Metabolomic and Transcriptomic Analyses Reveal the Effects of Grafting on Nutritional Properties in Eggplant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Sampling
2.2. Measurement of Physiological Traits of Eggplant Fruit
2.3. Widely Targeted Metabolite Identification and Quantification
2.4. RNA Sequencing (RNA-seq) Analyses and Differentially Expressed Genes (DEGs)
3. Results
3.1. Phenotype and Physiological Traits of Eggplant Fruit from Plants Grafted on Different Rootstocks
3.2. Widely Targeted Metabolomic Analysis
3.3. Significantly Differently Accumulated Bioactive Compounds between Sm64R-Grafted Eggplant and Self-Grafted Eggplant SG
3.4. Significantly Different Nitrogen Metabolism between Sm64R-Grafted Eggplant and Self-Grafted Eggplant SG
3.5. An Overview of the Transcriptomic Data
3.6. Correlation Analysis Based on Differentially Expressed Genes and Metabolites
3.7. Correlation Analyses of the Metabolomic and Transcriptomic Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naeem, M.Y.; Ugur, S. Technology. Nutritional content and health benefits of eggplant. Turk. J. Agric. Food Sci. Technol. 2019, 7, 31–36. [Google Scholar]
- Hanana, M.; Hamrouni, L.; Hamed, K.; Abdelly, C. Influence of the rootstock/scion combination on the grapevines behavior under salt stress. J. Plant Biochem. Physiol. 2015, 3, 1000154. [Google Scholar]
- Sen, A.; Chatterjee, R.; Bhaisare, P.; Subba, S. Grafting as an alternate tool for biotic and abiotic tolerance with improved growth and production of solanaceous vegetables: Challenges and scopes in india. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 121–135. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P.; Hassell, R.; Levi, A.; King, S.R.; Zhang, X. Grafting effects on vegetable quality. HortScience 2008, 43, 1670–1672. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Tian, H.; Wang, Y.; Jiang, H. Using a new hybrid rootstock significantly increases the grafted plant rate and watermelon yield. Int. Agrophys. 2019, 33, 97–106. [Google Scholar] [CrossRef]
