utilized [1,63,64,72]. Some other mobile phases have been reported like 0.15 M NaH2PO4 (pH = 4.2); 0.30 M NH4H2PO4 adjusted to pH 6.5 with concentrated ammonium hydroxide; 0.5 M (NH4)2SO4 adjusted to pH 7.25 with ammonium hydroxide; 0.3 M (NH4)2SO4 containing 10% methanol and 0.1–1.5 M MgSO4 solutions for the gradient analysis [84]. When using ion exclusion columns, sulfuric acid is always the chosen mobile phase. However, Zulkhairi et al. [68] used a mixture of this acid with acetonitrile. For reversed-phase columns, dihydrogen phosphate (H2PO4−) is frequently used. This ion can be used alone [35,84] or combined in a mixture with tertiary butyl alcohol (TBA) [55] or tetrabutylammonium hydrogen sulfate [36,39] and it is frequently buffered with phosphoric acid (circa pH 2) [36,55,58,84]. Other authors used different solutions as mobile phase, for example, tetrabutylammonium chloride [73] and sulfuric acid [56]. In addition, buffer solutions have a big impact on the retention of analytes [29], so there is frequent use of these solutions to maintain a stable pH. For reversed-phase and ion exclusion columns, acidic buffers from approximately pH 2–3 are commonly used. In contrast, for ion exchange columns, higher pH values are allowed (circa pH 5).
The flow rate varies from 0.4–2 mL/min, with 1 mL/min or less being more common. When it is mentioned, column temperature ranges from room temperature to 80 °C. However, usually lower temperatures than 80 °C, such as 20 °C [58], 25 °C [65,66], 30 °C [73], 33 °C [71], or 50 °C, are used [58,68,70]. The most-reported injection volumes are 5 or 20 μL.
The most usual detectors are ultraviolet (UV) or ultraviolet–visible (UV–Vis), with 210 nm as the most common wavelength. In contrast, some authors report measurements at 206 [35,84], 215 [40,56], and 220 nm [39,55]. The choice of wavelength is usually made considering the maximum absorbance of analytes and, thus, maximum sensitivity. However, there are other parameters which are also taken into account for this choice, such as analysis time. Other used detectors are diode array detectors (DADs) or photodiode array (PDA) [68], which also detect absorption in the UV to Vis region but can scan the entire range [86]; refractive index (RI) detectors [84]; conductivity detectors [17,59,60,71]; and amperometric detectors [1,63,64,72]. These instruments are chosen depending on the type of HPLC analysis, considering its analytes. For example, authors who employed ion exchange chromatography for oxalate measurement have utilized conductivity detectors, whereas researchers who applied the HPLC–enzyme reactor method (HPLC-ER) used an amperometric detector. This last method is based on the chromatographic separation of oxalate combined with enzymatic conversion to hydrogen peroxide by oxalate oxidase and its amperometric detection [72,87].
3.2.2. Spectrophotometric Conditions
Contrasting with HPLC, studies that describe spectrophotometric methods analyze much fewer samples in quantity and variety, whereas authors who applied HPLC present a large number of results of various foods. In the measurement of oxalates by spectrophotometry (Table 4), spinach and mushrooms are frequently studied. These observations might be due to HPLC’s ability for automatization and spectrophotometry’s laborious procedures when applied to a large number of samples.
Spectrophotometric conditions are quite peculiar. The studies analyzed for this matter include catalytic/kinetic methods. In other words, these procedures follow a spectrophotometric reaction which has some kind of oxalate intervention, either as a reagent [30,41,58,75,76,77], catalyst [31,37,38,74,78,79,80,81,82], or activator [54]. Some manuscripts mention other studies in which oxalate acts as an inhibitor [54,78]. These are indirect methods since they do not measure the absorbance of oxalate, but by measuring the absorbance of a substance within a system where oxalate has influence, it is possible to extrapolate oxalate content.
Most of these systems are redox reactions, using a wide range of different reagents (Table 4). For oxalate measurement, the most common type of redox reaction is oxidation in acidic media. However, there are a few reductions reported [58,75]. The use of potassium dichromate as an oxidant agent is frequently observed, but oxygen [77] and bromate [54] have also been reported. In contrast, oxalate has been used as a reducing agent [58,75].
In addition, other more complex procedures exist. For example, Matsubara et al. [77] studied a spectrophotometric method based on the oxidation of oxalate by oxygen in the presence of oxalate oxidase, forming hydrogen peroxide (H2O2) which forms a complex with TiO(tpypH4)4+ and TiO2(tpypH4)4+ that absorbs at 450 nm. Furthermore, Mo(VI) can form a stable complex with oxalate, [MoO3(Ox)]2−, which can have different forms in a solution in the pH range of 2–7, [Mo2O5(Ox)2]2−, or [Mo2O5(OH)(Ox)2]3−. Subsequently, this anionic complex associates with Toluidine blue (TBH2+) which has a maximum peak absorbance at 627 nm [30]. A new sensor material for solid-phase spectrophotometric determination of food oxalates was also developed. This method is based on the interaction of oxalates with a material of silica–titania xerogel with eriochrome cyanine R (Si-Ti/ECR) which causes sensor material discoloration, with absorbance being used as an analytical signal [41]. According to Tavallali et al. [76], the reaction of Reactive blue 4 (RB4)-Cu2+ with oxalate can be used for oxalate determination in food samples. The addition of oxalate to the RB4-Cu2+ complex increased the absorption band intensity at 607 nm and changed the color from sky blue to dark blue due to the regeneration of RB4 by the chelation of oxalate with Cu2+, since the binding constant of Cu2+ with oxalate is larger than that of Cu2+ with RB4.
Temperature, pH, and time of reactions are highly specific to each reaction, being parameters optimized before selecting the final procedure. The values of these parameters are chosen considering sensitivity and reproducibility [31,78]. Temperature, pH, and time of these reactions can range from approximately 20–90 °C, 3–7, and 8–60 min, respectively.
Reactions are monitored by measuring the absorbance of the reagents or products which are chromophore substances, such as crystal violet, Victoria blue, and brilliant cresyl blue, at maximum wavelength, λmax, the wavelength whose absorbance is maximum and producing maximum sensitivity. This measurement is always in the UV–Vis region (200–800 nm), mainly in the visible region (400–800 nm), since analyzed compounds are frequently colored and absorb this kind of light. For the construction of calibration graphs, oxalic acid as the standard solution was mainly used, but sodium oxalate [30,54,75,77,78,80] and potassium oxalate [58,76] were also reported.
