The Immunomodulatory Effect of β-Glucan Depends on the Composition of the Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
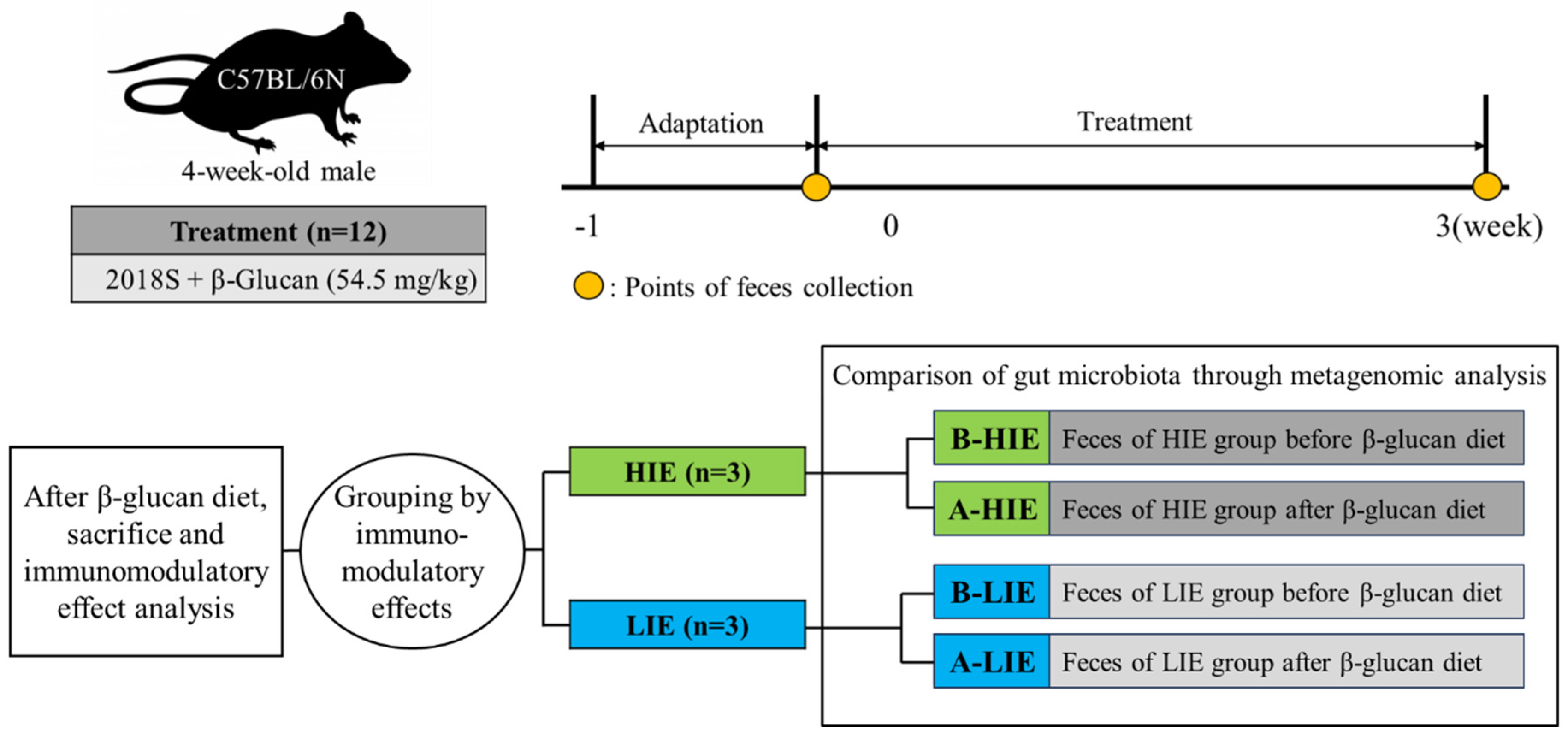
2.1. Experimental Design and Dosage Information
2.2. Classification into Groups with High and Low Immunomodulatory Effect
2.2.1. Measurement of the Intake of β-Glucan and Confirmation of Mouse Health Status
2.2.2. Analysis of Protein Expression Related to the Immunomodulatory Effects
2.3. Metagenomic Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. β-Glucan Intake Does Not Affect Body Weight and Biochemical Indices
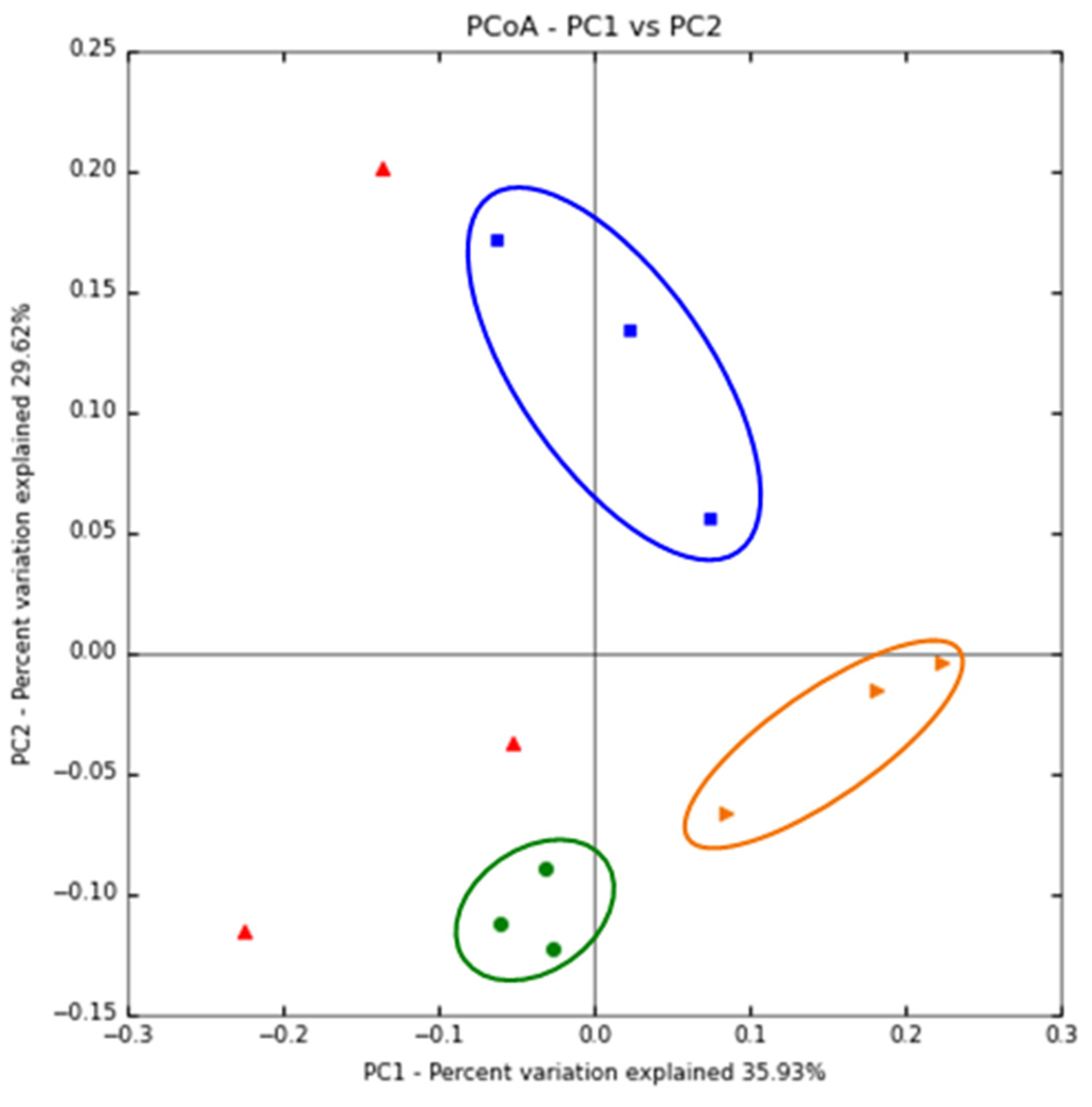
3.2. The Immunomodulatory Effect of β-Glucan Was Observed Differently in Each Mouse
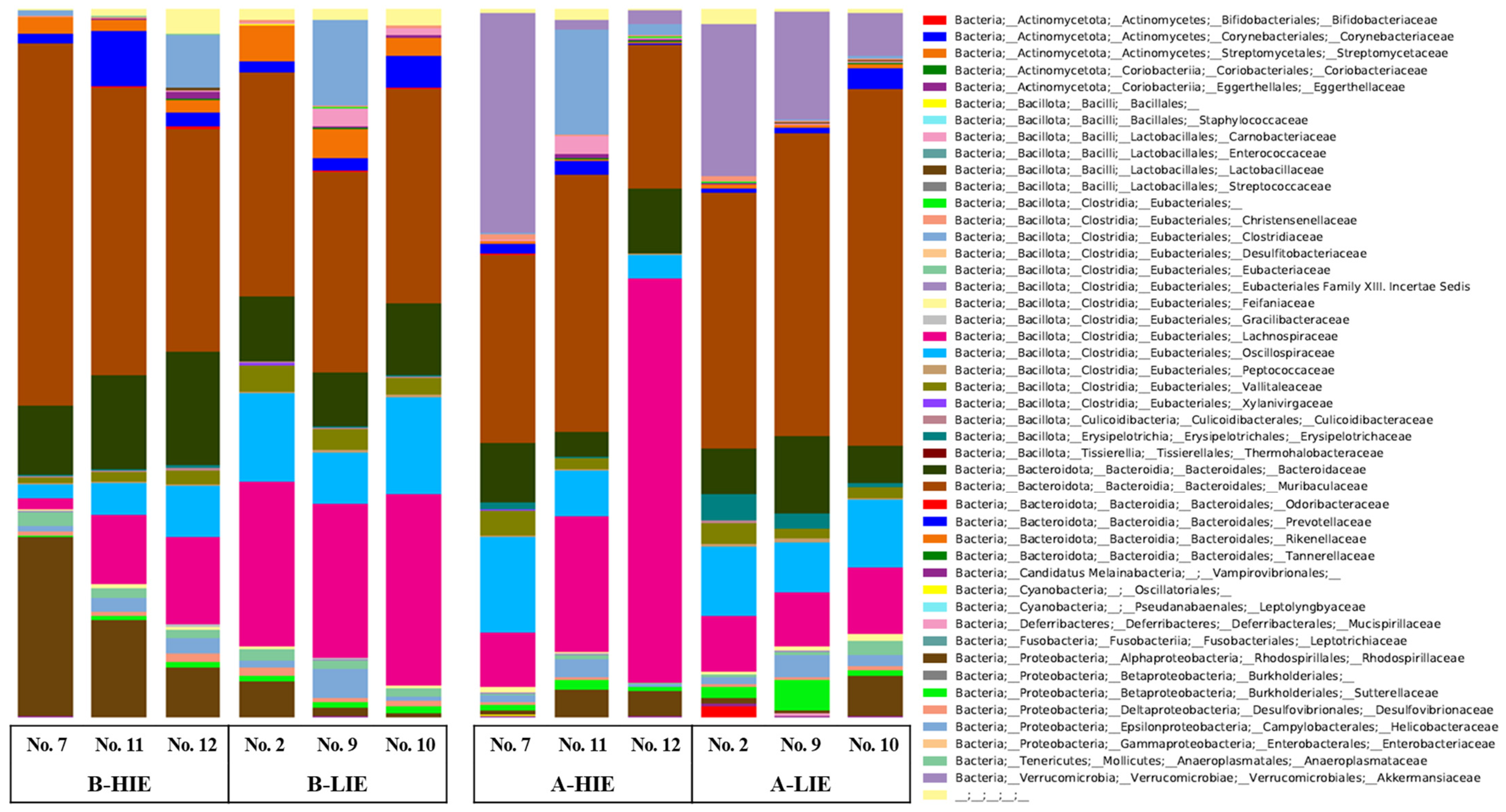
3.3. The Immunomodulatory Effect of β-Glucan Depends on the Gut Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ho Do, M.; Seo, Y.S.; Park, H.-Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef]
- Bobadilla, F.; Rodriguez-Tirado, C.; Imarai, M.; Galotto, M.J.; Andersson, R. Soluble β-1, 3/1, 6-glucan in seaweed from the southern hemisphere and its immunomodulatory effect. Carbohydr. Polym. 2013, 92, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary yeast beta-1, 3/1, 6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hong, J.T.; Kim, Y.; Han, S.-B. Stimulatory effect of β-glucans on immune cells. Immune Netw. 2011, 11, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y.C. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-functional activity relationship of β-glucans from the perspective of immunomodulation: A mini-review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Aldawoud, T.M.S.; Rizou, M.; Rowan, N.J.; Ibrahim, S.A. Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: A comprehensive review. Foods 2020, 9, 1701. [Google Scholar] [CrossRef]
- Son, E. Dietary habits of diabetes patients during the COVID-19 pandemic. J. Korean Diabetes 2021, 22, 161–167. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety. More than Half of Korean Adults Have Experience in Purchasing Health Functional Foods. Available online: https://www.korea.kr/news/pressReleas%0AeView.do?newsId=155840733 (accessed on 10 March 2023).
