Bioactive Potential of Aqueous Phenolic Extracts of Spices for Their Use in the Food Industry—A Systematic Review
Abstract
1. Introduction
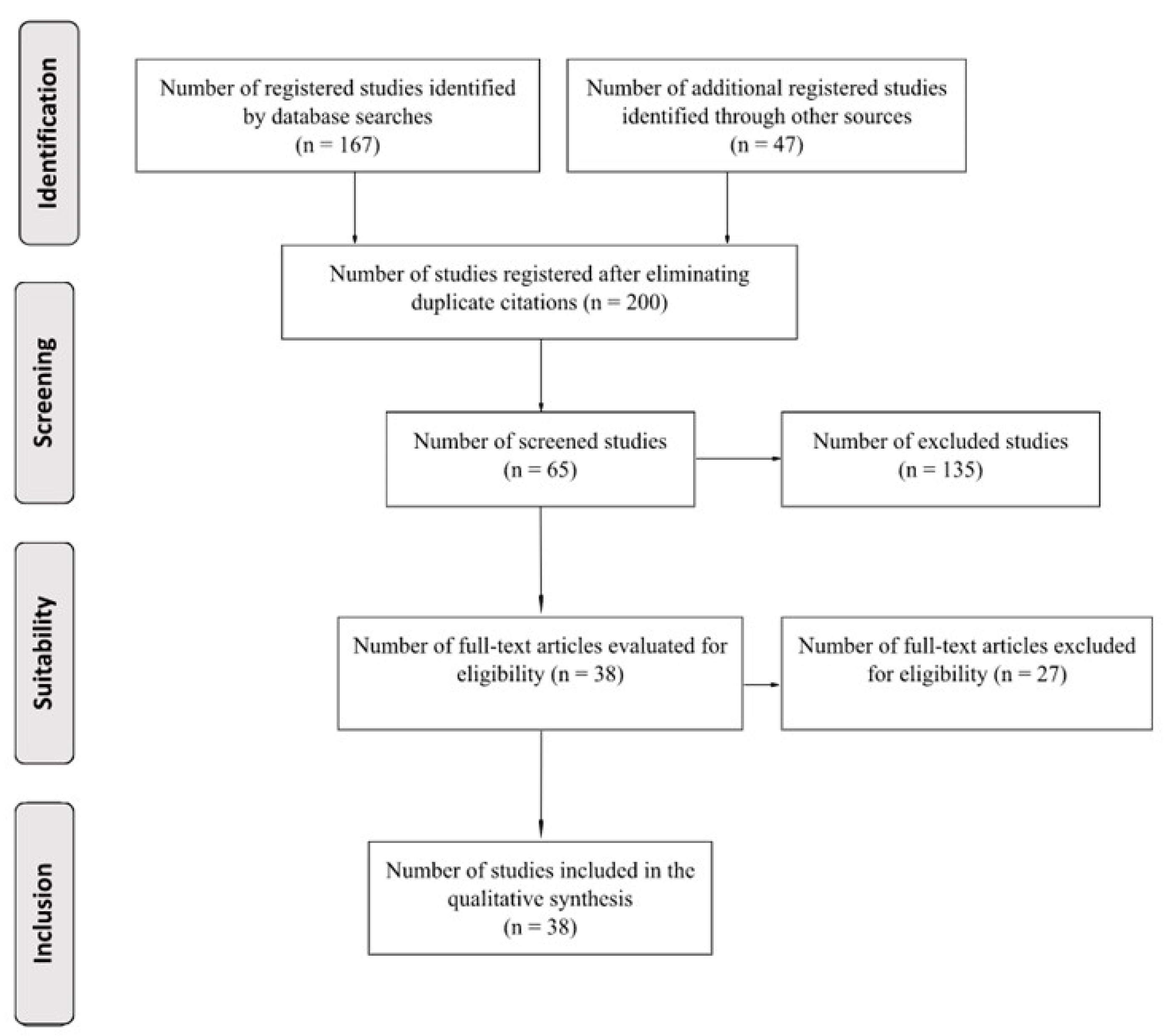
2. Materials and Methods
2.1. Search Strategy
2.2. Search Formulas and Keywords
3. Results and Discussion
3.1. Main Phenolic Compounds Identified in Medicinal and Aromatic Plants
3.2. Antioxidant Capacity Analysis in Spices
3.2.1. Main Antioxidant Methods Applied to Aromatic Plants
3.2.2. Antioxidant Activity and Main Phenolic Compounds Identified in Aromatic Plants
3.3. Antimicrobial Capacity of Phenolic Compounds from Aromatic Plants
3.4. Structure–Bioactivity Relationship
3.5. Comparison of Spice Extracts with Other Common Additives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorisation of food agro-industrial by-products: From the past to the present and perspectives. J. Environ. Manag. 2021, 299, 113571. [Google Scholar] [CrossRef]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-Based Phenolic Molecules as Natural Preservatives in Comminuted Meats: A Review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.; Muste, S.; Paucean, A.; Chis, S.; Man, S.; Salanta, L.; Marc, R.; Muresan, A.; Martis, G. Herbs and spices in terms of food preservation and shelf life. Hop Med. Plants 2019, 27, 57–65. [Google Scholar]
- Dougkas, A.; Vannereux, M.; Giboreau, A. The Impact of Herbs and Spices on Increasing the Appreciation and Intake of Low-Salt Legume-Based Meals. Nutrients 2019, 11, 2901. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and Spices- Biomarkers of Intake Based on Human Intervention Studies—A Systematic Review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Ju, R.; Chen, K.; Bhandari, B.; Wang, H. Advances in efficient extraction of essential oils from spices and its application in food industry: A critical review. Crit. Rev. Food Sci. Nutr. 2022, 1–22. [Google Scholar] [CrossRef]
- Omoarukhe, E.D.; Harbourne, N.; Jauregi, P. The Effect of Cinnamon and Ginger Spices on Anthocyanins in Sweetened Roselle Beverages. Beverages 2023, 9, 24. [Google Scholar] [CrossRef]
- Peters, J.C.; Marker, R.; Pan, Z.; Breen, J.A.; Hill, J.O. The Influence of Adding Spices to Reduced Sugar Foods on Overall Liking. J. Food Sci. 2018, 83, 814–821. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Baroi, A.M.; Ortan, A. Selected Aspects Related to Medicinal and Aromatic Plants as Alternative Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 1521. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Georgiev, M.I.; Sieniawska, E. Innovative Approaches for Recovery of Phytoconstituents from Medicinal/Aromatic Plants and Biotechnological Production. Molecules 2020, 25, 309. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; McClements, D.J.; Lorenzo, J.M. Encapsulation of Bioactive Phytochemicals in Plant-Based Matrices and Application as Additives in Meat and Meat Products. Molecules 2021, 26, 3984. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of Essential Oils Components and Polyphenols for Their Antioxidant Activity of Medicinal and Aromatic Plants Grown in Different Environmental Conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Guardiola, S.; Mach, N. Potencial terapéutico del Hibiscus sabdariffa: Una revisión de las evidencias científicas. Endocrinología y Nutrició 2014, 61, 274–295. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Regulation, European_Commission. No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Aditives. Off. J. Eur. Union 2008, 354, 16–33. [Google Scholar]
- Teng, X.; Zhang, M.; Devahastin, S. New developments on ultrasound-assisted processing and flavor detection of spices: A review. Ultrason. Sonochem. 2019, 55, 297–307. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Brajer, M.; Voća, S.; Galić, A.; Radman, S.; Rimac-Brnčić, S.; Xia, Q.; Zhu, Z.; Grimi, N.; Barba, F.J.; et al. Ultrasound as a Promising Tool for the Green Extraction of Specialized Metabolites from Some Culinary Spices. Molecules 2021, 26, 1866. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Binello, A.; Boffa, L.; Mulinacci, N.; Cravotto, G. Selective recovery of rosmarinic and carnosic acids from rosemary leaves under ultrasound- and microwave-assisted extraction procedures. C. R. Chim. 2016, 19, 699–706. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Ultrasonically assisted extraction of phenolic compounds from aromatic plants: Comparison with conventional extraction technics. J. Food Qual. 2006, 29, 567–582. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Stojanović, Z.; Segura Carretero, A.; Arráez Román, D.; Borrás, I.; Vasiljević, I. Development of a microwave-assisted extraction for the analysis of phenolic compounds from Rosmarinus officinalis. J. Food Eng. 2013, 119, 525–532. [Google Scholar] [CrossRef]
- Gouda, M.; El-Din Bekhit, A.; Tang, Y.; Huang, Y.; Huang, L.; He, Y.; Li, X. Recent innovations of ultrasound green technology in herbal phytochemistry: A review. Ultrason. Sonochem. 2021, 73, 105538. [Google Scholar] [CrossRef] [PubMed]
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The effect of Pulsed Electric Field as a pre-treatment step in Ultrasound Assisted Extraction of phenolic compounds from fresh rosemary and thyme by-products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102644. [Google Scholar] [CrossRef]
- Barbieri, J.B.; Goltz, C.; Batistão Cavalheiro, F.; Theodoro Toci, A.; Igarashi-Mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crops Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Josipović, R.; Knežević, Z.M.; Frece, J.; Markov, K.; Kazazić, S.; Mrvčić, J. Improved Properties and Microbiological Safety of Novel Cottage Cheese Containing Spices. Food Technol. Biotechnol. 2015, 53, 454–462. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 389103. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.N.; Moura, L.S.; Rosa, P.T.V.; Meireles, M.A.A. Supercritical fluid extraction from rosemary (Rosmarinus officinalis): Kinetic data, extract’s global yield, composition, and antioxidant activity. J. Supercrit. Fluids 2005, 35, 197–204. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Alcántara, C.; Žugčić, T.; Abdelkebir, R.; Collado, M.C.; García-Pérez, J.V.; Jambrak, A.R.; Gavahian, M.; Barba, F.J.; Lorenzo, J.M. Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary. Food Res. Int. 2020, 134, 109242. [Google Scholar] [CrossRef]