By reading spectrophotometric methods for the determination of oxalate content, it can be concluded that validation is a frequent concern. Thus, linearity range, determination coefficient (r2), and limit of detection (LOD) are often presented parameters (Table 4).
In Figure 2, a summary of the most commonly used conditions for extraction for HPLC and spectrophotometry analysis is provided. However, according to the literature, a wide variety of techniques and conditions are applied depending on the type of food, which makes it difficult to define a single method to measure oxalate in foods.

Figure 2.
Summary of the most commonly used conditions for extraction for HPLC and spectrophotometry measurement of oxalate in foods. (a)—These parameters are highly specific to each reaction (Table 4).
4. Oxalate Occurrence in Foods
Oxalate contents (mg/100 g FW) of various foods measured by chromatographic and spectrophotometric methods, such as in fruits, vegetables, mushrooms, legumes, pseudocereals, and aromatic plants, are collected in Table 5 in alphabetical order. Other studies were also taken into consideration; however, their oxalate contents were not included in this table because they were presented in dry weight and it was not possible to convert them into fresh weight owing to the lack of moisture or dry matter values [57,58,60,62,66,68].

Table 5.
Occurrence of oxalate (mg/100 g of fresh weight) in foods.
In general, fruits are considered low-oxalate foods (<30 mg total oxalate/100 g FW), except for star fruit (160 [35] and 295.4 [1] mg total oxalate/100 g FW), elderberry (72.1 mg total oxalate/100 g FW), and dried fig (95.1 mg total oxalate/100 g FW) [1]. However, it is to be noted that for elderberry and dried fig, the majority of oxalate content is insoluble (72.1 and 89.6 mg/100 g FW, respectively) and, thus, less harmful [1].
Regarding vegetables, there is much more variety in the values of oxalate content, ranging from not detected to high amounts of oxalate. For example, raw New Zealand spinach and typical spinach have been reported as extremely high in oxalates with values of 1764.7 [32] and 329.6–2350 mg total oxalate/100 g FW [32,33,34,35,36,37,38,39], respectively. Soluble oxalate concentration in Spinacia oleracea has been studied during the cultivation season. Oxalate content was higher in winter (1092.9 mg soluble oxalate/100 g FW) and lowest in fall (614.9 mg soluble oxalate/100 g FW), indicating that higher oxalate content is related to a longer growing period since this compound is an end product of some metabolisms and it increases in the vacuole with plant aging [73]. Also, rhubarb contained high oxalate content (1235 mg total oxalate/100 g FW) [1] as well as Swiss chard with 874 [1] and 1458.1 [40] mg total oxalate/100 g FW, having more in leaves than in stems [32], and sorrel with 1079 mg soluble oxalate/100 g FW [41]. Taro leaves yielded 300.2–721.9 mg total oxalate/100 g FW, depending on the type of cultivar and processing techniques: baking increased oxalate content compared to raw samples, due to concentrating effects, whereas baking with milk (a source of calcium) decreased oxalate content, especially soluble [34]. This decrease happens because oxalate ions can bind with calcium, precipitating and reducing soluble oxalate [45]. Conversely, there were some vegetables with small amounts of oxalate or it was even undetected, e.g., cabbage, broccoli, cauliflower, cucumber, kale, and pumpkin (Table 5).
Regarding legumes, soybean is considered high in oxalates, ranging between 124 and 497 mg total oxalate/100 g FW, depending on the kind of sample analyzed [36,61,63]. For different types of beans, oxalate values ranged between 13.9 and 547.9 mg total oxalate/100 g FW [1,36,61,63], whereas chickpeas [63] and lentils [1,63] yielded low oxalate content (<24 mg total oxalate/100 g FW) and oxalate in cowpea was not detected [61].
In the pseudocereals category, amaranth is considered a high-oxalate food (1510.8 mg total oxalate/100 g FW) [40] as well as green amaranth (circa 1940 mg total oxalate/100 g FW) and purple amaranth (circa 1354 mg total oxalate/100 g FW) [33,34].
For aromatic plants, two different species of parsley, water dropwort (also known as water celery), and coriander yielded 136 [1] and 270.7 [40], 93 [40], and circa 41 [33,34] mg total oxalate/100 g FW, respectively. Licorice was the highest-oxalate food reported in this review with 3569.3 mg total oxalate/100 g FW [63]. In contrast, arugula, cress, garlic, and green onion were some examples of aromatic plants which did not contain detectable oxalate (Table 5).
Differences between oxalate values for the same food, as observed for beans, lettuce, parsley, mushrooms, or spinach, can vary according to growth, ripeness, climate, region, soil conditions, and time of harvest. In addition to these conditions, which are harder to control, are sample preparation, which can lead to oxalate generation or its incomplete extraction, and analytical methods with different features that can have an impact on oxalate results [1,6,36,65].
It is important to consider the usual amount of consumption of these foods. For example, some aromatic plants, like parsley, contained high oxalate values but their daily intake is naturally much less than 100 g. Also, the type of consumption has to be taken into account because some foods are not generally eaten in the form in which they were analyzed (e.g., raw beans or mushrooms). It is well known that the same food prepared differently (raw, boiled, baked, fried, etc.) can lead to different oxalate results. The reported values for potato (Solanum tuberosum) are a great example to evidence the influence of cooking techniques on oxalate content [1]. In general, boiling has been associated with decreased oxalate content, especially soluble oxalates, due to its leaching and thermal degradation [45,47], as observed in spinach, New Zealand spinach, red and white beans, soybean, and rhubarb (Table 5).
5. Health Implications of Oxalates
Oxalate has been a concern for human health due to its antinutritive effects and potential nephrotoxicity for a long time [42,43]. In 1989, a fatality from oxalic acid poisoning was reported. A 53-year-old man, with other conditions, had eaten a sorrel soup with 6–8 g of oxalic acid [88]. A lethal dose of oxalic acid for adults was estimated as 10–15 g, although the ingestion of 4–5 g of oxalate was considered the minimum dose able to cause death [51,88]. As antinutrients, oxalates restrict the bioavailability of some nutrients since they can bind to minerals, like calcium, magnesium, or iron, reducing their absorption and use [3,44].
