Multicomponent Oleogels Prepared with High- and Low-Molecular-Weight Oleogelators: Ethylcellulose and Waxes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oleogels
2.3. Characterisation of Oleogels
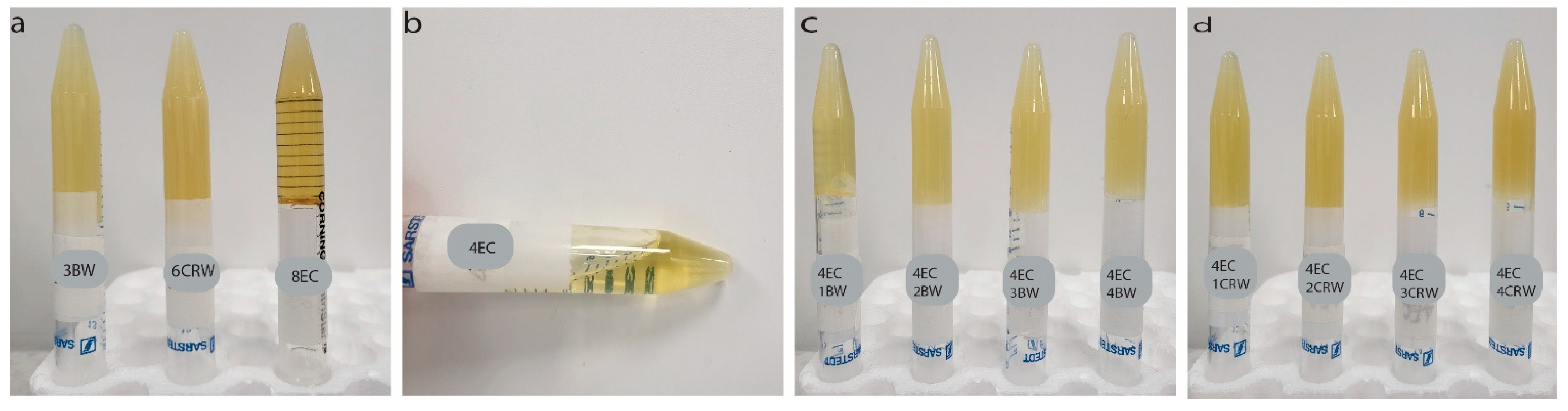
2.3.1. Visual Appearance and Morphological Analysis
2.3.2. Dynamic Rheology Measurements
Amplitude Sweep
Frequency Sweep
Thixotropy Behaviour
Temperature Rampng
2.3.3. Texture Measurement
2.3.4. Oil-Binding Capacity (OBC)
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. X-ray Diffraction (XRD)
2.3.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. Statistical Analysis
3. Results and Discussion
3.1. Visual Appearance and Morphological Properties
3.2. Dynamic Rheology Measurements
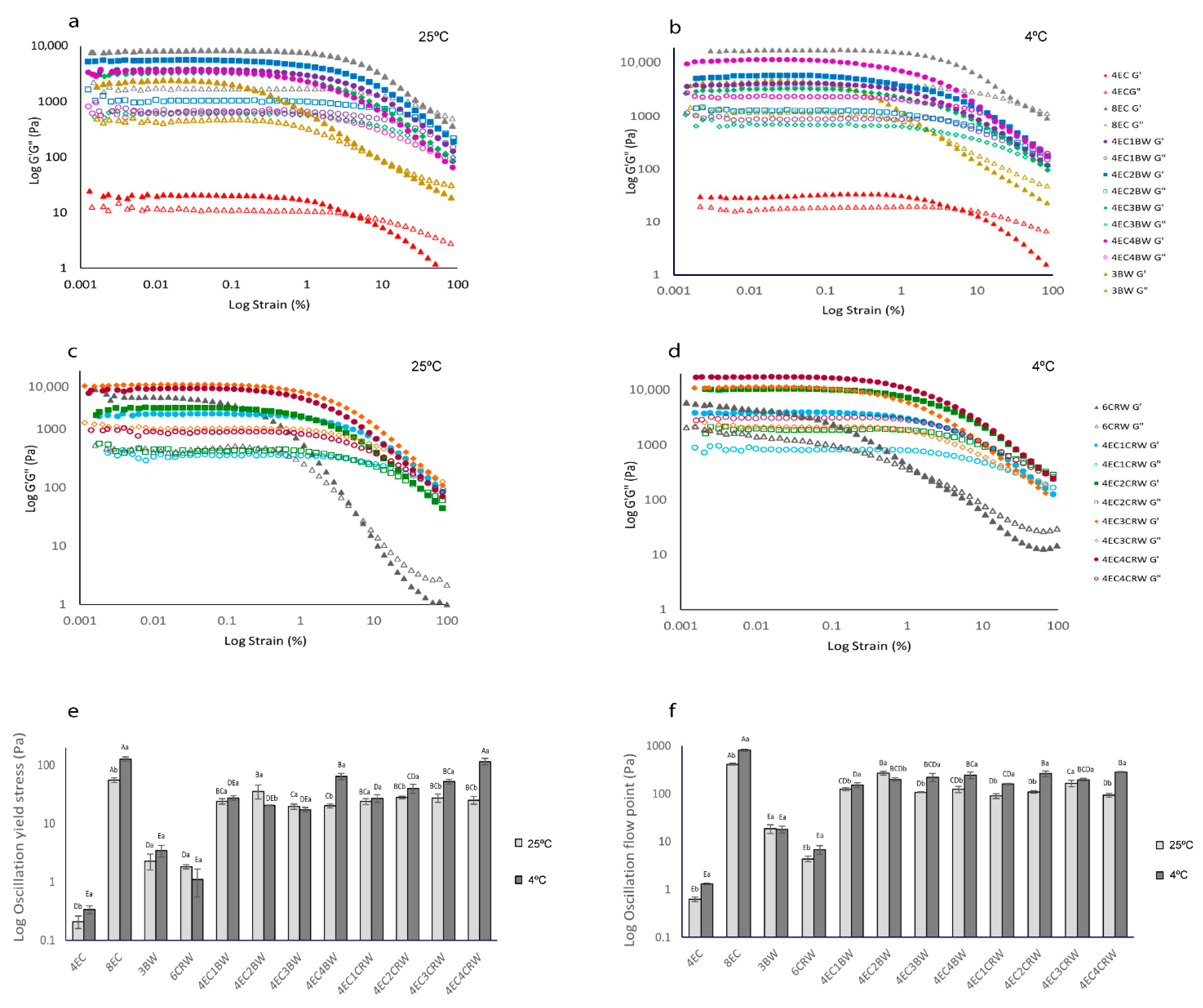
3.2.1. Amplitude Sweep
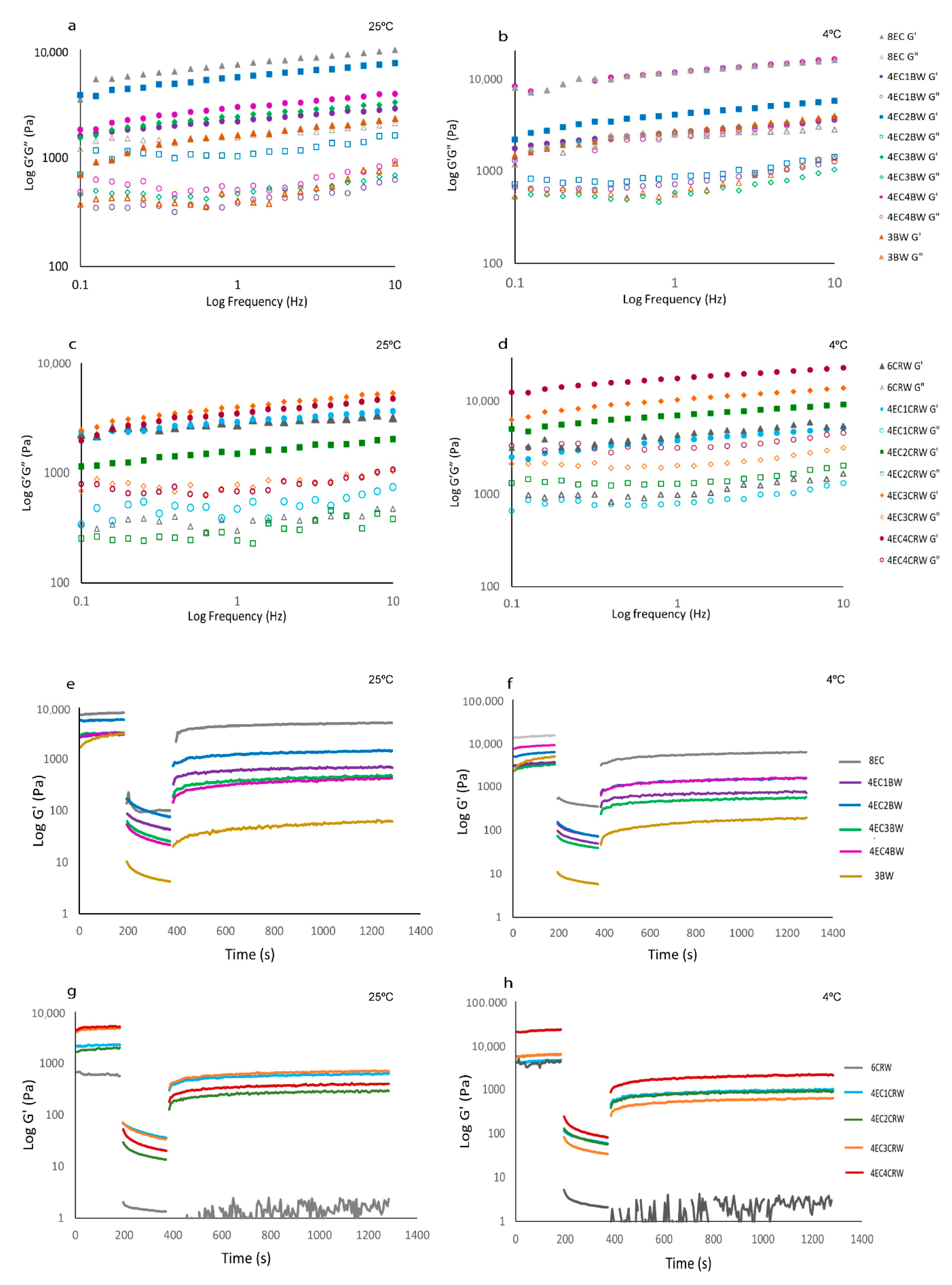
3.2.2. Frequency Sweep
3.2.3. Oscillatory Recovery
3.2.4. Temperature Ramping
3.3. Texture Measurement
3.4. Oil-Binding Capacity
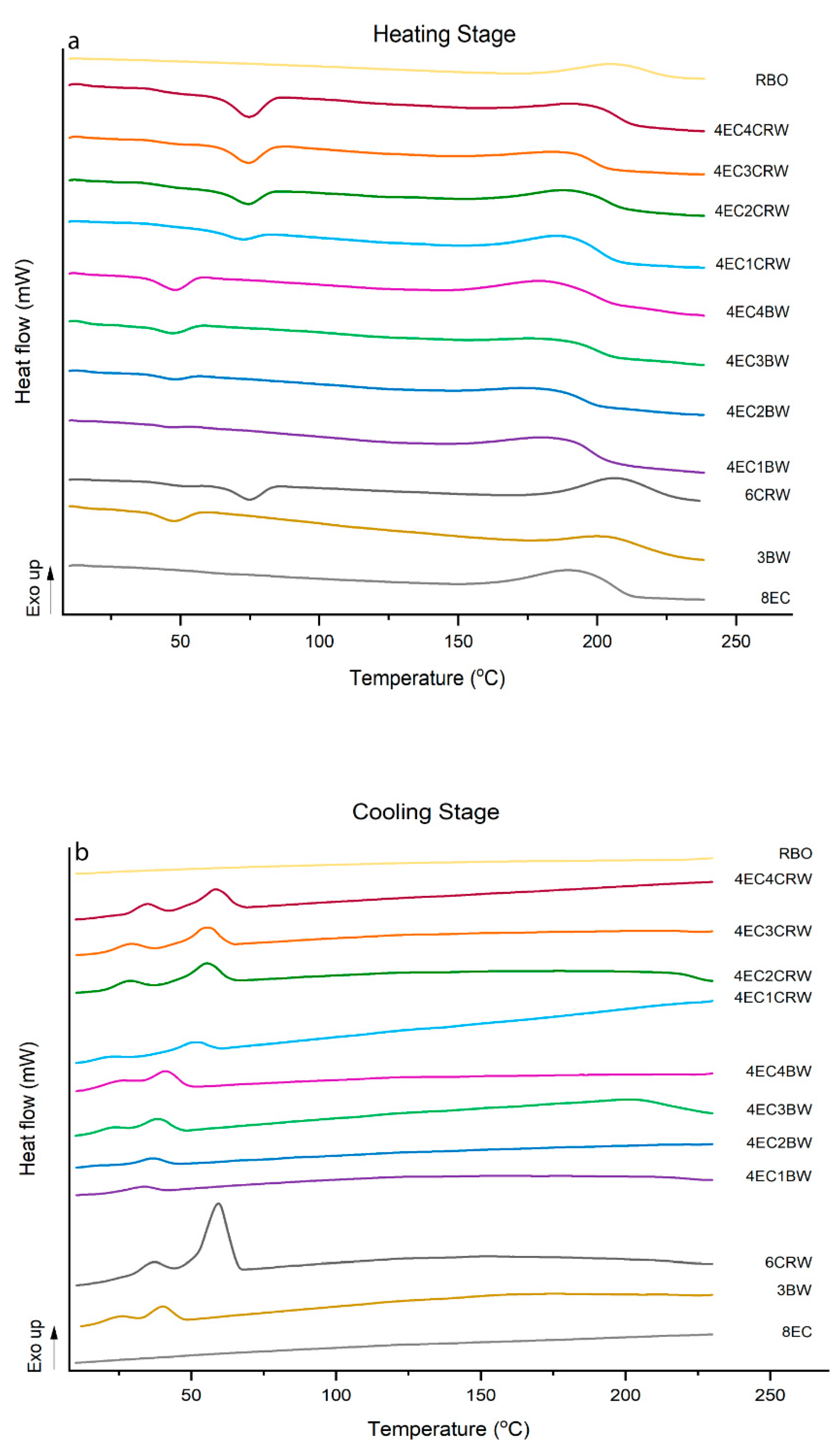
3.5. Thermal Analysis
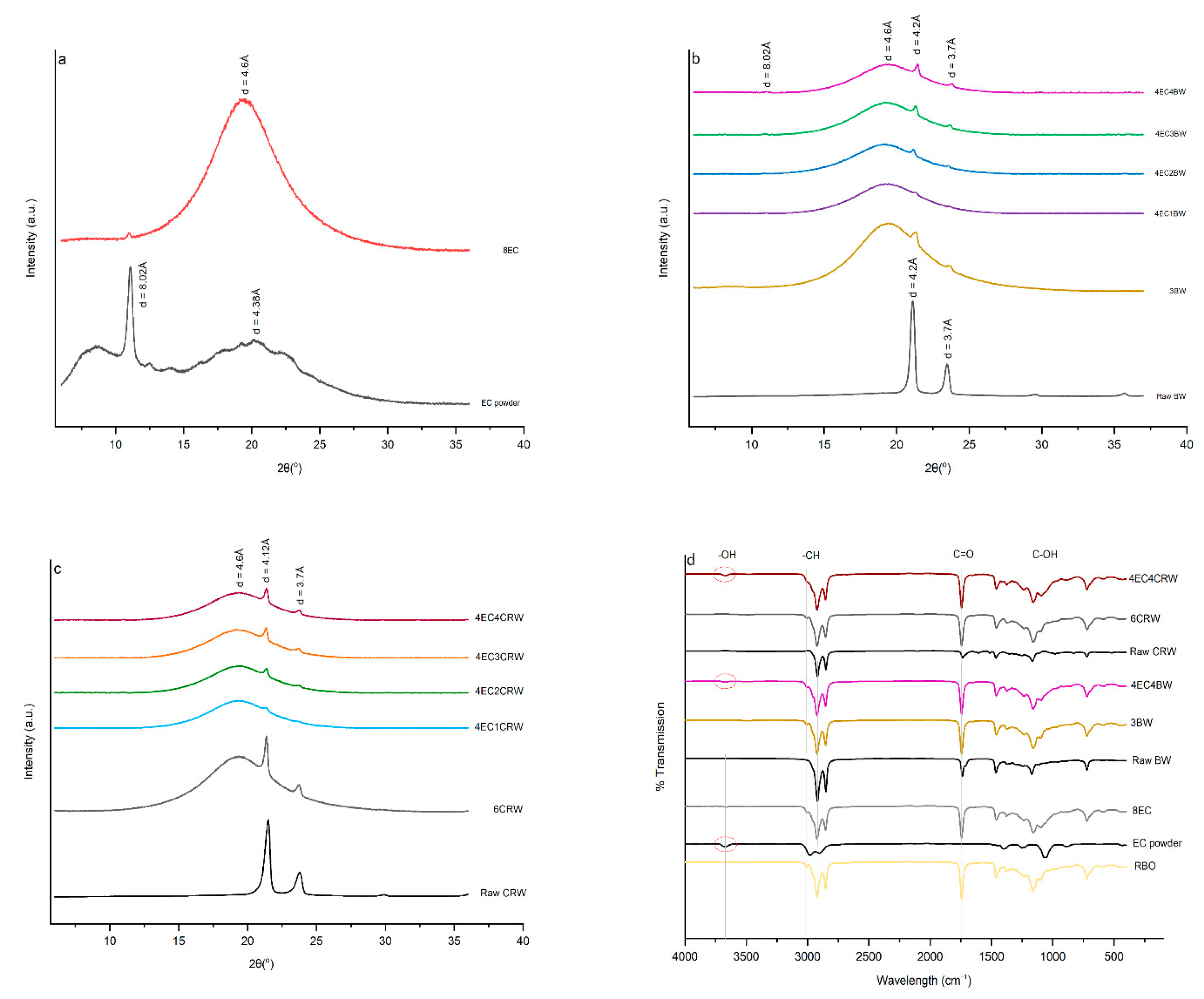
3.6. XRD
3.7. FTIR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Healthy Diet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 17 May 2022).
- Co, E.D.; Marangoni, A.G. Oleogels: An Introduction. In Edible Oleogels: Structure and Health Implications, 2nd ed.; Garti, N., Marangoni Alejandro, G., Eds.; AOCS Press: Urbana, IL, USA, 2018. [Google Scholar]
- Martins, A.J.; Vicente, A.A.; Pastrana, L.M.; Cerqueira, M.A. Oleogels for development of health-promoting food products. Food Sci. Hum. Wellness 2020, 9, 31–39. [Google Scholar] [CrossRef]
- Wang, Z.; Chandrapala, J.; Truong, T.; Farahnaky, A. Oleogels prepared with low molecular weight gelators: Texture, rheology and sensory properties, a review. Crit. Rev. Food Sci. Nutr. 2022, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Scholten, E. Edible oleogels: How suitable are proteins as a structurant? Curr. Opin. Food Sci. 2019, 27, 36–42. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Marangoni, A.G.; Davidovich-Pinhas, M. Ethylcellulose oleogels. In Edible Oleogels: Structure and Health Implications, 2nd ed.; Garti, N., Marangoni Alejandro, G., Eds.; AOCS Press: Urbana, IL, USA, 2018. [Google Scholar]