- Aslam, A.; Zhao, S.; Azam, M.; Lu, X.; He, N.; Li, B.; Dou, J.; Zhu, H.; Liu, W. Comparative analysis of primary metabolites and transcriptome changes between ungrafted and pumpkin-grafted watermelon during fruit development. PeerJ 2020, 8, 8259. [Google Scholar] [CrossRef]
- Musa, I.; Rafii, M.Y.; Ahmad, K.; Ramlee, S.I.; Md Hatta, M.A.; Oladosu, Y.; Muhammad, I.I.; Chukwu, S.C.; Mat Sulaiman, N.N.; Ayanda, A.F. Effects of grafting on morphophysiological and yield characteristic of eggplant (Solanum melongena L.) grafted onto wild relative rootstocks. Plants 2020, 9, 1583. [Google Scholar] [CrossRef]
- Djidonou, D.; Leskovar, D.I.; Joshi, M.; Jifon, J.; Avila, C.A.; Masabni, J.; Wallace, R.W.; Crosby, K. Stability of yield and its components in grafted tomato tested across multiple environments in texas. Sci. Rep. 2020, 10, 13535. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Diao, M. Characteristics of the sugar metabolism in leaves and fruits of grafted watermelon during fruit development. Plant Physiol. Commun. 2006, 42, 835. [Google Scholar]
- Habibi, F.; Liu, T.; Shahid, M.A.; Schaffer, B.; Sarkhosh, A. Physiological, biochemical, and molecular responses of fruit trees to root zone hypoxia. Environ. Exp. Bot. 2023, 206, 105179. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Vetrano, F.; Mineo, V.; Planeta, D.; D’Anna, F. Effect of grafting on yield and quality of eggplant (Solanum melongena L.). Sci. Hortic. 2013, 149, 108–114. [Google Scholar] [CrossRef]