The sources of oxalates in our body can be exogenous or endogenous (Figure 3). Exogenous oxalate sources are mainly plant foods, like vegetables, grains, legumes, and fruits, among others. When these types of foods are ingested, oxalate is absorbed in multiple parts of our gastrointestinal tract, namely the stomach, small intestine, and large intestine. However, the absorption depends on its availability, among other individual features. Insoluble oxalates are excreted in feces since they are less bioavailable and, therefore, pose a lower health risk. In contrast, soluble oxalates are absorbed through the intestines and colon (5–10% of ingested oxalate, under normal conditions), going into the bloodstream.
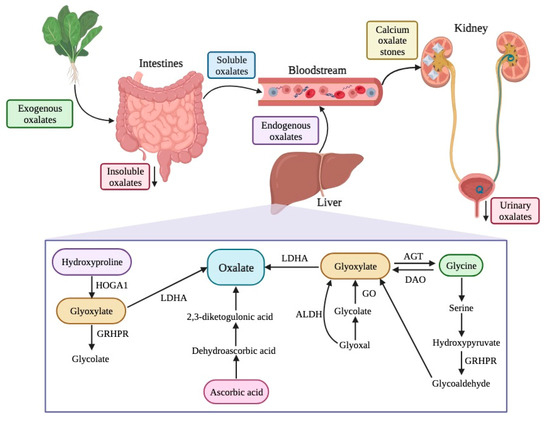
Figure 3.
Sources, endogenous pathways, and excretion of oxalates in the human body. HOGA1—4-hydroxy-2-oxoglutarate aldolase type 1; GRHPR—glyoxylate reductase/hydroxypyruvate reductase; LDHA—lactate dehydrogenase A; ALDH—aldehyde dehydrogenase; GO—glycolate oxidase; AGT—alanine/glyoxylate aminotransferase; DAO—D-amino acid oxidase.
Since absorption of oxalates is related to the amount of soluble oxalates, which are more bioavailable, a simultaneous consumption of oxalate with calcium or magnesium can reduce its bioavailability and absorption due to the formation and fecal excretion of insoluble salts, lowering the health risk [1,45,47,52,89]. It has been reported that men with less than 755 mg/day of calcium intake had a higher risk of kidney stone formation, whereas men with a median calcium intake or above had a lower risk [3]. Therefore, dietary calcium intake has been inversely associated with kidney stone formation [3,52,90,91].
Also, it has been observed that intestinal absorption of oxalates in individuals with a history of stone formation was expressly higher than in healthy individuals (9.2% and 6.7%, respectively) [1]. Gastrointestinal health influences oxalate absorption as well, with soluble oxalate being excessively absorbed due to intestinal malfunction.
Despite these facts, oxalate is not typically consumed daily in high concentrations and there are other constituents in foods which have a protective role against kidney stone formation, such as phytate, potassium, calcium, and antioxidant phytochemicals like polyphenols [3]. Also, boiling, steaming, soaking, and processing with calcium sources are some procedures to reduce the content of soluble oxalates, the most harmful oxalates [45,52].
Concerning the endogenous production of oxalates, the liver is the primary source. There are different pathways for oxalate production, including the metabolism of protein (through amino acids, like tyrosine, tryptophan, phenylalanine, and hydroxyproline), ascorbic acid, and precursors of oxalate (such as L-glycerate glycollate and glyoxylate) [92,93]. Glyoxylate is an important intermediary product in several reactions and, for its metabolization into oxalate, enzymes like glycolate oxidase and lactate dehydrogenase are needed.
Free oxalates are delivered to the kidney and can be excreted, increasing urinary oxalates, or can chelate with calcium ions there, resulting in calcium oxalate crystals, which can cause serious health issues like kidney stones, also known as nephrolithiasis or urolithiasis (Figure 3) [3,45,46,47,48,91].
This crystallization in the kidney infiltrates vessel walls and can lead to renal tubular obstruction, vascular necrosis, and hemorrhage, which can cause anuria, uremia, electrolyte disturbances, or even rupture and kidney failure [48,51]. Calcium oxalate and its relationship with kidney stone formation have been amply studied, with calcium oxalate being one of the most common types of human kidney stone reported, followed by calcium phosphate [46,51,90,94,95].
Hyperoxaluria is a metabolic disease that leads to excessive urinary oxalate excretion (>40–45 mg/day) [89,96], being an indicator of possible kidney stone formation [91]. The most reliable way to assess daily oxalate intake is through 24 h urine collection; however, there are also food frequency questionnaires whose credibility is debated [89,91].
Hyperoxaluria can be divided into primary hyperoxaluria (PH1) and secondary hyperoxaluria (PH2). PH1 is a group of rare autosomal recessive diseases that negatively affect key enzymes of oxalate metabolism, leading to an overproduction of oxalates in the liver [50]. When renal function declines and excess oxalate exists in the bloodstream, a phenomenon known as systemic oxalosis occurs and calcium oxalate crystals deposit in various organs, tissues, and bones [50,96,97]. Severe damage in the eyes, joints [98], myocardium, skin [99], oral tissues [96], and bone marrow [49] is reported. Oxalate can also be associated with acute kidney injury, a tubular obstruction due to calcium oxalate crystal deposition, and with chronic kidney disease progression, but further studies are necessary [89].
Patients with hyperoxaluria, especially PH1, from a clinical point of view, frequently present severe bone pain, pathological fractures, and bone deformations. This is frequently associated with the fact that calcium oxalate crystals may deposit within bones, tendons, cartilage, and synovium, causing oxalate arthritis. Then, the calcium oxalate crystals may enter into the synovial fluid, where an inflammatory response will arise, leading to joint effusions and arthralgias [100,101].
PH2 results from increased intestinal absorption of dietary oxalates and can also lead to excessive urinary oxalate [48,51,102]. A high intake of foods rich in oxalate, enteric hyperoxaluria, oxalate-degrading mechanisms, and SLC4 and SLC26 ionic exchangers are linked with PH2. Dietary oxalate plays an important role in PH2, contributing up to 72% of the urinary oxalate excreted [17]. Enteric hyperoxaluria is a form of PH2 that is linked with malabsorption syndromes due to disease or resection of the gastrointestinal tract. In foods, oxalate is usually complexed with calcium, resulting in insoluble oxalate, which is difficult to absorb. Nevertheless, in fat malabsorption conditions, the amount of free oxalates can increase, due to the capacity of free fats to bind calcium. Therefore, PH2 is linked with several conditions that cause fat malabsorption, such as inflammatory bowel disease, celiac disease, short bowel syndrome, and bariatric surgery, among others [100].