- Laredo, T.; Barbut, S.; Marangoni, A.G. Molecular interactions of polymer oleogelation. Soft Matter 2011, 7, 2734–2743. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Barbut, S.; Quinton, M.; Marangoni, A.G. Towards the development of a predictive model of the formulation-dependent mechanical behaviour of edible oil-based ethylcellulose oleogels. J. Food Eng. 2014, 143, 114–122. [Google Scholar] [CrossRef]
- Giacintucci, V.; Di Mattia, C.D.; Sacchetti, G.; Flamminii, F.; Gravelle, A.J.; Baylis, B.; Dutcher, J.R.; Marangoni, A.G.; Pittia, P. Ethylcellulose oleogels with extra virgin olive oil: The role of oil minor components on microstructure and mechanical strength. Food Hydrocoll. 2018, 84, 508–514. [Google Scholar] [CrossRef]
- Fu, H.; Lo, Y.M.; Yan, M.; Li, P.; Cao, Y. Characterization of thermo-oxidative behavior of ethylcellulose oleogels. Food Chem. 2020, 305, 125470. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, A.; Laufer, S.; Rosen-Kligvasser, J.; Lesmes, U.; Davidovich-Pinhas, M. Impact of different oil gelators and oleogelation mechanisms on digestive lipolysis of canola oil oleogels. Food Hydrocoll. 2019, 97, 105218. [Google Scholar] [CrossRef]
- Alongi, M.; Lucci, P.; Clodoveo, M.L.; Schena, F.P.; Calligaris, S. Oleogelation of extra virgin olive oil by different oleogelators affects the physical properties and the stability of bioactive compounds. Food Chem. 2022, 368, 130779. [Google Scholar] [CrossRef]
- Bemer, H.L.; Limbaugh, M.; Cramer, E.D.; Harper, W.J.; Maleky, F. Vegetable organogels incorporation in cream cheese products. Food Res. Int. 2016, 85, 67–75. [Google Scholar] [CrossRef]