- Miao, L.; Di, Q.; Sun, T.; Li, Y.; Duan, Y.; Wang, J.; Yan, Y.; He, C.; Wang, C.; Yu, X. Integrated metabolome and transcriptome analysis provide insights into the effects of grafting on fruit flavor of cucumber with different rootstocks. Int. J. Mol. Sci. 2019, 20, 3592. [Google Scholar] [CrossRef] [PubMed]
- Aslam, W.; Noor, R.S.; Hussain, F.; Ameen, M.; Ullah, S.; Chen, H. Evaluating morphological growth, yield, and postharvest fruit quality of cucumber (Cucumis Sativus L.) grafted on cucurbitaceous rootstocks. Agriculture 2020, 10, 101. [Google Scholar] [CrossRef]
- Zhang, S.W.; Zong, Y.J.; Fang, C.Y.; Huang, S.H.; Li, J.; Xu, J.H.; Wang, Y.F.; Liu, C.H. Optimization of anthrone colorimetric method for rapid determination of soluble sugar in barley leaves. Food Res. Dev. 2020, 41, 196–200. [Google Scholar]
- Yemm, E.W.; Folkes, B.F. The amino acids of cytoplasmic and chloroplastic proteins of barley. Biochem. J. 1953, 55, 700. [Google Scholar] [CrossRef]
- García-Ruiz, A.; González-Rompinelli, E.M.; Bartolomé, B.; Moreno-Arribas, M.V. Potential of wine-associated lactic acid bacteria to degrade biogenic amines. Int. J. Food Microbiol. 2011, 148, 115–120. [Google Scholar] [CrossRef]
- Johnson, J.; Collins, T.; Walsh, K.; Naiker, M. Solvent extractions and spectrophotometric protocols for measuring the total anthocyanin, phenols and antioxidant content in plums. Chem. Pap. 2020, 74, 4481–4492. [Google Scholar] [CrossRef]
- Li, Y.G.; Tanner, G.; Larkin, P. The dmaca–hcl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic composition and antioxidant properties of different peach [Prunus persica (L.) Batsch] cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Leng, P.; Wang, C.; Liu, S.; Hu, Y. Effect of abscisic acid (ABA) on melon fruit ripening and softening. J. China Agric. Univ. 2010, 15, 25–32. [Google Scholar]