The gut microbiome plays an important role since some bacterial species can degrade oxalate to obtain carbon and energy and therefore reduce the concentration of oxalates in blood and urine, minimizing the formation of kidney stones [91,92,93,103,104].
The gut microbiota is usually similar between individuals; however, it can be affected by the age of individuals, by the diet, and by the use of antibiotics, among other factors. Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” and are being abundantly used as preventive therapeutic agents for several diseases, since they have been implicated in the stabilization of gut microbiota and enhancement of immune responses [105].
The best-known oxalate-degrading microorganism is Oxalobacter formigenes, but others are also able to degrade oxalate into carbon dioxide and formate, namely Escherichia coli, Bifidobacterium spp., and Lactobacillus spp. [93,104]. O. formigenes is a Gram-negative anaerobic bacterium isolated from human feces and other animals that utilizes intestinal oxalate as a carbon source, through formyl-CoA transferase and oxalyl-CoA decarboxylase enzymes, and metabolizes the oxalate into carbon dioxide and formate, contributing to regulating oxalate homeostasis [103]. However, its application as a probiotic is limited due to its nutritious requirements, but also because it has less colonization ability and is sensitive to the use of certain antibiotics and drugs [106]. Moreover, the therapeutic use of O. formigenes can be compromised, for example, in patients with PH1 and patients with cystic fibrosis. To date, the best conditions (pH, sugar concentration), as well as the adequate amount of these supplements, are not clear and more research is still needed [93].
6. Conclusions
Various methods have been employed for the determination of oxalate in foods. Particularly, extraction and analytical conditions of HPLC and UV–Vis spectrophotometry were reviewed. Despite having different features, both methods have similar extraction procedures. Among other extraction parameters, temperature remains a controversial question because it can lead to oxalate formation from precursors or failure to dissolve all pre-existing insoluble oxalates. Furthermore, a considerable quantity of different HPLC and spectrophotometry methods were gathered and analyzed, concluding that there is a huge variety of procedures.
This review also compared the oxalate content (mg/100 g FW) of a wide range of foods, measured by HPLC and spectrophotometry. The results showed that spinach, New Zealand spinach, rhubarb, Swiss chard, taro leaves, sorrel, soybean, amaranth, parsley, and licorice contained high oxalate levels and can be considered high-oxalate foods, especially some green leafy vegetables. In contrast, others can be referred to as low-oxalate foods: fruits (except for star fruit, elderberry, and dried fig), cabbage, broccoli, cauliflower, cucumber, kale, pumpkin, chickpeas, lentils, cowpea, arugula, cress, garlic, and green onion. Nevertheless, there are some procedures to reduce oxalate, in particular soluble oxalate, such as boiling, steaming, soaking, and processing with calcium sources.
Despite a clear relationship between dietary oxalate, calcium oxalate, and kidney stone risk, the connection might be more complex than previously thought due to the impact of cooking techniques, calcium intake, endogenously produced oxalate, and gastrointestinal health. Foods which contain oxalates, such as fruits and vegetables, have a wide range of beneficial compounds that might outweigh possible negative implications on human health. Additionally, systemic oxalosis does not seem to be related to dietary oxalate, but to previous pathologic conditions of individuals such as primary hyperoxaluria. Hence, regular consumption of high-oxalate foods by healthy individuals as a part of a balanced and diversified diet does not appear to cause health issues if daily consumption is from 50–200 mg/day, whereas for individuals susceptible to kidney stone formation dietary modification is crucial for its prevention. For these individuals, it is recommended to limit the consumption of high-oxalate foods to less than 40–50 mg oxalate/day since they can present a health threat in these cases.
Author Contributions
Conceptualization, N.S. and T.G.A.; methodology, N.S. and M.A.S.; data collection, N.S. and T.G.A.; writing—original draft preparation, N.S., T.G.A. and M.A.S.; writing—review and editing, M.E.F. and H.S.C.; supervision, M.E.F. and H.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from Foundation for Science and Technology (FCT) under the projects Food4DIAB (EXPL/BAA-AGR/1382/2021); UIDB/50006/2020; UIDP/50006/2020 and by AgriFood XXI I&D&I project (NORTE-01-0145-FEDER-000041) co-financed by European Regional Development Fund (ERDF), through the NORTE 2020 (Programa Operacional Regional do Norte 2014/2020).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hönow, R.; Hesse, A. Comparison of Extraction Methods for the Determination of Soluble and Total Oxalate in Foods by HPLC-Enzyme-Reactor. Food Chem. 2002, 78, 511–521. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Luo, Y.; Shi, H.; Li, Q.; Pinchu, C.; Li, X.; Yang, J.; Fan, W. Oxalate in Plants: Metabolism, Function, Regulation, and Application. J. Agric. Food Chem. 2022, 70, 16037–16049. [Google Scholar] [CrossRef] [PubMed]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef] [PubMed]