- Ye, X.; Li, P.; Lo, Y.M.; Fu, H.; Cao, Y. Development of novel shortenings structured by ethylcellulose oleogels. J. Food Sci. 2019, 84, 1456–1464. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Amaral, J.T.; Ribeiro, M.; Sebastião, E.E.; Vargas, C.; de Lima Franzen, F.; Schneider, G.; Lorenzo, J.M.; Fries LL, M.; Cichoski, A.J.; et al. Fat replacement by oleogel rich in oleic acid and its impact on the technological, nutritional, oxidative, and sensory properties of Bologna-type sausages. Meat Sci. 2019, 149, 141–148. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli SA, H.; Sharifimehr, S. Physicochemical properties of beef burger after partial incorporation of ethylcellulose oleogel instead of animal fat. J. Food Sci. Technol. 2021, 58, 4775–4784. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martínez, A.; Charó-Alonso, M.A.; Marangoni, A.G.; Toro-Vazquez, J.F. Monoglyceride organogels developed in vegetable oil with and without ethylcellulose. Food Res. Int. 2015, 72, 37–46. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. Influencing the crystallization behavior of binary mixtures of stearyl alcohol and stearic acid (SOSA) using ethylcellulose. Food Res. Int. 2017, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stortz, T.A.; Marangoni, A.G. Ethylcellulose solvent substitution method of preparing heat resistant chocolate. Food Res. Int. 2013, 51, 797–803. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.K.; Pérez-Martínez, J.D.; Gallegos-Infante, J.A.; Toro-Vazquez, J.F.; Ornelas-Paz, J.J. Rheological properties of ethyl cellulose-monoglyceride-candelilla wax oleogel vis-a-vis edible shortenings. Carbohydr. Polym. 2021, 252, 117171. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-micro structure characterization and molecular properties of emulsion-templated polysaccharide oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Yu, J.; Gao, Y.; Mao, L. Rheology and Tribology of Ethylcellulose-Based Oleogels and W/O Emulsions as Fat Substitutes: Role of Glycerol Monostearate. Foods 2022, 11, 2364. [Google Scholar] [CrossRef]
- Zampouni, K.; Soniadis, A.; Moschakis, T.; Biliaderis, C.G.; Lazaridou, A.; Katsanidis, E. Crystalline microstructure and physicochemical properties of olive oil oleogels formulated with monoglycerides and phytosterols. Food Sci. Technol. 2022, 154, 112815. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Herrero, A.M.; Herranz, B.; Álvarez, M.D.; Jiménez-Colmenero, F.; Cofrades, S. Characterization of ethyl cellulose and beeswax oleogels and their suitability as fat replacers in healthier lipid pâtés development. Food Hydrocoll. 2019, 87, 960–969. [Google Scholar] [CrossRef]
- Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Makarenko, M.; Semin, M.; Kochetkova, A. The Influence of Edible Oils’ Composition on the Properties of Beeswax-Based Oleogels. Gels 2022, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Zetzl, A.K.; Gravelle, A.J.; Kurylowicz, M.; Dutcher, J.; Barbut, S.; Marangoni, A.G. Microstructure of ethylcellulose oleogels and its relationship to mechanical properties. Food Struct. 2014, 2, 27–40. [Google Scholar] [CrossRef]
- Blake, A.I.; Toro-Vazquez, J.F.; Hwang, H.S. Wax Oleogels. In Edible Oleogels: Structure and Health Implications, 2nd ed.; Garti, N., Marangoni Alejandro, G., Eds.; AOCS Press: Urbana, IL, USA, 2018. [Google Scholar]
- Trujillo-Ramirez, D.; Lobato-Calleros, C.; Jaime Vernon-Carter, E.; Alvarez-Ramirez, J. Cooling rate, sorbitan and glyceryl monostearate gelators elicit different microstructural, viscoelastic and textural properties in chia seed oleogels. Food Res. Int. 2019, 119, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, H.; Liu, W.; Li, C.; Wang, S. The effect of cooling rate on the microstructure and macroscopic properties of rice bran wax oleogels. J. Oleo Sci. 2021, 70, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.D.; Van de Walle, D.; Dewettinck, K.; Patel, A.R. Evaluating the oil-gelling properties of natural waxes in rice bran oil: Rheological, thermal, and microstructural study. J. Am. Oil Chem. Soc. 2015, 92, 801–811. [Google Scholar] [CrossRef]
- Barroso, N.G.; Okuro, P.K.; Ribeiro AP, B.; Cunha, R.L. Tailoring properties of mixed-component oleogels: Wax and monoglyceride interactions towards flaxseed oil structuring. Gels 2020, 6, 5. [Google Scholar] [CrossRef]