- Kaleem, M.M.; Nawaz, M.A.; Ding, X.; Wen, S.; Shireen, F.; Cheng, J.; Bie, Z. Comparative analysis of pumpkin rootstocks mediated impact on melon sensory fruit quality through integration of non-targeted metabolomics and sensory evaluation. Plant Physiol. Biochem. 2022, 192, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Ramos, L.; Moreno, C.; Zúñiga-Paredes, J.C.; Carlosama-Yepez, M.; Ruales, P. Plant foods by-products as sources of health-promoting agents for animal production: A review focusing on the tropics. Agron. J. 2016, 108, 1759–1774. [Google Scholar] [CrossRef]
- Yang, Q.M.; Pan, X.H.; Kong, W.B.; Yang, H.; Su, Y.D.; Li, Z.; Zhang, Y.N.; Yang, Y.L.; Lan, D.; Liu, G.A. Antioxidant activities of malt extract from barley (Hordeum vulgare L.) toward various oxidative stress in vitro and in vivo. Food Chem. 2010, 118, 84–89. [Google Scholar]
- Kaushik, P.; Andújar, I.; Vilanova, S.; Plazas, M.; Gramazio, P.; Herraiz, F.J.; Brar, N.S.; Prohens, J. Breeding vegetables with increased content in bioactive phenolic acids. Molecules 2015, 20, 18464–18481. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F. A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Yokoyama, W.E.; Hong, Y.J.; Prohens, J.A. Solasonine and α-solamargine contents of gboma (Solanum macrocarpon L.) and scarlet (Solanum aethiopicum L.) eggplants. J. Agric. Food Chem. 2010, 58, 5502–5508. [Google Scholar] [CrossRef]
- Hussain, N.; Yasmeen, A.; Bilal, M. The application of ammonium sulphate and amino acid on cotton: Effects on can improve growth, yield, quality and nitrogen absorption. Braz. J. Microbiol. 2021, 82, 240133. [Google Scholar] [CrossRef]