- Nabet, N.; Gilbert-López, B.; Madani, K.; Herrero, M.; Ibáñez, E.; Mendiola, J.A. Optimization of microwave-assisted extraction recovery of bioactive compounds from Origanum glandulosum and Thymus fontanesii. Ind. Crops Prod. 2019, 129, 395–404. [Google Scholar] [CrossRef]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef]
- Rosarior, V.L.; Lim, P.S.; Wong, W.K.; Yue, C.S.; Yam, H.C.; Tan, S.A. Antioxidant-rich Clove Extract, A Strong Antimicrobial Agent against Urinary Tract Infections-causing Bacteria in vitro. Trop. Life Sci. Res. 2021, 32, 45. [Google Scholar] [CrossRef]
- Rocío Teruel, M.; Garrido, M.D.; Espinosa, M.C.; Linares, M.B. Effect of different format-solvent rosemary extracts (Rosmarinus officinalis) on frozen chicken nuggets quality. Food Chem. 2015, 172, 40–46. [Google Scholar] [CrossRef]
- Thorsen, M.A.; Hildebrandt, K.S. Quantitative determination of phenolic diterpenes in rosemary extracts: Aspects of accurate quantification. J. Chromatogr. A 2003, 995, 119–125. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef]
- Borrás Linares, I.; Arráez-Román, D.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 7682–7690. [Google Scholar] [CrossRef]
- El-Maati, M.F.A.; Mahgoub, S.A.; Labib, S.M.; Al-Gaby, A.M.A.; Ramadan, M.F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur. J. Integr. Med. 2016, 8, 494–504. [Google Scholar] [CrossRef]
- Kim, I.S.; Yang, M.R.; Lee, O.H.; Kang, S.N. Antioxidant Activities of Hot Water Extracts from Various Spices. Int. J. Mol. Sci. 2011, 12, 4120–4131. [Google Scholar] [CrossRef] [PubMed]
- Kivilompolo, M.; Hyötyläinen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. A 2007, 1145, 155–164. [Google Scholar] [CrossRef]
- Nikolin, B.; Imamović, B.; Medanhodzić-Vuk, S.; Sober, M. High perfomance liquid chromatography in pharmaceutical analyses. Bosn. J. Basic Med. Sci. 2004, 4, 5. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Li, X.; Cao, X.; Li, Y.; Cao, H.; Men, Y. Multistage Extraction of Star Anise and Black Pepper Derivatives for Antibacterial, Antioxidant, and Anticancer Activity. Front. Chem. 2021, 9, 660138. [Google Scholar] [CrossRef] [PubMed]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef]
- Miron, T.L.; Plaza, M.; Bahrim, G.; Ibáñez, E.; Herrero, M. Chemical composition of bioactive pressurized extracts of Romanian aromatic plants. J. Chromatogr. A 2011, 1218, 4918–4927. [Google Scholar] [CrossRef]
- Yoo, K.M.; Kim, D.O.; Lee, C.Y. Evaluation of different methods of antioxidant measurement. Food Sci. Biotechnol. 2007, 16, 177–182. [Google Scholar]