- Lim, J.; Jeong, S.; Oh, I.K.; Lee, S. Evaluation of soybean oil-carnauba wax oleogels as an alternative to high saturated fat frying media for instant fried noodles. LWT Food Sci. Technol. 2017, 84, 788–794. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Int. 2016, 84, 170–179. [Google Scholar] [CrossRef]
- Haj Eisa, A.; Laufer, S.; Rosen-Kligvasser, J.; Davidovich-Pinhas, M. Stabilization of Ethyl-Cellulose Oleogel Network Using Lauric Acid. Eur. J. Lipid Sci. Technol. 2020, 122, 1900044. [Google Scholar] [CrossRef]
- Ahmadi, P.; Tabibiazar, M.; Roufegarinejad, L.; Babazadeh, A. Development of behenic acid-ethyl cellulose oleogel stabilized Pickering emulsions as low calorie fat replacer. Int. J. Biol. Macromol. 2020, 150, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S.; Wood, J.; Marangoni, A. Potential use of organogels to replace animal fat in comminuted meat products. Meat Sci. 2016, 122, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, I.; Doan, C.D.; Van de Walle, D.; Danthine, S.; Rimaux, T.; Dewettinck, K. Sequential crystallization of high and low melting waxes to improve oil structuring in wax-based oleogels. RSC Adv. 2017, 7, 12113–12125. [Google Scholar] [CrossRef]
- Öǧütcü, M.; Yilmaz, E. Oleogels of virgin olive oil with carnauba wax and monoglyceride as spreadable products. Grasas Aceites 2014, 65, e040. [Google Scholar] [CrossRef]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Lobo FC, M.; Gomes, J.M.; Reis, R.L. Tailoring Natural-Based Oleogels Combining Ethylcellulose and Virgin Coconut Oil. Polymers 2022, 14, 2473. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.; Kim, D.A.; Marangoni Alejandro, G. Edible Oleogels: Structure and Health Implications; AOCS Press: Urbana, IL, USA, 2011. [Google Scholar]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. The gelation of oil using ethyl cellulose. Carbohydr. Polym. 2015, 117, 869–878. [Google Scholar] [CrossRef]
- Li, L.; Liu, G. Corn oil-based oleogels with different gelation mechanisms as novel cocoa butter alternatives in dark chocolate. J. Food Eng. 2019, 263, 114–122. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, H.-S.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli SA, H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Saleh AS, M.; Yang, H.; Wang, N.; Wang, P.; Yue, X.; Xiao, Z. Functional characteristics of oleogel prepared from sunflower oil with β-sitosterol and stearic acid. J. Am. Oil Chem. Soc. 2017, 94, 1153–1164. [Google Scholar] [CrossRef]