- Hall, R.D.; Brouwer, I.D.; Fitzgerald, M.A. Plant metabolomics and its potential application for human nutrition. Physiol. Plant. 2008, 132, 162–175. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Zhou, Y.; Zhou, S.; Zhang, S.; Tong, H.; Zhao, A. Transcriptome and metabolome profiling unveiled mechanisms of tea (Camellia sinensis) quality improvement by moderate drought on pre-harvest shoots. Phytochemistry 2020, 180, 112515. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, J.; Wang, W.; Hu, T.; Hu, H.; Bao, C. A high-quality chromosome-level genome assembly reveals genetics for important traits in eggplant. Hortic. Res. 2020, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.C.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock–scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; D’anna, F.; Palazzolo, E.; Mennella, G.; Rotino, G.L. Hybrids and allied species as potential rootstocks for eggplant: Effect of grafting on vigour, yield and overall fruit quality traits. Sci. Hortic. 2018, 228, 81–90. [Google Scholar] [CrossRef]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Braga, P.C.; Scalzo, R.L.; Dal Sasso, M.; Lattuada, N.; Greco, V.; Fibiani, M. Characterization and antioxidant activity of semi-purified extracts and pure delphinidin-glycosides from eggplant peel (Solanum melongena L.). J. Funct. Foods 2016, 20, 411–421. [Google Scholar] [CrossRef]
- Prohens, J.; Whitaker, B.D.; Plazas, M.; Vilanova, S.; Hurtado, M.; Blasco, M.; Gramazio, P.; Stommel, J.R. Genetic diversity in morphological characters and phenolic acids content resulting from an interspecific cross between eggplant, Solanum melongena, and its wild ancestor (S. incanum). Ann. Appl. Biol. 2013, 162, 242–257. [Google Scholar] [CrossRef]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Urías-Orona, V.; Muy-Rangel, M.D.; Heredia, J.B. Structure and content of phenolics in eggplant (Solanum melongena)—A review. S. Afr. J. Bot. 2017, 111, 161–169. [Google Scholar] [CrossRef]