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Molinero, N.; Ruiz, L.; Sánchez, B.; Margolles, A.; Delgado, S. Intestinal bacteria interplay with bile and cholesterol metabolism: Implications on host physiology. Front. Physiol. 2019, 10, 185. [Google Scholar] [CrossRef]
- Ramos, S.; Martín, M.Á. Impact of diet on gut microbiota. Curr. Opin. Food Sci. 2021, 37, 83–90. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety. Status of Recognized Functional Ingredients for Health Functional Food. 2016. Available online: https://www.khsa.or.kr/assets/extra/hfood/01.pdf (accessed on 7 March 2023).
- Underwood, W.; Anthony, R. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Anim. Welf. 2013, 22, 412. [Google Scholar]
- Charles River. C57BL/6 Mouse Biochemistry. 2019. Available online: https://www.criver.com/sites/default/files/resources/C57BL6MouseModelInformationSheet.pdf (accessed on 15 March 2023).
- Lala, V.; Zubair, M.; Minter, D. Liver Function Tests. 2023. Available online: https://www.statpearls.com/articlelibrary/viewarticle/20995/ (accessed on 30 July 2023).
- Jain, K.K. Role of biomarkers in healthcare. In The Handbook of Biomarkers; Springer: New York, NY, USA; Humana Press: Totowa, NJ, USA, 2017; pp. 147–175. [Google Scholar]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.; Paik, H.-D.; Park, E. Antioxidant and immune-modulating activities of egg yolk protein extracts. Food Sci. Anim. Resour. 2022, 42, 321. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Ruthes, A.C.; van Arkel, J.; Chanput, W.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J.L.D. Polysaccharides from Agaricus Bisporus and Agaricus Brasiliensis show similarities in their structures and their immunomodulatory effects on human monocytic THP-1 cells. BMC Complement. Altern. Med. 2011, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.R.; Webster, N.R. Cytokines and the immunomodulatory function of the vagus nerve. Br. J. Anaesth. 