- Adili, L.; Roufegarinejad, L.; Tabibiazar, M.; Hamishehkar, H.; Alizadeh, A. Development and characterization of reinforced ethyl cellulose based oleogel with adipic acid: Its application in cake and beef burger. LWT Food Sci. Technol. 2020, 126, 109277. [Google Scholar] [CrossRef]
- Pang, M.; Shi, Z.; Lei, Z.; Ge, Y.; Jiang, S.; Cao, L. Structure and thermal properties of beeswax-based oleogels with different types of vegetable oil. Grasas Aceites 2020, 71, e380. [Google Scholar] [CrossRef]




| Sample Code | EC (w/w) | Beeswax (w/w) | Carnauba Wax (w/w) | Oil (w/w) |
|---|---|---|---|---|
| Controls | ||||
| 4EC | 4 | 0 | 0 | 96 |
| 8EC | 8 | 0 | 0 | 92 |
| 3BW | 3 | 0 | 0 | 97 |
| 6CRW | 6 | 0 | 0 | 94 |
| Treatments | ||||
| 4EC1BW | 4 | 1 | 0 | 95 |
| 4EC2BW | 4 | 2 | 0 | 94 |
| 4EC3BW | 4 | 3 | 0 | 93 |
| 4EC4BW | 4 | 4 | 0 | 92 |
| 4EC1CRW | 4 | 0 | 1 | 95 |
| 4EC2CRW | 4 | 0 | 2 | 94 |
| 4EC3CRW | 4 | 0 | 3 | 93 |
| 4EC4CRW | 4 | 0 | 4 | 92 |
| DSC Parameters | 8EC | 3BW | 6CRW | 4EC + BW | 4EC + CRW | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4EC1BW | 4EC2BW | 4EC3BW | 4EC4BW | 4EC1CRW | 4EC2CRW | 4EC3CRW | 4EC4CRW | ||||
| 25 °C | |||||||||||
| Melting | |||||||||||
| Tm—onset (°C) | - | 38.77 ± 0.37 DEa | 65.22 ± 0.13 Aa | 39.45 ± 1.2 Da | 39.34 ± 0.67 DEa | 36.73 ± 0.28 Fb | 38.35 ± 0.16 Ea | 61.05 ± 0.3 Ca | 64.05 ± 0.27 Ba | 64.35 ± 0.14 ABa | 64.54 ± 0.13 ABa |
| Tm—peak (°C) | - | 47.18 ± 0.33 Da | 74.89 ± 0.23 Aa | 46.09 ± 0.37 Ea | 47.56 ± 0.56 Da | 47.64 ± 0.29 Da | 48.98 ± 0.68 Ca | 71.86 ± 0.2 Ba | 74.2 ± 0.16 Aa | 74.33 ± 0.17 Aa | 74.53 ± 0.09 Aa |
| Tm—endset (°C) | - | 58.87 ± 0.45 Ca | 85.32 ± 0.11 Bb | 53.58 ± 1.55 Ea | 55.73 ± 0.87 Db | 59.76 ± 0.14 Ca | 59.16 ± 1.38 Cb | 83.45 ± 1.21 Ba | 85.13 ± 0.84 Ba | 87.63 ± 1.05 Aa | 87.54 ± 0.28 Aa |