- Moon, J.-K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity in foods. In Handbook of Antioxidants for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 287–333. [Google Scholar]
- Çelik, G.; Kılıç, G.; Kanbolat, Ş.; Özlem Şener, S.; Karaköse, M.; Yaylı, N.; Karaoğlu, Ş.A. Biological activity, and volatile and phenolic compounds from five Lamiaceae species. Flavour Fragr. J. 2021, 36, 223–232. [Google Scholar] [CrossRef]
- Hcini, K.; Bahi, A.; Zarroug, M.B.; Ben Farhat, M.; Lozano-Pérez, A.A.; Cenis, J.L.; Quílez, M.; Stambouli-Essassi, S.; Jordán, M.J. Polyphenolic Profile of Tunisian Thyme (Thymbra capitata L.) Post-Distilled Residues: Evaluation of Total Phenolic Content and Phenolic Compounds and Their Contribution to Antioxidant Activity. Molecules 2022, 27, 8791. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, P.I.; Vasileva, I.N.; Parzhanova, A.B.; Chalova, V.I.; Ivanova, S.D.; Slavov, A.M. Factors affecting the amount of biologically active substances in extracts of Bulgarian medical plants typical of Western Rhodopes. Bulg. Chem. Commun. 2022, 54, 74–80. [Google Scholar]
- Horbańczuk, O.K.; Kurek, M.A.; Atanasov, A.G.; Brnčić, M.; Brnčić, S.R. The Effect of Natural Antioxidants on Quality and Shelf Life of Beef and Beef Products. Food Technol. Biotechnol. 2019, 57, 439. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.R.; Byrne, D.V.; Bertelsen, G.; Skibsted, L.H. The antioxidative activity of plant extracts in cooked pork patties as evaluated by descriptive sensory profiling and chemical analysis. Meat Sci. 2004, 68, 485–495. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Sewalt, V.J.H.; Robbins, K.L.; Houser, T.A. Comparison of a natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2005, 69, 289–296. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sharma, G.K.; Radhakrishna, K.; Bawa, A.S. Antioxidant potential of synthetic and natural antioxidants and its effect on warmed-over-flavour in different species of meat. Food Chem. 2007, 105, 908–916. [Google Scholar] [CrossRef]
- Zahid, M.A.; Choi, J.Y.; Seo, J.K.; Parvin, R.; Ko, J.; Yang, H.S. Effects of clove extract on oxidative stability and sensory attributes in cooked beef patties at refrigerated storage. Meat Sci. 2020, 161, 107972. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kayama, H.; Ueda, Y.; Takahashi, T.; Asahara, T.; Tsuji, H.; Tsuji, N.M.; Kiyono, H.; Ma, J.S.; Kusu, T.; et al. Probiotic Bifidobacterium breve Induces IL-10-Producing Tr1 Cells in the Colon. PLoS Pathog. 2012, 8, e1002714. [Google Scholar] [CrossRef] [PubMed]
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef]
- Bouymajane, A.; Filali, F.R.; El Majdoub, Y.O.; Ouadik, M.; Abdelilah, R.; Cavò, E.; Miceli, N.; Taviano, M.F.; Mondello, L.; Cacciola, F. Phenolic Compounds, Antioxidant and Antibacterial Activities of Extracts from Aerial Parts of Thymus zygis subsp. gracilis, Mentha suaveolens and Sideritis incana from Morocco. Chem. Biodivers. 2022, 19, e202101018. [Google Scholar] [CrossRef]