2009, 102, 453–462. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, X.; Yang, Y.; Cao, G. β-Glucan from Saccharomyces cerevisiae is involved in immunostimulation of ovine ruminal explants. Can. J. Vet. Res. 2020, 84, 283–293. [Google Scholar] [PubMed]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef]
- Ha, T.-Y. The role of regulatory T cells in cancer. Immune Netw. 2009, 9, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Feranchuk, S.; Belkova, N.; Potapova, U.; Kuzmin, D.; Belikov, S. Evaluating the use of diversity indices to distinguish between microbial communities with different traits. Res. Microbiol. 2018, 169, 254–261. [Google Scholar] [CrossRef]
- Gorelick, R. Combining richness and abundance into a single diversity index using matrix analogues of Shannon’s and Simpson’s indices. Ecography 2006, 29, 525–530. [Google Scholar] [CrossRef]
- Myhrstad, M.C.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation—Current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Zhou, N.; McDonald, D.; Costello, E.K.; Knight, R. Forensic identification using skin bacterial communities. Proc. Natl. Acad. Sci. USA 2010, 107, 6477–6481. [Google Scholar] [CrossRef]
- Gower, J.C. Principal coordinates analysis. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–7. [Google Scholar]
- Nie, C.; Yan, X.; Xie, X.; Zhang, Z.; Zhu, J.; Wang, Y.; Wang, X.; Xu, N.; Luo, Y.; Sa, Z.; et al. Structure of β-glucan from Tibetan hull-less barley and its in vitro fermentation by human gut microbiota. Chem. Biol. Technol. Agric. 2021, 8, 12. [Google Scholar] [CrossRef]
- Velikonja, A.; Lipoglavsek, L.; Zorec, M.; Orel, R.; Avgustin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef] [PubMed]
- De Giani, A.; Bovio, F.; Forcella, M.E.; Lasagni, M.; Fusi, P.; Di Gennaro, P. Prebiotic effect of maitake extract on a probiotic consortium and its action after microbial fermentation on colorectal cell lines. Foods 2021, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Takashima, A.; Bergstresser, P.R.; Ariizumi, K. Identification of a human homologue of the dendritic cell-associated C-type lectin-1, dectin-1. Gene 2001, 272, 51–60. [Google Scholar] [CrossRef] [PubMed]