| ΔH (J/g) | - | 1.74 ± 0.15 Ea | 6.37 ± 1.09 Aa | 0.25 ± 0.12 Ga | 0.75 ± 0.08 FGb | 1.74 ± 0.14 Ea | 2.63 ± 0.09 Da | 1.44 ± 0.08 EFa | 2.79 ± 0.38 CDa | 3.58 ± 0.16 Ca | 4.82 ± 0.07 Ba |
| 4 °C | |||||||||||
| Tm—onset (°C) | - | 36.99 ± 0.22 Db | 64.25 ± 0.08 Ab | 32.15 ± 2.3 Eb | 38.28 ± 0.62 CDa | 39.07 ± 0.51 Ca | 37.93 ± 0.08 CDb | 61.49 ± 0.5 Ba | 64.5 ± 0.23 Aa | 63.92 ± 0.61 Aa | 64.58 ± 0.06 Aa |
| Tm—peak (°C) | - | 45.76 ± 0.36 Eb | 74.39 ± 0.19 Ab | 40.05 ± 1.2 Fb | 46.33 ± 0.1 Eb | 47.82 ± 0.39 Da | 49.61 ± 0.17 Ca | 71.24 ± 0.4 Bb | 74.3 ± 0.19 Aa | 73.75 ± 0.18 Ab | 74.12 ± 0.06 Ab |
| Tm—endset (°C) | - | 57.6 ± 0.91 Da | 86.47 ± 0.37 Aa | 49.46 ± 0.41 Eb | 57.98 ± 0.29 Da | 59.03 ± 0.59 Da | 61.55 ± 0.3 Ca | 81.05 ± 0.3 Bb | 86.71 ± 1.07 Aa | 87.55 ± 1.07 Aa | 87.34 ± 0.59 Aa |
| ΔH (J/g) | - | 1.56 ± 0.21 Eb | 7.04 ± 0.41 Aa | 0.24 ± 0.06 Ga | 0.95 ± 0.04 Fa | 1.53 ± 0.07 Ea | 2.26 ± 0.09 Db | 1.01 ± 0.04 Fb | 2.15 ± 0.11 Db | 3.55 ± 0.21 Ca | 4.31 ± 0.31 Bb |
| DSC Parameters | 8EC | 3BW | 6CRW | 4EC + BW | 4EC + CRW | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4EC1BW | 4EC2BW | 4EC3BW | 4EC4BW | 4EC1CRW | 4EC2CRW | 4EC3CRW | 4EC4CRW | ||||
| 25 °C | |||||||||||
| Crystallisation | |||||||||||
| Tc—onset (°C) | - | 47.52 ± 0.75 EFa | 64.76 ± 1.37 ABa | 39.36 ± 0.94 Ha | 43.26 ± 0.15 Ga | 46 ± 0.66 Fa | 48.35 ± 1.65 Ea | 58.62 ± 0.2 Da | 62.34 ± 0.74 Ca | 63.56± 0.79 BCa | 66.2 ± 0.12 Aa |
| Tc—peak 1 (°C) | - | 40.69 ± 0.89 Ea | 56.83 ± 1.02 Bb | 32.76 ± 0.51 Ha | 36.57 ± 0.06 Ga | 38.14 ± 0.12 Fa | 40.78 ± 0.61 Ea | 51.23 ± 0.5 Da | 54.49 ± 0.57 Ca | 56.63 ± 1.02 Ba | 58.21 ± 0.08 Aa |
| Tc—peak 2 (°C) | - | 25.04 ± 0.24 Da | 35.88 ± 0.71 Ab | - | 19.41 ± 1.29 Fa | 22.8 ± 0.33 Ea | 25.32 ± 0.44 Da | 22.95 ± 0.67 Ea | 27.44 ± 0.99 Ca | 30.09 ± 0.94 Ba | 34.57 ± 0.09 Aa |
| Tc—endset (°C) | - | 14.65 ± 0.38 Eb | 18.47 ± 1.19 Cb | 19.48 ± 3.09 BCa | 13.03 ± 0.74 Eb | 14.53 ± 0.08 Eb | 14.24 ± 0.86 Ea | 15.13 ± 0.33 DEa | 17.59 ± 0.18 CDa | 21.37 ± 1.4 Ba | 25.86 ± 0.32 Aa |