- Mascoloti Spréa, R.; Caleja, C.; Pinela, J.; Finimundy, T.C.; Calhelha, R.C.; Kostić, M.; Sokovic, M.; Prieto, M.A.; Pereira, E.; Amaral, J.S.; et al. Comparative study on the phenolic composition and in vitro bioactivity of medicinal and aromatic plants from the Lamiaceae family. Food Res. Int. 2022, 161, 111875. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H. Bin Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly)phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Chang, J.S.; Wang, K.C.; Yeh, C.F.; Shieh, D.E.; Chiang, L.C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 145, 146–151. [Google Scholar] [CrossRef]
- Žitek, T.; Borjan, D.; Golle, A.; Knez, Ž.; Knez, M. Optimization of Extraction of Phenolic Compounds with Antimicrobial Properties from Origanum vulgare. Processes 2021, 9, 1032. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, X.; Zhou, N.; Li, J.; Liu, J.; Yue, J.; Hao, X.; Gan, M.; Lin, P.; Shang, X. Chemical characterization of the polar antibacterial fraction of the ethanol extract from Rosmarinus officinalis. Food Chem. 2021, 344, 128674. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef]
- Ali, H.M.; Abo-Shady, A.; Sharaf Eldeen, H.A.; Soror, H.A.; Shousha, W.G.; Abdel-Barry, O.A.; Saleh, A.M. Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem. Cent. J. 2013, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Siquet, C.; Paiva-Martins, F.; Lima, J.L.F.C.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy- and trihydroxyphenolic acids-A structure–activity relationship study. Free Radic. Res. 2009, 40, 433–442. [Google Scholar] [CrossRef]
- Muhammad, A.; Tel-Çayan, G.; Öztürk, M.; Duru, M.E.; Nadeem, S.; Anis, I.; Ng, S.W.; Shah, M.R. Phytochemicals from Dodonaea viscosa and their antioxidant and anticholinesterase activities with structure–activity relationships. Pharm. Biol. 2016, 54, 1649–1655. [Google Scholar] [CrossRef]
- Tohma, H.; Köksal, E.; Kılıç, Ö.; Alan, Y.; Abdullah Yılmaz, M.; Gülçin, İ.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS Analysis of the Phenolic Compounds, Antioxidant and Antimicrobial Activities of Salvia L. Species. Antioxidants 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, N.; Matsuda, M.; Kubo, I. Characterisation of the antioxidant activity of flavonoids. Food Chem. 2012, 131, 541–545. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols-A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Showing All Polyphenols Found in Cloves—Phenol-Explorer. Available online: http://phenol-explorer.eu/contents/food/797 (accessed on 4 August 2023).
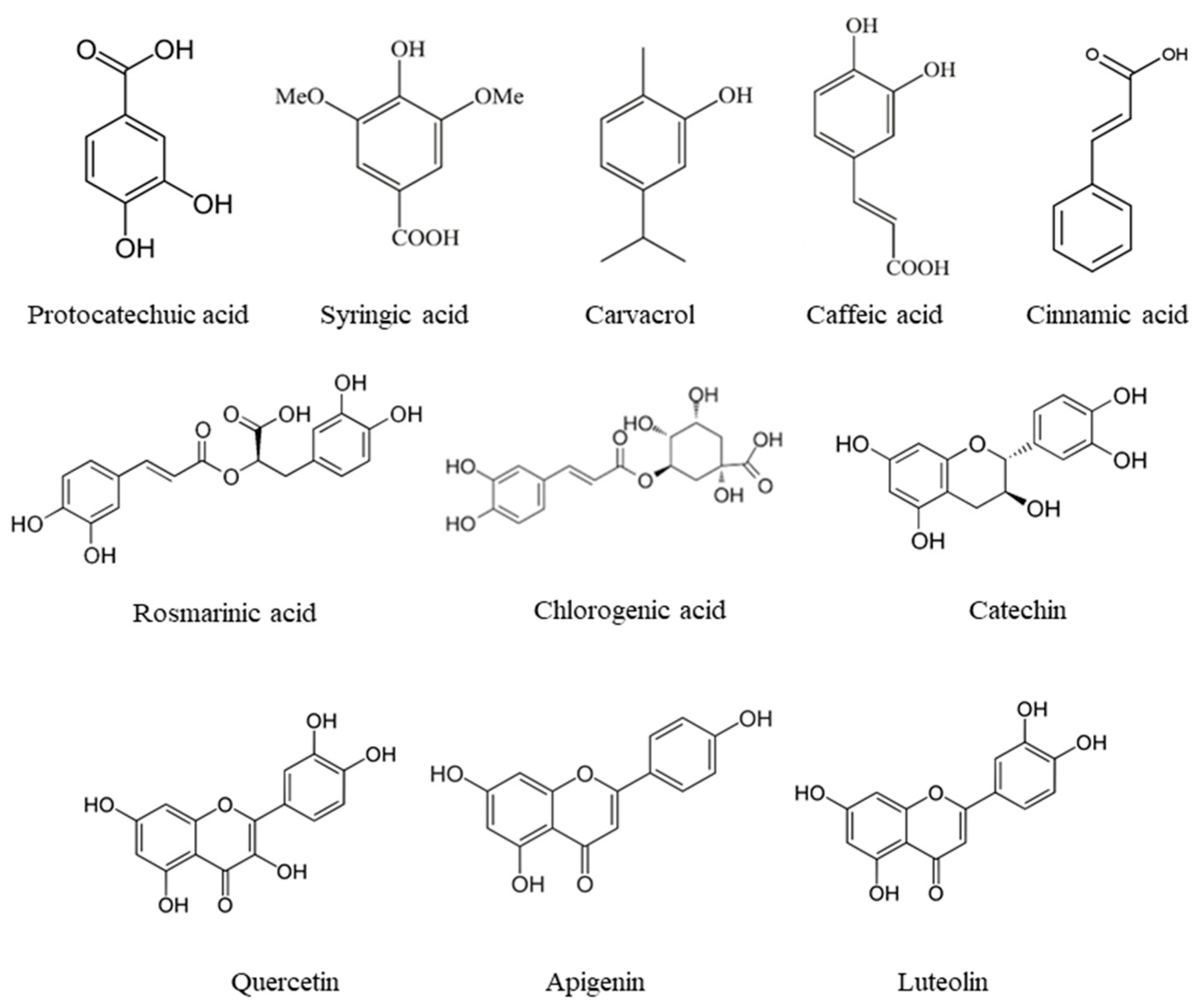
| Spice Extract | Phenolic Compound | Extraction Technique | Extraction Conditions | Characterization Technique | References |
|---|---|---|---|---|---|
| Oregano | Glycosylated flavonoids: kaempferol-O-glucuronide and luteolin-7-O glucuronide. | MAE. | 100% water, 42 °C and 2 min. extraction time. | Folin–Ciocalteu. | [38] |