- de Oliviera Nascimento, L.; Massari, P.; Wetzler, L. The role of TLR2 in infection and immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Chan, G.C.-F.; Chan, W.K.; Sze, D.M.-Y. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Julia, P.J.; Munoz-Munoz, J.; van Sinderen, D. A comprehensive review on the impact of β-glucan metabolism by Bacteroides and Bifidobacterium species as members of the gut microbiota. Int. J. Biol. Macromol. 2021, 181, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.O.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A.; et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Miller, R.A.; Schmidt, T.M. Muribaculaceae genomes assembled from metagenomes suggest genetic drivers of differential response to acarbose treatment in mice. Msphere 2021, 6, e0085121. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Y.; Wang, Y.; Wang, X.; Xu, J.; Zhao, H.; Zheng, D.; Sun, L.; Tai, G.; Zhou, Y.; et al. β-1, 6-Glucan from Pleurotus eryngii modulates the immunity and gut microbiota. Front. Immunol. 2021, 13, 859923. [Google Scholar] [CrossRef]
- Rodrigues, H.G.; Sato, F.T.; Curi, R.; Vinolo, M.A. Fatty acids as modulators of neutrophil recruitment, function and survival. Eur. J. Pharmacol. 2016, 785, 50–58. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Kiššová, Z.; Tkáčiková, Ľ.; Mudroňová, D.; Bhide, M.R. Immunomodulatory effect of Lactobacillus reuteri (Limosilactobacillus reuteri) and its exopolysaccharides investigated on epithelial cell line IPEC-J2 challenged with Salmonella Typhimurium. Life 2022, 12, 1955. [Google Scholar] [CrossRef]