| ΔH (J/g) | - | 2.63 ± 0.1 Ea | 9.47 ± 0.85 Aa | 0.5 ± 0.13 Ga | 1.34 ± 0.04 Fa | 2.5 ± 0.12 Ea | 3.61 ± 0.19 Da | 1.59 ± 0.12 Fa | 3.59 ± 0.39 Da | 4.57 ± 0.39 Ca | 5.98 ± 0.05 Ba |
| 4 °C | |||||||||||
| Tc—onset (°C) | - | 47.09 ± 0.32 Ea | 66.83 ± 1.9 Aa | 39.75 ± 0.42 Ha | 43.73 ± 0.38 Ga | 45.47 ± 0.34 Fa | 47.41 ± 0.22 Ea | 57.67 ± 0.36 Db | 61.35 ± 0.53 Ca | 64.67 ± 0.25 Ba | 65.57 ± 0.39 ABa |
| Tc—peak 1 (°C) | - | 40 ± 0.54 Ea | 60.1 ± 2.11 Aa | 33.29 ± 0.51 Ha | 36.96 ± 0.54 Ga | 38.04 ± 0.16 FGa | 39.32 ± 0.29 EFb | 50.71 ± 0.17 Da | 54.16 ± 0.43 Ca | 57.78 ± 0.33 Ba | 58.15 ± 0.57 Ba |
| Tc—peak 2 (°C) | - | 25.24 ± 0.04 Da | 38.05 ± 1.01 Aa | - | 19.73 ± 0.56 Fa | 22.38 ± 0.81 Ea | 25.12 ± 0.71 Da | 21.64 ± 0.52 Ea | 26.78 ± 1.02 Da | 31.63 ± 1.36 Ca | 33.67 ± 0.31 Bb |
| Tc—endset (°C) | - | 15.77 ± 0.4 Fa | 27.91 ± 1.05 Aa | 20.37 ± 0.35 Da | 14.74 ± 0.49 Fa | 14.78 ± 0.09 Fa | 16 ± 0.7 Fa | 15.81 ± 0.87 Fa | 18.11 ± 0.89 Ea | 23.02 ± 1.33 Ca | 26.34 ± 0.24 Ba |
| ΔH (J/g) | - | 2.62 ± 0.13 Ea | 8.56 ± 0.34 Aa | 0.55 ± 0.06 Ga | 1.44 ± 0.07 Fa | 2.15 ± 0.22 Ea | 3.33 ± 0.15 Da | 1.43 ± 0.07 Fa | 2.68 ± 0.49 DEa | 5.03 ± 0.36 Ca | 5.77 ± 0.68 Ba |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chandrapala, J.; Truong, T.; Farahnaky, A. Multicomponent Oleogels Prepared with High- and Low-Molecular-Weight Oleogelators: Ethylcellulose and Waxes. Foods 2023, 12, 3093. https://doi.org/10.3390/foods12163093
Wang Z, Chandrapala J, Truong T, Farahnaky A. Multicomponent Oleogels Prepared with High- and Low-Molecular-Weight Oleogelators: Ethylcellulose and Waxes. Foods. 2023; 12(16):3093. https://doi.org/10.3390/foods12163093
Chicago/Turabian StyleWang, Ziyu, Jayani Chandrapala, Tuyen Truong, and Asgar Farahnaky. 2023. "Multicomponent Oleogels Prepared with High- and Low-Molecular-Weight Oleogelators: Ethylcellulose and Waxes" Foods 12, no. 16: 3093. https://doi.org/10.3390/foods12163093
APA StyleWang, Z., Chandrapala, J., Truong, T., & Farahnaky, A. (2023). Multicomponent Oleogels Prepared with High- and Low-Molecular-Weight Oleogelators: Ethylcellulose and Waxes. Foods, 12(16), 3093. https://doi.org/10.3390/foods12163093