| Rosmarinic, caffeic, chlorogenic, ferulic, gallic, p-coumaric, syringic and vanillic acids. | Dynamic sonication assisted ethanol extraction. | 60% ethanol (v/v) at room temperature, 0.25 h. | LC × LC-MS. | [48] | |
| Caffeic and caffeic-O-hexoside, protocatechic, rosmarinic, 3-, 4- and 5-O-caffeoylquinic, coumaroylquinic, ferulic-O-hexoside, ferulic, p-coumaric, homovanillic-O-hexoside, gallic acids, syringic, p- and m-hydroxybenzoic, kaempferol-3-O-glucoside, kaempferol, and quercetin. | SPE. | Hydroalcoholic solvent. | Folin–Ciocalteu, HPLC LTQ-Orbitrap mass spectrometry. | [43] | |
| Gallic acid, caffeic acid, gentisic acid, p-coumaric acid, vannyl acid, ferulic acid, syringic acid, catechin, rutin, quercetin, apigenin, naringenin, and eriodictyol | UAE. | 60% methanol, 60% acetone, water and ethyl acetate/water [60:30, v/v]. | RP-HPLC and Folin–Ciocalteu. | [24] | |
| Rosemary | Carnosoic acid and rosmarinic acid. | SFE. | 40 °C and 300 bar. | GC-FID, TLC | [32] |
| Rosmarinic acid, caffeine, 7-methylrosmanol, rutin, naringin, and ferulic acid. | UAE. | Glicerol:choline chloride (1: 2 v/w) and water (10% w/w), lactic acid:choline chloride (1:3 v/w) and water (10% w/w), 1,2-propanediol:choline chloride (1:2 v/w) and water (10% w/w): deep eutectic solvents compared to pure ethanol. | Folin–-Ciocalteu. | [29] | |
| Phenolic acids (gallic acid, rosmarinic acid, p-coumaric acid), flavonoids (quercetin 3-O-galactoside, kaempferol 3-O-glucuronide), and terpenoids (rosmanol, carnosol, carnosic acid). | UAE and CE. | 100% water and 50:50 ethanol:water. | Folin–Ciocalteu and Triple TOF-LC-MS-MS. | [37] | |
| Flavonoids (cirsimaritin, genkwanin), rosmarinic acid and terpenoids (rosmanol, carnosol, carnosic acid). | UAE and MAE. | UAE: 140 W MAE: N2 20 bar, 100 °C. | HPLC-DAD-MS-TOF. | [23] | |
| Total polyphenols, flavonoids, anthocyanins, monomeric and condensed anthocyanins. | MAE. | Methanol: water (70:30, v/v), 70 °C. | Folin–Ciocalteu. | [26] | |
| Flavonoids (luteolin, apigenin, diosmetin, cirsimaritin, genkwanin) and terpenoids (rosmanol, carnosol, carnosic acid). | SFE and PLE. | SFE: 150 bar 6.6% ethanol and 400 bar with CO2, 40 °C. PLE: ethanol 150 °C and water 100 °C and 200 °C, 20 min. | HPLC-DAD-MS. | [45] | |
| Caffeic and caffeic-O-hexoside, protocatechic, rosmarinic, 3-, 4- and 5-O-caffeoylquinic, coumaroylquinic, ferulic-O-hexoside, ferulic, p-coumaric, homovanillic-O-hexoside, gallic acids, syringic, p- and m-hydroxybenzoic, kaempferol-3-O-glucoside, kaempferol, and quercetin. | SPE. | Hydroalcoholic solvent. | Folin–Ciocalteu. HPLC + LTQ-Orbitrap mass spectrometry. | [43] | |
| Thyme | Derivatives of hydroxycinnamic acid: caffeic acid hexoside, rosmarinic acid and derivatives of salvianolic acid-A. Glycosylated flavonoids: luteolin-7-O-glucuronide and kaempferol-O glucuronide. | MAE. | 150 °C, 50% ethanol and 9.5 min. | Folin–Ciocalteu. | [38] |