- Laiño, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: New insights into molecular interactions with host cells. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.; Cui, J.; Guo, T.; Zhang, J.; Li, B. Alleviative effects of exopolysaccharide produced by Lactobacillus helveticus KLDS1. 8701 on dextran sulfate sodium-induced colitis in mice. Microorganisms 2021, 9, 2086. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; de Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; Devries, J.W.; Furda, I. Determination of insoluble and soluble dietary fiber in foods and food products: Collaborative study. J. AOAC Int. 1992, 75, 360–367. [Google Scholar] [CrossRef]
- Dikeman, C.L.; Murphy, M.R.; Fahey, G.C., Jr. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J. Nutr. 2006, 136, 913–919. [Google Scholar] [CrossRef]
- Mäkelä, N.; Brinck, O.; Sontag-Strohm, T. Viscosity of β-glucan from oat products at the intestinal phase of the gastrointestinal model. Food Hydrocoll. 2020, 100, 105422. [Google Scholar] [CrossRef]
- Han, X.; Ma, Y.; Ding, S.; Fang, J.; Liu, G. Regulation of dietary fiber on intestinal microorganisms and its effects on animal health. Anim. Nutr. 2023, in press. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Tan, H.; Zhong, Y.; Nie, S. Akkermansia muciniphila, an important link between dietary fiber and host health. Curr. Opin. Food Sci. 2022, 47, 100905. [Google Scholar] [CrossRef]
- Ryan, P.M.; London, L.E.; Bjorndahl, T.C.; Mandal, R.; Murphy, K.; Fitzgerald, G.F.; Shanahan, F.; Ross, R.P.; Wishart, D.S.; Cplice, N.M.; et al. Microbiome and metabolome modifying effects of several cardiovascular disease interventions in apo-E−/− mice. Microbiome 2017, 5, 30. [Google Scholar] [CrossRef]
- Herath, M.; Hosie, S.; Bornstein, J.C.; Franks, A.E.; Hill-Yardin, E.L. The role of the gastrointestinal mucus system in intestinal homeostasis: Implications for neurological disorders. Front. Cell. Infect. Microbiol. 2020, 10, 248. [Google Scholar] [CrossRef]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Ashrafian, F.; Behrouzi, A.; Badi, S.A.; Davari, M.; Jamnani, F.R.; Fateh, A.; Vaziri, F.; Siadat, S.D. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol. Hepatol. Bed Bench 2019, 12, 163–168. [Google Scholar] [PubMed]