- Okombo, J.; Liebman, M. Oxalate Content of Selected Breads and Crackers. J. Food Compos. Anal. 2010, 23, 118–121. [Google Scholar] [CrossRef]
- Al-Wahsh, I.A.; Wu, Y.; Liebman, M. A Comparison of Two Extraction Methods for Food Oxalate Assessment. J. Food Res. 2012, 1, 233. [Google Scholar] [CrossRef]
- Ruan, Q.-Y.; Zheng, X.-Q.; Chen, B.-L.; Xiao, Y.; Peng, X.-X.; Leung, D.W.M.; Liu, E.-E. Determination of Total Oxalate Contents of a Great Variety of Foods Commonly Available in Southern China Using an Oxalate Oxidase Prepared from Wheat Bran. J. Food Compos. Anal. 2013, 32, 6–11. [Google Scholar] [CrossRef]
- Mou, B. Evaluation of Oxalate Concentration in the U.S. Spinach Germplasm Collection. HortScience 2008, 43, 1690–1693. [Google Scholar] [CrossRef]
- Kasidas, G.P.; Rose, G.A. Oxalate Content of Some Commond Foods: Determination by an Enzymatic Method. J. Hum. Nutr. 1980, 34, 255–266. [Google Scholar] [CrossRef]
- Milardović, S.; Grabarić, Z.; Rumenjak, V.; Jukić, M. Rapid Determination of Oxalate by an Amperometric Oxalate Oxidase-Based Electrode. Electroanalysis 2000, 12, 1051–1058. [Google Scholar] [CrossRef]
- Bohn, T.; Davidsson, L.; Walczyk, T.; Hurrell, R.F. Fractional Magnesium Absorption Is Significantly Lower in Human Subjects from a Meal Served with an Oxalate-Rich Vegetable, Spinach, as Compared with a Meal Served with Kale, a Vegetable with a Low Oxalate Content. Br. J. Nutr. 2004, 91, 601–606. [Google Scholar] [CrossRef]
- Chai, W.; Liebman, M. Effect of Different Cooking Methods on Vegetable Oxalate Content. J. Agric. Food Chem. 2005, 53, 3027–3030. [Google Scholar] [CrossRef]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; Tomás, V.; Casajús, R. FIow Injection SpectrofIuorimetric Determination of Oxalate Based on Its Enhancing Effect on the Oxidation of Rhodamine B by Dichromate. Analyst 1995, 120, 2111–2114. [Google Scholar] [CrossRef]
- Shaidarova, L.G.; Leksina, Y.A.; Chelnokova, I.A.; Gedmina, A.V.; Budnikov, H.C. Flow Injection Amperometric Determination of Oxalic Acid on Graphite Electrode Covered by Nafion Film with Included Palladium Nanoparticles. Turk. Online J. Des. Art Commun. 2017, 7, 1879–1886. [Google Scholar]
- Pundir, C.S.; Chauhan, N.; Rajneesh; Verma, M.; Ravi, A. Novel Amperometric Biosensor for Oxalate Determination Using Multi-Walled Carbon Nanotube-Gold Nanoparticle Composite. Sens. Actuators B Chem. 2011, 155, 796–803. [Google Scholar] [CrossRef]
- Šljukić, B.; Baron, R.; Compton, R.G. Electrochemical Determination of Oxalate at Pyrolytic Graphite Electrodes. Electroanalysis 2007, 19, 918–922. [Google Scholar] [CrossRef]
- Fakhari, A.R.; Rafiee, B.; Ahmar, H.; Bagheri, A. Electrocatalytic Determination of Oxalic Acid by TiO2 Nanoparticles/Multiwalled Carbon Nanotubes Modified Electrode. Anal. Methods 2012, 4, 3314–3319. [Google Scholar] [CrossRef]
- Holmes, R.P.; Kennedy, M. Estimation of the Oxalate Content of Foods and Daily Oxalate Intake. Kidney Int. 2000, 57, 1662–1667. [Google Scholar] [CrossRef]
- Merusi, C.; Corradini, C.; Cavazza, A.; Borromei, C.; Salvadeo, P. Determination of Nitrates, Nitrites and Oxalates in Food Products by Capillary Electrophoresis with PH-Dependent Electroosmotic Flow Reversal. Food Chem. 2010, 120, 615–620. [Google Scholar] [CrossRef]
- Sotomayor, M.D.P.T.; Raimundo, I.M., Jr.; Oliveira Neto, G.O.; Kubota, L.T. Bi-Enzymatic Optode Detection System for Oxalate Determination Based on a Natural Source of Enzyme. Anal. Chim. Acta 2001, 447, 33–40. [Google Scholar] [CrossRef]
- Ojinnaka, M.C.; Ebinyasi, C.S.; Ihemeje, A.; Okorie, S.U. Nutritional Evaluation of Complementary Food Gruels Formulated from Blends of Soybean Flour and Ginger Modified Cocoyam Starch. Adv. J. Food Sci. Technol. 2013, 5, 1325–1330. [Google Scholar] [CrossRef]
- Adeniyi, S.A.; Orjiekwe, C.L.; Ehiagbonare, J.E. Determination of Alkaloids and Oxalates in Some Selected Food Samples in Nigeria. Afr. J. Biotechnol. 2009, 8, 110–112. [Google Scholar]
- Gouveia, C.S.S.; Ganança, J.F.T.; Lebot, V.; de Carvalho, M.Â.A.P. Quantitation of Oxalates in Corms and Shoots of Colocasia Esculenta (L.) Schott under Drought Conditions. Acta Physiol. Plant 2018, 40, 214. [Google Scholar] [CrossRef]
- Ikese, O.; Ubwa, S.; Sunday, A.; Lenka, J.; Inalegwu, J.; Ubwa, S.; Ocheje, M.; Inegedu, A. Proximate Composition, Antinutrients and Some Functional Properties of a Potential Infant Food Made from Wheat and Groundnut. Int. J. Food Sci. Nutr. 2016, 1, 59–63. [Google Scholar]
- Iwuozor, K.O. Qualitative and Quantitative Determination of Anti-Nutritional Factors of Five Wine Samples. Adv. J. Chem. 2019, 2, 136–146. [Google Scholar] [CrossRef]
- Baker, C.J.L. The Determination of Oxalates in Fresh Plant MateriaI. Analyst 1952, 77, 340–344. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wei, J.; Sun, M.; Tian, Y.; Li, Z. Effects of Nitrogen and Calcium Nutrition on Oxalate Contents, Forms, and Distribution in Spinach. J. Plant Nutr. 2009, 32, 2123–2139. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Zhang, Y.; Shao, J.Z.; Du, S. Effects of Nitrogen Levels and Nitrate/Ammonium Ratios on Oxalate Concentrations of Different Forms in Edible Parts of Spinach. J. Plant Nutr. 2005, 28, 2011–2025. [Google Scholar] [CrossRef]
- Ohkawa, H. Gas Chromatographic Determination of Oxalic Acid in Foods. J. Assoc. Off. Anal. Chem. 1985, 68, 108–111. [Google Scholar] [CrossRef]