| Rosmarenic acid, methyl rosmarnate, caffeic acid, cinnamic acid, chlorogenic acid, quinic acid, and flavonoids such as ferulic acid, apigenin, luteolin, and quercetin. | Maceration. | Metanol, ethanol, diethyl ether and hexane, 72 h. | Folin–Ciocalteu and HPLC. | [34] | |
| Caffeic and caffeic-O-hexoside, protocatechic, rosmarinic, 3-, 4- and 5-O-caffeoylquinic, coumaroylquinic, ferulic-O-hexoside, ferulic, p-coumaric, homovanillic-O-hexoside, gallic acids, syringic, p- and m-hydroxybenzoic, kaempferol-3-O-glucoside, kaempferol, and quercetin. | SPE. | Hydroalcoholic solvent. | Folin–Ciocalteu. HPLC + LTQ-Orbitrap mass spectrometry. | [43] | |
| p-coumarinic acid, kaempferol, epigenin, ferulic acid, luteolin, rosmarinic acid, gallic acid, epigenin, eugenol, quercetin. | UAE and CE. | Water 100% and 50:50 ethanol:water. | Folin–Ciocalteu and Triple TOF-LC-MS-MS. | [37] | |
| Ginger | Total soluble solids, total polyphenols, flavonoids and non-flavonoids, total carotenoids, and vitamin C. | UAE. | Water as solvent, 40–70 °C and 30 min | Folin–Ciocalteu. | [22] |
| Pepper | Capsaicinoids (nordihydrocapsaicin, capsaicin, dihydrocapsaicin, homocapsaicin, and homodihydrocapsaicin). | UAE. | Methanol as solvent, 50 °C and 10 min. | HPLC-fluoresncence. | [44] |
| Piperine and piperine acid, anisic acid, methyl anisate, shikimic acid, methyl shikimate. | Oxidative or steam distilled. | Piperine extraction: Ethanol, 80 °C, 2 h. Piperine acid extraction: Piperine dissolved in KOH/ethanol at ¼ (v/v), at 8 °C, 15 h. | 1H NMR, 13C NMR, FTIR, and HPLC. | [50] | |
| Clove | Tannins, phenolic compounds, terpenoids, glycosides. cardiac. TPC (mg/g): Kaempeforl 5.839, Catechin 0.0184, Gallic acid 0.0169. | Maceration. | 80% ethanol at a ratio of 1:5 (w:v) 24 h. | GC-MS and HPLC-DAD. | [40] |
| References | Spice | TPC (mg GAE/g. DW) | ABTS (mmol TE/g. DW) | DPPH (mmol TE/g. DW) | FRAP (mmol Fe2+/mg.) | Phenolic Compounds |
|---|---|---|---|---|---|---|
| [36] | Oregano (Origanum vulgare L.) | 101.7 ± 0.10 | 1.010 ± 0.009 | - | - | Phenolic acids (caffeic acid, p-coumaric acid, rosmarinic acid, caffeoyl derivatives), volatile compounds (carvacrol), and other flavonoids. |
| Rosemary (Rosmarinus officinalis L.) | 50.7 ± 0.36 | 0.378 ± 0.00021 | - | - | Phenolic acids (caffeic acid, rosmarinic acid, caffeoyl derivatives), phenolic diterpenes (carnosic acid, carnosol, epirosmanol), volatile compounds (carvacrol) and other flavonoids. | |
| Thyme (Thymus vulgaris L.) | 45.2 ± 0.06 | 0.381 ± 0.00003 | - | - | Phenolic acids (gallic acid, caffeic acid, rosmarinic acid), volatile compounds (thymol), phenolic diterpenes, flavonoids. | |
| Clove | 143.8 ± 0.06 | 1.67 ± 0.00024 | - | - | Phenolic acids (gallic acid), flavonol glucosides, phenolic volatile oils (eugenol, acetyl eugenol), tannins. | |
| [30] | Rosemary | 5.15 ± 0.08 | - | 1.30 ± 0.03 | 2.64 ± 0.09 | caffeic acid, rosmarinic acid and flavones. |
| Pepper | 1.77 ± 0.02 | - | 1.199 ± 0.044 | 0.81 ± 0.62 | flavones and flavones. | |
| [41] | Rosemary | 0.204 ± 0.002 | 8.12 ± 0.17 | - | - | carnosic acid, carnosol (liquid methanol). |