- Plazas, M.; Andujar, I.; Vilanova, S.; Hurtado, M.; Gramazio, P.; Herraiz, F.J.; Prohens, J. Breeding for chlorogenic acid content in eggplant: Interest and prospects. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 26–35. [Google Scholar] [CrossRef]
- Li, D.; Qian, J.; Li, W.; Yu, N.; Gan, G.; Jiang, Y.; Li, W.; Liang, X.; Chen, R.; Mo, Y. A high-quality genome assembly of the eggplant provides insights into the molecular basis of disease resistance and chlorogenic acid synthesis. Mol. Ecol. Resour. 2021, 21, 1274–1286. [Google Scholar] [CrossRef]
- Huang, R.; Fang, W.; Xie, X.; Liu, Y.; Xu, C. Identification of key astringent compounds in aronia berry juice. Food Chem. 2022, 393, 133431. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.J.; Martínez-Esplá, A.; Gironés-Vilaplana, A.; Santos-Lax, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A. Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. Lwt 2019, 103, 139–146. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in phenolic compounds and antioxidant activity during development of ‘qiangcuili’and ‘cuihongli’fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biol. Technol. 2003, 30, 113–121. [Google Scholar] [CrossRef]
- Li, X.; Tieman, D.; Liu, Z.; Chen, K.; Klee, H.J. Identification of a lipase gene with a role in tomato fruit short--chain fatty acid--derived flavor volatiles by genome-wide association. Plant J. 2020, 104, 631–644. [Google Scholar] [CrossRef]
- Charpentier, C.; Aussenac, J.; Charpentier, M.; Prome, J.-C.; Duteurtre, B.; Feuillat, M. Release of nucleotides and nucleosides during yeast autolysis: Kinetics and potential impact on flavor. J. Agric. Food Chem. 2005, 53, 3000–3007. [Google Scholar] [CrossRef]
- Phan, C.W.; Wang, J.-K.; Cheah, S.C.; Naidu, M.; David, P.; Sabaratnam, V. A review on the nucleic acid constituents in mushrooms: Nucleobases, nucleosides and nucleotides. Crit. Rev. Biotechnol. 2018, 38, 762–777. [Google Scholar] [CrossRef]