- Alvarez, C.S.; Badia, J.; Bosch, M.; Giménez, R.; Baldomà, L. Outer membrane vesicles and soluble factors released by probiotic Escherichia coli Nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front. Microbiol. 2016, 7, 1981. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, G.; Zhu, L.; Sun, Z.; Jiang, Y.; Gao, M.J.; Zhan, X. Study of growth, metabolism, and morphology of Akkermansia muciniphila with an in vitro advanced bionic intestinal reactor. BMC Microbiol. 2021, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Ghotaslou, R.; Nabizadeh, E.; Memar, M.Y.; Law, W.M.H.; Ozma, M.A.; Abdi, M.; Yekani, M.; Kadkhoda, H.; Hosseinpour, R.; Bafadam, S.; et al. The metabolic, protective, and immune functions of Akkermansia muciniphila. Microbiol. Res. 2022, 266, 127245. [Google Scholar] [CrossRef] [PubMed]

| Markers | Concentration |
|---|---|
| AST | 52.6 ± 3.3 U/L |
| ALT | 26 ± 1.2 U/L |
| ALP | 147 ± 4.5 U/L |
| Total protein | 5.4 ± 0.1 g/dL |
| Albumin | 3.4 ± 0.0 g/dL |
| Total bilirubin | 0.5 ± 0.1 mg/dL |
| BUN | 23.3 ± 1.0 mg/dL |
| Creatine | 0.4 ± 0.0 mg/dL |
| Glucose | 157 ± 8.7 mg/dL |
| Mouse Object No. | COX-2 | IL-6 | IL-10 | iNOS | TNF-α | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | Score * | Value | Score | Value | Score | Value | Score | Value | Score | ||
| 1 | 0.087 | 1 | 0.655 | 11 | 0.131 | 11 | 0.138 | 1 | 0.120 | 2 | 26 |
| 2 | 0.102 | 2 | 0.529 | 8 | 0.233 | 7 | 0.200 | 3 | 0.168 | 6 | 26 |
| 3 | 0.151 | 6 | 0.773 | 12 | 0.267 | 5 | 0.240 | 4 | 0.170 | 7 | 34 |
| 4 | 0.193 | 9 | 0.388 | 5 | 0.349 | 1 | 0.308 | 6 | 0.299 | 12 | 33 |
| 5 | 0.170 | 7 | 0.449 | 7 | 0.286 | 4 | 0.258 | 5 | 0.231 | 11 | 34 |
| 6 | 0.111 | 3 | 0.602 | 10 | 0.147 | 10 | 0.165 | 2 | 0.151 | 4 | 29 |
| 7 | 0.307 | 11 | 0.545 | 9 | 0.221 | 8 | 0.529 | 10 | 0.162 | 5 | 43 |
| 8 | 0.182 | 8 | 0.369 | 4 | 0.306 | 2 | 0.462 | 9 | 0.182 | 8 | 31 |
| 9 | 0.118 | 4 | 0.228 | 1 | 0.300 | 3 | 0.395 | 7 | 0.196 | 9 | 24 |
| 10 | 0.144 | 5 | 0.410 | 6 | 0.254 | 6 | 0.424 | 8 | 0.114 | 1 | 26 |
| 11 | 0.378 | 12 | 0.332 | 2 | 0.187 | 9 | 0.532 | 11 | 0.217 | 10 | 44 |