- Dong, M.W. Modern HPLC for Practicing Scientists; Wiley-Interscience: Hoboken, NJ, USA, 2006; ISBN 9780471727897. [Google Scholar]
- Altunay, N.; Gürkan, R. A Simple, Low-Cost, and Useful Preconcentration Method for Quantification of Soluble, Insoluble, and Total Oxalate in Selected Vegetables Through Spectrophotometry. Food Anal. Methods 2016, 9, 950–965. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Abbasi, S.; Rezaei, B. Kinetic Spectrophotometric Method for the Determination of Oxalic Acid by Its Catalytic Effect on the Oxidation of Safranine by Dichromate. Spectrochim. Acta Part A 2001, 57, 1833–1838. [Google Scholar] [CrossRef]
- Savage, G.P.; Vanhanen, L.; Mason, S.M.; Ross, A.B. Effect of Cooking on the Soluble and Insoluble Oxalate Content of Some New Zealand Foods. J. Food Compos. Anal. 2000, 13, 201–206. [Google Scholar] [CrossRef]
- Radek, M.; Savage, G.P. Oxalates in Some Indian Green Leafy Vegetables. Int. J. Food Sci. Nutr. 2008, 59, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Savage, G.P.; Mårtensson, L. Comparison of the Estimates of the Oxalate Content of Taro Leaves and Corms and a Selection of Indian Vegetables Following Hot Water, Hot Acid and in Vitro Extraction Methods. J. Food Compos. Anal. 2010, 23, 113–117. [Google Scholar] [CrossRef]
- Wilson, C.W.; Shaw, P.E.; Knight, R.J. Analysis of Oxalic Acid in Carambola (Averrhoa Carambola L.) and Spinach by High-Performance Liquid Chromatography. J. Agric. Food Chem. 1982, 30, 1106–1108. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Israr, B.; Bhatty, N.; Ali, A. Effect of Cooking on Soluble and Insoluble Oxalate Contents in Selected Pakistani Vegetables and Beans. Int. J. Food Prop. 2011, 14, 241–249. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Kazemzadeh, A. Flow Injection Spectrophotometric Determination of Ultra Trace Amounts of Oxalic Acid. Fresenius J. Anal. Chem. 2000, 367, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-L.; Zhao, M.-X.; Liao, L.-X. Catalytic Spectrophotometric Methods for the Determination of Oxalic Acid. Anal. Chim. Acta 1996, 320, 139–143. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhou, K.; Hu, Y.; Jin, R.; Lu, L.L.; Jin, C.W.; Lin, X.Y. Oxalate Synthesis in Leaves Is Associated with Root Uptake of Nitrate and Its Assimilation in Spinach (Spinacia oleracea L.) Plants. J. Sci. Food Agric. 2014, 95, 2105–2116. [Google Scholar] [CrossRef]
- Kim, D.-J.; Kim, H.; Kim, M.; Lee, J. Analysis of Oxalic Acid of Various Vegetables Consumed in Korea. Food Sci. Biotechnol. 2007, 16, 650–654. [Google Scholar]
- Morosanova, M.A.; Samodelov, Z.V.; Morosanova, E.I. Determination of Food Oxalates Using Silica–Titania Xerogel Modified with Eriochrome Cyanine R. Sensors 2018, 18, 864. [Google Scholar] [CrossRef]
- Fatoki, O.S. Determination of Oxalic Acid in Vegetables. In Modern Methods of Plant Analysis: Vegetables and Vegetable Products; Springer Berlin: Heidelberg, Germany, 1994; Volume 16, pp. 161–167. [Google Scholar]
- Singh, P.P.; Kothari, L.K.; Sharma, D.C.; Saxena, S.N. Nutritional Value of Foods in Relation to Their Oxalic Acid Content. Am. J. Clin. Nutr. 1972, 25, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Natesh, H.N.; Abbey, L.; Asiedu, S.K. An Overview of Nutritional and Anti Nutritional Factors in Green Leafy Vegetables. Hortic. Int. J. 2017, 1, 58–65. [Google Scholar] [CrossRef]
- Huynh, N.K.; Nguyen, D.H.M.; Nguyen, H.V.H. Effects of Processing on Oxalate Contents in Plant Foods: A Review. J. Food Compos. Anal. 2022, 112, 104685. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent Advances on the Mechanisms of Kidney Stone Formation (Review). Int. J. Mol. Med. 2021, 48, 149. [Google Scholar] [CrossRef] [PubMed]
- Massey, L.K. Food Oxalate: Factors Affecting Measurement, Biological Variation, and Bioavailability. J. Am. Diet. Assoc. 2007, 107, 1191–1194. [Google Scholar] [CrossRef]
- Witting, C.; Langman, C.B.; Assimos, D.; Baum, M.A.; Kausz, A.; Milliner, D.; Tasian, G.; Worcester, E.; Allain, M.; West, M.; et al. Pathophysiology and Treatment of Enteric Hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2021, 16, 487–495. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, R.N.; Pani, K.C.; Paul, P. Bone Marrow Oxalosis: An Unusual Cause of Cytopenia in End-Stage Renal Disease; Report of Two Cases. Indian J. Pathol. Microbiol. 2018, 61, 268–270. [Google Scholar] [CrossRef]
- Fogo, A.B.; Lusco, M.A.; Najafian, B.; Alpers, C.E. AJKD Atlas of Renal Pathology: Oxalosis. Am. J. Kidney Dis. 2017, 69, e13–e14. [Google Scholar] [CrossRef]
- Noonan, S.C.; Savage, G.P. Oxalate Content of Foods and Its Effect on Humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74. [Google Scholar] [CrossRef]
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, Goitrogens, Phytates and Oxalates, Friends or Foe? J. Funct. Foods 2022, 89, 104938. [Google Scholar] [CrossRef]
- Karamad, D.; Khosravi-Darani, K.; Hosseini, H.; Tavasoli, S. Analytical Procedures and Methods Validation for Oxalate Content Estimation. Biointerface Res. Appl. Chem. 2019, 9, 4305–4310. [Google Scholar] [CrossRef] [PubMed]
- Chamjangali, M.A.; Keley, V.; Bagherian, G. Kinetic Spectrophotometric Method for the Determination of Trace Amounts of Oxalate by an Activation Effect. Anal. Sci. 2006, 22, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Libert, B.O. Rapid Determination of Oxalic Acid by Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. 1981, 210, 540–543. [Google Scholar] [CrossRef]