- Chen, J.N.; Zhang, Y.Y.; Huang, X.H.; Dong, M.; Dong, X.P.; Zhou, D.Y.; Zhu, B.W.; Qin, L. Integrated volatolomics and metabolomics analysis reveals the characteristic flavor formation in chouguiyu, a traditional fermented mandarin fish of China. Food Chem. 2023, 418, 135874. [Google Scholar] [CrossRef]
- Reis Rocha, R.A.; Reis Rocha, L.C.; Ribeiro, M.N.; Lima Ribeiro, A.P.; Alves Da Rocha, R.; Souza Carneiro, J.D.D. Effect of the food matrix on the capacity of flavor enhancers in intensifying salty taste. J. Food Sci. 2021, 86, 1022–1032. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Swarup, S. Metabolomics for evaluating flavor-associated metabolites in plant-based products. Metabolites 2020, 10, 197. [Google Scholar] [CrossRef]
- Zhu, G.; Gou, J.; Klee, H.; Huang, S. Next-gen approaches to flavor-related metabolism. Annu. Rev. Plant Biol. 2019, 70, 187–212. [Google Scholar] [CrossRef] [PubMed]

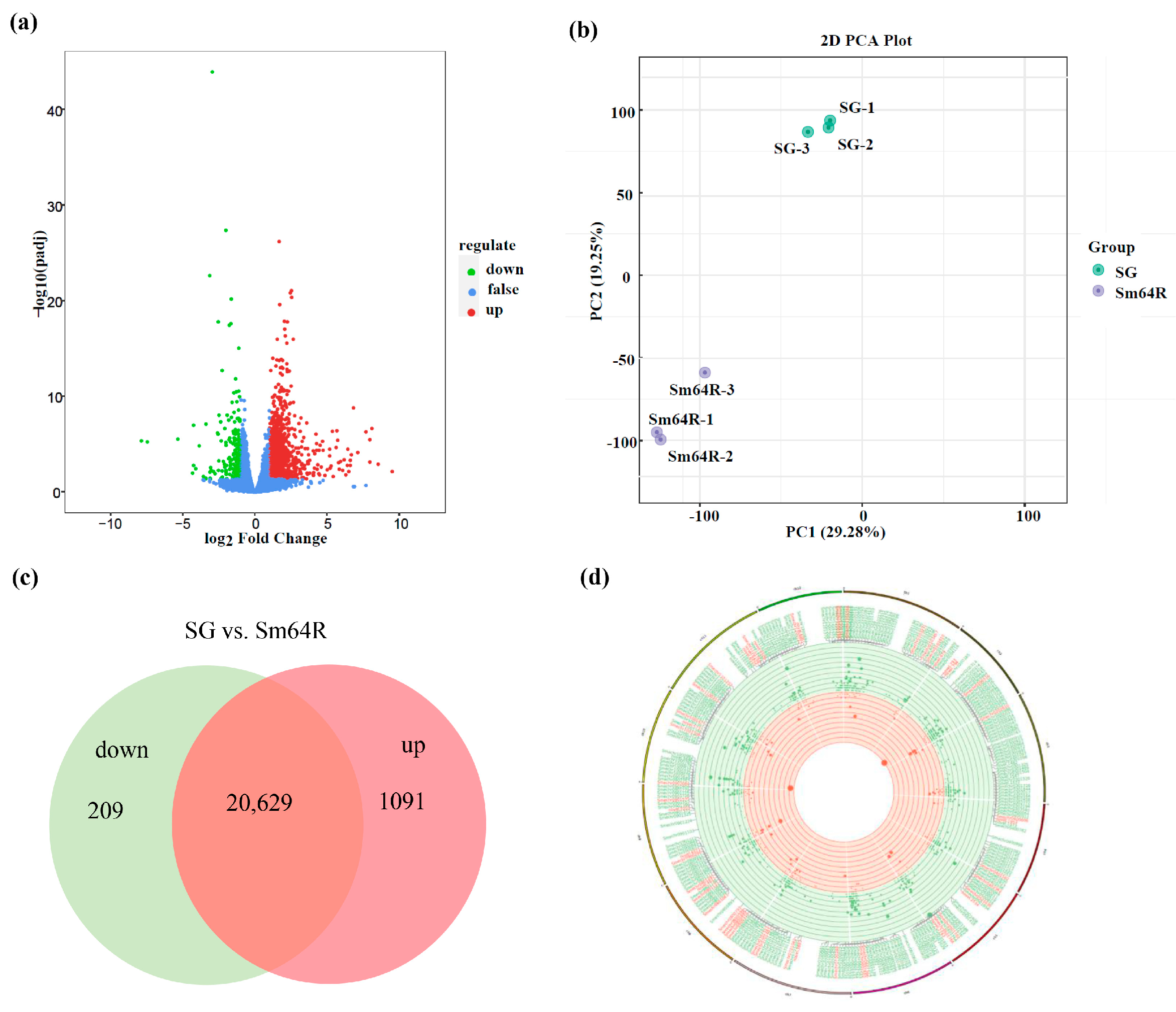
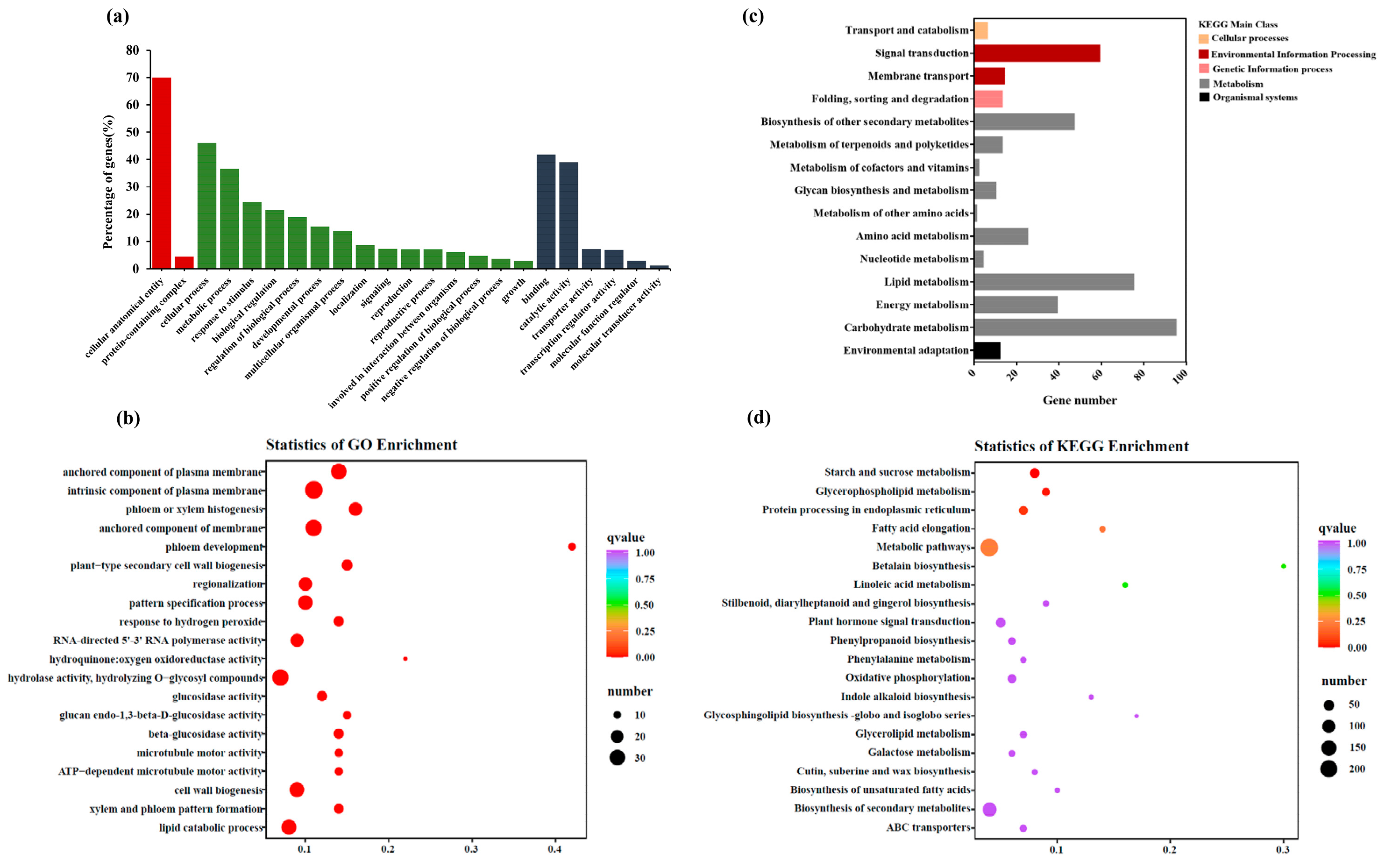
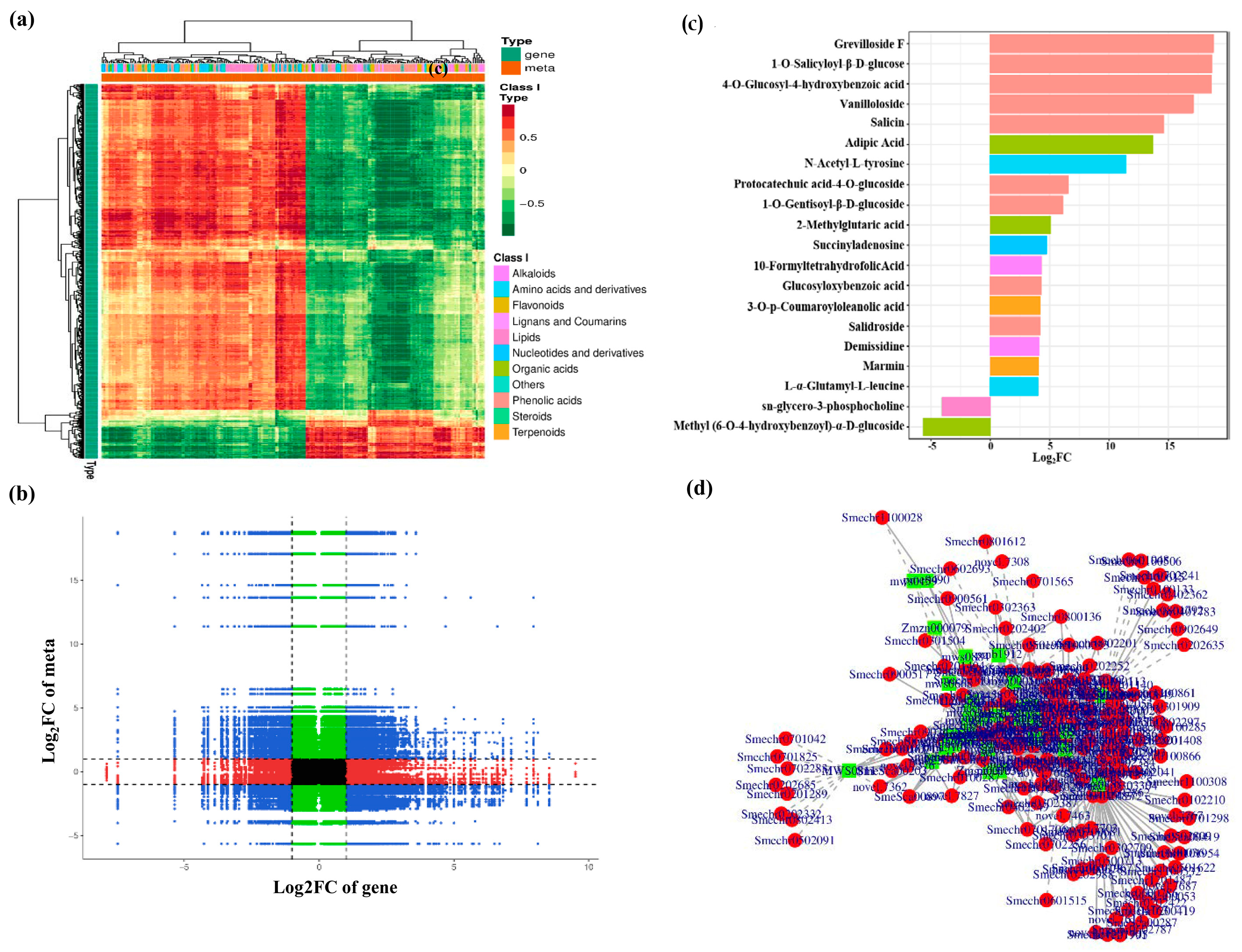
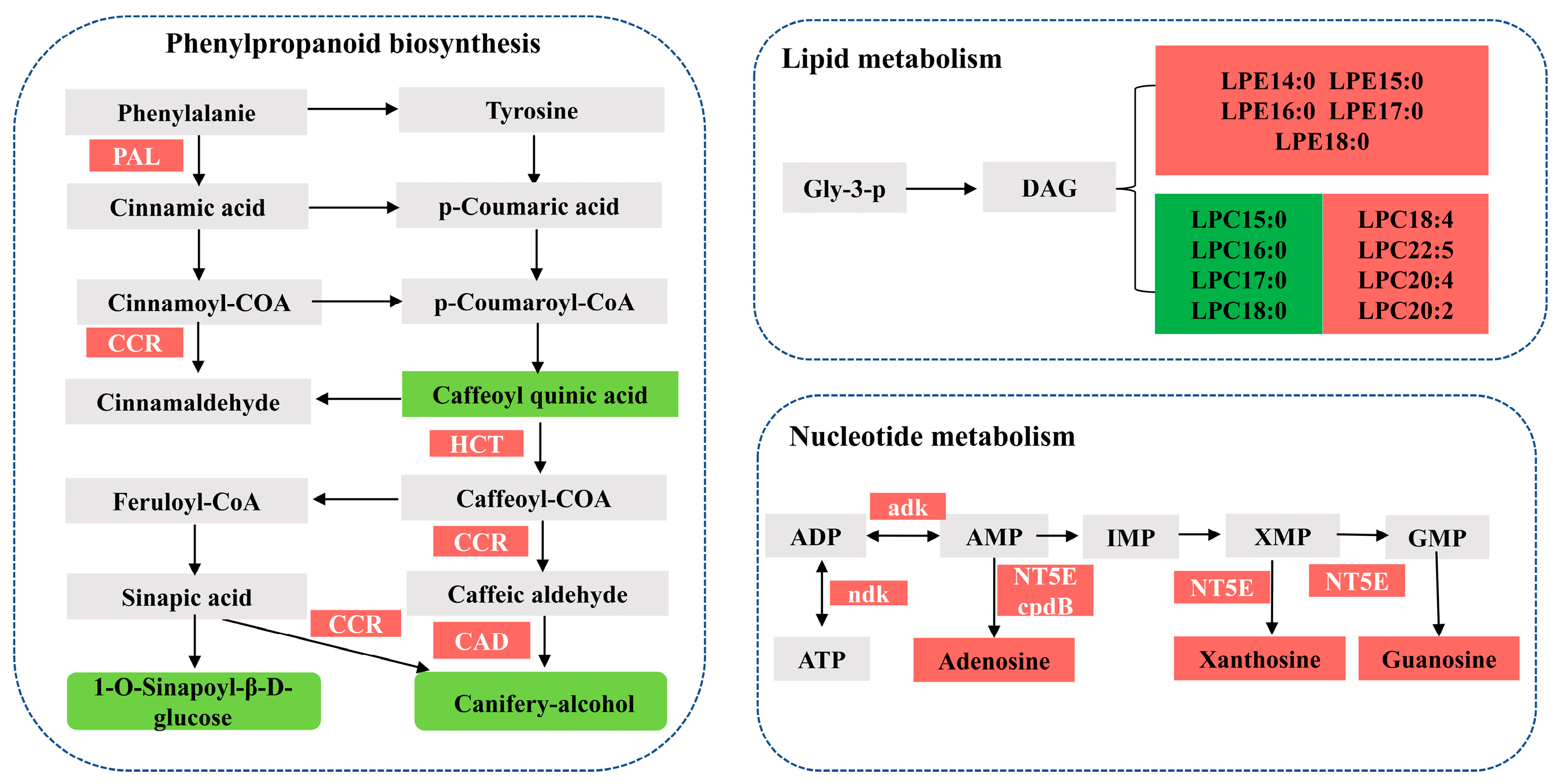
| Grafting Combinations | Fruit Length (cm) | Fruit Diameter (cm) | Fruit Weight (g) | Hardness (N) | Total Soluble Solid (Brix0) | Titratable Acid (mg/g) |
|---|---|---|---|---|---|---|
| Sa | 33.68 ± 0.20 b | 2.33 ± 0.03 b | 98.4 ± 0.52 b | 6.39 ± 0.11 a | 14.36 ± 0.30 b | 1.58 ± 0.03 c |
| SS | 33.64 ± 0.19 b | 2.40 ± 0.02 b | 99.2 ± 0.54 b | 6.37 ± 0.11 a | 13.38 ± 0.17 b | 1.76 ± 0.03 c |
| TOR | 38.20 ± 0.16 a | 2.58 ± 0.02 a | 107.58 ± 0.78 a | 6.18 ± 0.18 a | 13.42 ± 0.24 b | 1.38 ± 0.02 c |
| Sm64R | 39.10 ± 0.17 a | 2.61 ± 0.09 a | 110.02 ± 0.68 a | 6.29 ± 0.15 a | 15.78 ± 0.21 a | 1.67 ± 0.02 b |
| SG | 33.73 ± 0.22 b | 2.40 ± 0.02 b | 97.45 ± 0.47 b | 6.26 ± 0.09 a | 14.00 ± 0.16 b | 1.80 ± 0.02 a |