| 12 | 0.227 | 10 | 0.365 | 3 | 0.110 | 12 | 0.648 | 12 | 0.133 | 3 | 40 |
| Protein | HIE | LIE |
|---|---|---|
| COX-2/β-actin | 0.30 ± 0.04 | 0.12 ± 0.01 * |
| IL-6/β-actin | 0.41 ± 0.07 | 0.39 ± 0.09 |
| IL-10/β-actin | 0.17 ± 0.03 | 0.26 ± 0.02 |
| iNOS/β-actin | 0.57 ± 0.04 | 0.34 ± 0.07 * |
| TNF-α/β-actin | 0.17 ± 0.02 | 0.16 ± 0.02 |
| Phylum | Groups | |||
|---|---|---|---|---|
| B-HIE | B-LIE | A-HIE | A-LIE | |
| Actinomycetota | 0.09 ± 0.01 | 0.07 ± 0.02 | 0.16 ± 0.07 | 0.73 ± 0.56 |
| Bacillota (Synonym Firmicutes) | 34.82 ± 0.39 | 46.42 ± 2.85 | 44.03 ± 10.85 | 30.33 ± 1.31 |
| Bacteroidota (Synonym Bacteroidetes) | 59.96 ± 4.11 a | 45.75 ± 1.79 b | 36.34 ± 3.52 c | 52.68 ± 4.59 a |
| Candidatus Melainabacteria | 0.38 ± 0.32 | 0.22 ± 0.07 | 0.26 ± 0.17 | 0.08 ± 0.05 |
| Cyanobacteria | 0.03 ± 0.03 | 0.04 ± 0.02 | - * | - |
| Deferribacteres | 0.03 ± 0.02 | 1.29 ± 0.73 | 0.98 ± 0.83 | 0.07 ± 0.03 |
| Fusobacteria | - | 0.002 ± 0.002 | - | - |
| Proteobacteria | 3.03 ± 2.46 | 4.39 ± 4.05 | 6.00 ± 4.54 | 0.80 ± 0.14 |
| Tenericutes | 0.10 ± 0.06 | 0.03 ± 0.02 | 0.05 ± 0.03 | 0.01 ± 0.01 |
| Verrucomicrobia | - | - | 11.46 ± 9.83 | 14.23 ± 4.50 |
| Uncultured | 1.56 ± 0.96 | 1.79 ± 0.28 | 0.73 ± 0.39 | 1.06 ± 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, M.; Yoon, Y.; Lee, J. The Immunomodulatory Effect of β-Glucan Depends on the Composition of the Gut Microbiota. Foods 2023, 12, 3148. https://doi.org/10.3390/foods12173148
Sung M, Yoon Y, Lee J. The Immunomodulatory Effect of β-Glucan Depends on the Composition of the Gut Microbiota. Foods. 2023; 12(17):3148. https://doi.org/10.3390/foods12173148
Chicago/Turabian StyleSung, Miseon, Yohan Yoon, and Jeeyeon Lee. 2023. "The Immunomodulatory Effect of β-Glucan Depends on the Composition of the Gut Microbiota" Foods 12, no. 17: 3148. https://doi.org/10.3390/foods12173148
APA StyleSung, M., Yoon, Y., & Lee, J. (2023). The Immunomodulatory Effect of β-Glucan Depends on the Composition of the Gut Microbiota. Foods, 12(17), 3148. https://doi.org/10.3390/foods12173148