- Arias-Rico, J.; Macías-León, F.J.; Alanís-García, E.; Cruz-Cansino, N.d.S.; Jaramillo-Morales, O.A.; Barrera-Gálvez, R.; Ramírez-Moreno, E. Study of Edible Plants: Effects of Boiling on Nutritional, Antioxidant, and Physicochemical Properties. Foods 2020, 9, 599. [Google Scholar] [CrossRef]
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Extraction and Natural Bioactive Molecules Characterization in Spinach, Kale and Purslane: A Comparative Study. Molecules 2021, 26, 2515. [Google Scholar] [CrossRef]
- Kassie, W.; Washe, A.P.; Etsay, H. Spectrophotometric Determination of Oxalic Acid in Dietary Sources Through Catalytic Titration with Hexavalent Chromium. Food Sci. Qual. Manag. 2019, 83, 30–38. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Serio, F.; Todaro, E. A Survey of Nitrate and Oxalate Content in Fresh Vegetables. J. Sci. Food Agric. 1999, 79, 1882–1888. [Google Scholar] [CrossRef]
- Elia, A.; Santamaria, P.; Serio, F. Nitrogen Nutrition, Yield and Quality of Spinach. J. Sci. Food Agric. 1998, 76, 341–346. [Google Scholar] [CrossRef]
- Judprasong, K.; Charoenkiatkul, S.; Sungpuag, P.; Vasanachitt, K.; Nakjamanong, Y. Total and Soluble Oxalate Contents in Thai Vegetables, Cereal Grains and Legume Seeds and Their Changes after Cooking. J. Food Compos. Anal. 2006, 19, 340–347. [Google Scholar] [CrossRef]
- Ombódi, A.; Kosuge, S.; Saigusa, M. Effects of Polyolefin-Coated Fertilizer on Nutritional Quality of Spinach Plants. J. Plant Nutr. 2000, 23, 1495–1504. [Google Scholar] [CrossRef]
- Siener, R.; Seidler, A.; Hönow, R. Oxalate-Rich Foods. Food Sci. Technol. 2021, 41, 169–173. [Google Scholar] [CrossRef]
- Siener, R.; Hönow, R.; Voss, S.; Seidler, A.; Hesse, A. Oxalate Content of Cereals and Cereal Products. J. Agric. Food Chem. 2006, 54, 3008–3011. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, D.S.; Vanhanen, L.P.; Savage, G.P. Evaluation of Extraction Parameters for Total Oxalate Determination in Spinach Using Design of Experiment Analysis. J. Food Compos. Anal. 2016, 51, 9–14. [Google Scholar] [CrossRef]
- Ghosh Das, S.; Savage, G.P. Oxalate Content of Indian Spinach Dishes Cooked in a Wok. J. Food Compos. Anal. 2013, 30, 125–129. [Google Scholar] [CrossRef]
- Vanhanen, L.; Savage, G. Comparison of Oxalate Contents and Recovery from Two Green Juices Prepared Using a Masticating Juicer or a High Speed Blender. NFS J. 2015, 1, 20–23. [Google Scholar] [CrossRef]
- Zulkhairi, A.M.; Razali, M.; Umikalsum, M.B.; Norfaizal, G.M.; Athirah, A.A.; Aisyah, M.N.S. Determination of Oxalates in Corms of Selected Taro (Colocasia esculenta) Varieties in Malaysia Using Ultra High-Performance Liquid Chromatography. Asian J. Chem. Sci. 2020, 7, 28–37. [Google Scholar] [CrossRef]
- Jaworska, G. Nitrates, Nitrites, and Oxalates in Products of Spinach and New Zealand Spinach: Effect of Technological Measures and Storage Time on the Level of Nitrates, Nitrites, and Oxalates in Frozen and Canned Products of Spinach and New Zealand Spinach. Food Chem. 2005, 93, 395–401. [Google Scholar] [CrossRef]
- Charrier, M.J.S.; Savage, G.P.; Vanhanen, L. Oxalate Content and Calcium Binding Capacity of Tea and Herbal Teas. Asia Pac. J. Clin. Nutr. 2002, 11, 298–301. [Google Scholar] [CrossRef]
- Yusenko, E.; Polyntseva, E.; Lyzhova, A.; Kalyakina, O. Determination of Oxalate and Some Inorganic Anions in Green and Black Tea. Proc. Latv. Acad. Sci. Sect. B 2013, 67, 429–432. [Google Scholar] [CrossRef]
- Siener, R.; Seidler, A.; Voss, S.; Hesse, A. Oxalate Content of Beverages. J. Food Compos. Anal. 2017, 63, 184–188. [Google Scholar] [CrossRef]
- Kaminishi, A.; Kita, N. Seasonal Change of Nitrate and Oxalate Concentration in Relation to the Growth Rate of Spinach Cultivars. HortScience 2006, 41, 1589–1595. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Emadi, M. Spectrophotometric Reaction Rate Method for Determination of Oxalic Acid in Food Based on Its Enhancing Effect on the Oxidation of Pyrocathecol Violet by Dichromate. Anal. Lett. 2004, 37, 321–332. [Google Scholar] [CrossRef]
- Tabe, M.; Fujimoto, T.; Nakahara, R.; Yamaguchi, T.; Fujita, Y. Spectrophotometric Determination of Oxalate Ion with N,N′-Diethyl-N,N′-[[4,4′-Dihydroxy-1,1′-Binaphthalene]-3,3’-Diyl]Bisbenzamide and Copper(II). Anal. Sci. 2007, 23, 601–604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tavallali, H.; Deilamy-Rad, G.; Mosallanejad, N. Development of a New Colorimetric Chemosensor for Selective Determination of Urinary and Vegetable Oxalate Concentration through an Indicator Displacement Assay (IDA) in Aqueous Media. Food Technol. Biotechnol. 2018, 56, 329–336. [Google Scholar] [CrossRef]
- Matsubara, C.; Yokoi, Y.; Tsuji, M.; Takamura, K. Flow Injection Analysis of Oxalate in Foods Using Titanium(IV)-Porphyrin Reagent. Anal. Sci. 1995, 11, 245–249. [Google Scholar] [CrossRef]
- Chamjangali, M.A.; Sharif-Razavian, L.; Yousefi, M.; Amin, A.H. Determination of Trace Amounts of Oxalate in Vegetable and Water Samples Using a New Kinetic-Catalytic Reaction System. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 112–116. [Google Scholar] [CrossRef]
- Xu, X.-Q.; Zhang, Z.-Q. Kinetic Spectrophotometric Determination of Oxalic Acid Based on the Catalytic Oxidation of Bromophenol Blue by Dichromate. Mikrochim. Acta 2000, 135, 169–172. [Google Scholar] [CrossRef]