| Grafting Combinations | Flavonoids (ug/g) | Anthocyanin (ug/g) | Phenolic Acid (ug/g) | Total Amino Acid (ug/g) | Total Sugar (mg/g) | Vitamin C (mg/g) |
|---|---|---|---|---|---|---|
| Sa | 25.30 ± 1.10 d | 2.52 ± 0.23 c | 155.46 ± 1.33 b | 12.64 ± 0.18 b | 23.16 ± 0.47 c | 3.61 ± 0.06 d |
| SS | 38.63 ± 1.13 c | 6.30 ± 0.34 a | 154.05 ± 3.61 b | 12.86 ± 0.51 b | 18.65 ± 0.29 d | 6.12 ± 0.04 ab |
| TOR | 38.46 ± 0.42 c | 5.07 ± 0.39 b | 139.97 ± 3.73 c | 13.82 ± 0.22 a | 23.95 ± 0.78 c | 5.89 ± 0.20 b |
| Sm64R | 42.60 ± 0.89 b | 6.18 ± 0.35 a | 207.36 ± 4.53 a | 14.85 ± 0.23 a | 26.74 ± 0.32 b | 6.28 ± 0.12 a |
| SG | 47.01 ± 1.41 a | 6.49 ± 0.13 a | 153.46 ± 2.18 b | 12.31 ± 0.42 b | 29.34 ± 1.15 a | 4.30 ± 0.12 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Wang, W.; Hu, T.; Hu, H.; Wang, J.; Wei, Q.; Bao, C. Metabolomic and Transcriptomic Analyses Reveal the Effects of Grafting on Nutritional Properties in Eggplant. Foods 2023, 12, 3082. https://doi.org/10.3390/foods12163082
Yan Y, Wang W, Hu T, Hu H, Wang J, Wei Q, Bao C. Metabolomic and Transcriptomic Analyses Reveal the Effects of Grafting on Nutritional Properties in Eggplant. Foods. 2023; 12(16):3082. https://doi.org/10.3390/foods12163082
Chicago/Turabian StyleYan, Yaqin, Wuhong Wang, Tianhua Hu, Haijiao Hu, Jinglei Wang, Qingzhen Wei, and Chonglai Bao. 2023. "Metabolomic and Transcriptomic Analyses Reveal the Effects of Grafting on Nutritional Properties in Eggplant" Foods 12, no. 16: 3082. https://doi.org/10.3390/foods12163082
APA StyleYan, Y., Wang, W., Hu, T., Hu, H., Wang, J., Wei, Q., & Bao, C. (2023). Metabolomic and Transcriptomic Analyses Reveal the Effects of Grafting on Nutritional Properties in Eggplant. Foods, 12(16), 3082. https://doi.org/10.3390/foods12163082