- Hassouna, M.E.M.; Elsuccary, S.A.A. Determination of Oxalate Based on Its Enhancing Effect on the Oxidation of Mn(II) by Periodate. Talanta 2002, 56, 193–202. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Xing, G.-M.; Li, Z.-X. Quantitative Determination of Oxalic Acid Using Victoria Blue B Based on a Catalytic Kinetic Spectrophotometric Method. Microchim. Acta 2004, 144, 199–205. [Google Scholar] [CrossRef]
- Safavi, A.; Banazadeh, A.R. Catalytic Determination of Traces of Oxalic Acid in Vegetables and Water Samples Using a Novel Optode. Food Chem. 2007, 105, 1106–1111. [Google Scholar] [CrossRef]
- Holloway, W.D.; Argall, M.E.; Jealous, W.T.; Lee, J.A.; Bradbury, J.H. Organic Acids and Calcium Oxalate in Tropical Root Crops. J. Agric. Food Chem. 1989, 37, 337–341. [Google Scholar] [CrossRef]
- Buslig, B.S.; Wilson, C.W.; Shaw, P.E. High-Performance Liquid Chromatographic Separation of Carboxylic Acids with Anion-Exchange and Reverse-Phase Columns. J. Agric. Food Chem. 1982, 30, 342–345. [Google Scholar] [CrossRef]
- Glod, B.K. Ion Exclusion Chromatography: Parameters Influencing Retention. Neurochem. Res. 1997, 22, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M. HPLC Detectors: A Brief Review. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1130–1150. [Google Scholar] [CrossRef]
- Hönow, R.; Bongartz, D.; Hesse, A. An Improved HPLC-Enzyme-Reactor Method for the Determination of Oxalic Acid in Complex Matrices. Clin. Chim. Acta 1997, 261, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Xirgu, J.; Salgado, A.; Peracaula, R.; Reig, R.; Sanz, P. Fatal Oxalic Acid Poisoning from Sorrel Soup. Lancet. 1989, 334, 1524. [Google Scholar] [CrossRef]
- Bargagli, M.; Tio, M.C.; Waikar, S.S.; Ferraro, P.M. Dietary Oxalate Intake and Kidney Outcomes. Nutrients 2020, 12, 2673. [Google Scholar] [CrossRef]
- Taylor, E.N.; Curhan, G.C. Oxalate Intake and the Risk for Nephrolithiasis. J. Am. Soc. Nephrol. 2007, 18, 2198–2204. [Google Scholar] [CrossRef]
- Mitchell, T.; Kumar, P.; Reddy, T.; Wood, K.D.; Knight, J.; Assimos, D.G.; Holmes, R.P. Dietary Oxalate and Kidney Stone Formation. Am. J. Physiol.-Ren. Physiol. 2019, 316, 409–413. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.H.; Chi, Z.P.; Huang, R.; Huang, H.; Liu, G.Y.; Zhang, Y.F.; Yang, H.S.; Lin, J.H.; Yang, T.H.; et al. The Handling of Oxalate in the Body and the Origin of Oxalate in Calcium Oxalate Stones. Urol. Int. 2020, 104, 167–176. [Google Scholar] [CrossRef]
- Karamad, D.; Khosravi-Darani, K.; Khaneghah, A.M.; Miller, A.W. Probiotic Oxalate-Degrading Bacteria: New Insight of Environmental Variables and Expression of the Oxc and Frc Genes on Oxalate Degradation Activity. Foods 2022, 11, 2876. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, B.; Liu, J.; Yao, W.; Xia, D.; Li, L.; Chen, Z.; Ye, Z.; Yu, X. Analysis of Altered MicroRNA Expression Profiles in Proximal Renal Tubular Cells in Response to Calcium Oxalate Monohydrate Crystal Adhesion: Implications for Kidney Stone Disease. PLoS ONE 2014, 9, e0101306. [Google Scholar] [CrossRef] [PubMed]
- Ryall, R.L.; Fleming, D.E.; Doyle, I.R.; Evans, N.A.; Dean, C.J.; Marshall, V.R. Intracrystalline Proteins and the Hidden Ultrastructure of Calcium Oxalate Urinary Crystals: Implications for Kidney Stone Formation. J. Struct. Biol. 2001, 134, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Panis, V.; Tosios, K.I.; Gagari, E.; Griffin, T.J.; Damoulis, P.D. Severe Periodontitis in a Patient With Hyperoxaluria and Oxalosis: A Case Report and Review of the Literature. J. Periodontol. 2010, 81, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Farlay, D.; Abelin-Genevois, K.; Lebourg, L.; Cochat, P.; Boivin, G. Bone Impairment in Oxalosis: An Ultrastructural Bone Analysis. Bone 2015, 81, 161–167. [Google Scholar] [CrossRef]
- D’Costa, M.R.; Winkler, N.S.; Milliner, D.S.; Norby, S.M.; Hickson, L.T.J.; Lieske, J.C. Oxalosis Associated With High-Dose Vitamin C Ingestion in a Peritoneal Dialysis Patient. Am. J. Kidney Dis. 2019, 74, 417–420. [Google Scholar] [CrossRef]
- Blackmon, J.A.; Jeffy, B.G.; Malone, J.C.; Knable, A.L., Jr. Oxalosis Involving the Skin: Case Report and Literature Review. Arch. Dermatol. 2011, 147, 1302–1305. [Google Scholar] [CrossRef]
- Lorenz, E.C.; Michet, C.J.; Milliner, D.S.; Lieske, J.C. Update on Oxalate Crystal Disease. Curr. Rheumatol. Rep. 2013, 15, 340. [Google Scholar] [CrossRef]
- Bacchetta, J.; Boivin, G.; Cochat, P. Bone Impairment in Primary Hyperoxaluria: A Review. Pediatr. Nephrol. 2016, 31, 1–6. [Google Scholar] [CrossRef]
- Bhasin, B. Primary and Secondary Hyperoxaluria: Understanding the Enigma. World J. Nephrol. 2015, 4, 235–244. [Google Scholar] [CrossRef]
- Soliman, N.R.; Effat, B.A.M.; Mehanna, N.S.; Tawfik, N.F.; Ibrahim, M.K. Activity of Probiotics from Food Origin for Oxalate Degradation. Arch. Microbiol. 2021, 203, 5017–5028. [Google Scholar] [CrossRef]
- Ermer, T.; Nazzal, L.; Tio, M.C.; Waikar, S.; Aronson, P.S.; Knauf, F. Oxalate Homeostasis. Nat. Rev. Nephrol. 2023, 19, 123–138. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Gomathi, S.; Sasikumar, P.; Anbazhagan, K.; Sasikumar, S.; Kavitha, M.; Selvi, M.S.; Selvam, G.S. Screening of Indigenous Oxalate Degrading Lactic Acid Bacteria from Human Faeces and South Indian Fermented Foods: Assessment of Probiotic Potential. Sci. World J. 2014, 2014, 648059. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).