| [43] | Oregano | - | 1.34 ± 0.13 | 0.78 ± 0.07 | - | Phenolic acids (rosmarinic, vanillinic, syringic, caffeic, protocatechic, chlorogenic, coumaric acids) and flavonoids (apigenin, hesperetin, naringenin, quercetin, among others). |
| Rosemary | - | 2.39 ± 0.17 | 1.98 ± 0.17 | - | Phenolic acids (rosmarinic, vanillinic, syringic, caffeic, protocatechic, chlorogenic, coumaric acids) and flavonoids (apigenin, hesperetin, naringenin, quercetin, among others). | |
| Thyme | - | 1.38 ± 0.13 | 1.15 ± 0.06 | - | Phenolic acids (rosmarinic, vanillinic, syringic, caffeic, protocatechic, chlorogenic, coumaric acids) and flavonoids (apigenin, hesperetin, naringenin, quercetin, among others). | |
| [38] | Oregano | - | 0.361 ± 0.14 | 5.54 ± 0.52 b | - | Phenolic acids (caffeic and rosmarinic acids), flavonoids (gallocatechin, galangin, lucenin-2, luteolin-O-hexoside, and luteolin-7-O-glucuronide). |
| Thyme | - | 3.92 ± 0.61 | 8.58 ± 1.75 b | - | Phenolic acids (Caffeic acid hexoside, rosmarinic acid and derivatives of salvianolic acid A), flavonoids (luteolin-7-O-glucuronide and kaempferol-o-glucuronide). | |
| [52] | Thyme (Thymus serpyllum) | 112.27 ± 16.75 | 271 ± 8 | - | - | Phenolic acids (rosmarinic, syringic, vanillinic, chlorogenic, p-coumaric, caffeic acids), flavonoids (luteolin glucoside, luteolin-glucuronide, eriodictyol-glucuronide, apigenin-glucuronide). |
| [51] | Thyme (Thymus vulgaris) | 15.13 ± 0.313 | - | 29.22 ± 0.385 b | 30.88 ± 0.02 a | Phenolic acids (cinnamic, rosmarinic, chlorogenic, syringic, coumaric acids), flavonoids (rutin, quercetin). |
| [56] | Thyme | 109 ± 1.98 | - | 8.43 ± 0.0009 b | 348 ± 7.89 c | Simple phenols phenols (phenolic acids and coumarins) and polyphenols (flavonoids, stilbenes, lignans, tannins). |
| [57] | Thyme | 126.7 ± 34.3 | - | 42.97 ± 2.10 b | 50.21 ± 2.400 | Phenolic acids (rosmarinic, caffeic acids), flavonoids (luteolin.7.O.neohesperidoside, apigenin-7-Neohesperidoside, apigenin-7-glucuronide). |
| [58] | Thyme | 86.19 ± 0.36 | - | 218.97 ± 0.28 | 1.11 ± 0.00085 | Flavonoids (including flavones, flavanones, isoflavones, flavonols, and anthocyanidins) |
| Spice | Antioxidant Capacity Method | Food Matrix | Common Additives | Observed Effect | References |
|---|---|---|---|---|---|
| Rosemary (Rosmarinus officinalis) | TBARS, hexanal, vitamin E, and sensory panel. | Pork patties precooked during storage under retail conditions (10 days, 4 °C, atmospheric air). | Vitamin E | Extracts improved the retardation of lipid oxidation during meat processing | [60] |
| Rosemary (Rosmarinus officinalis) | FRAP, ABTS. | Frozen chicken nuggets. | - | Extracts improved lipid oxidative stability without altering physical or chemical characteristics during storage | [41] |
| Rosemary (Rosmarinus officinalis) | TBARS, color, and sensory panel. | Pork sausages frozen, precooked, frozen, and chilled fresh pork sausages. | BHA/BHT. | Extract was more effective than BHA/BHT for preventing a TBARS increase and red color loss in raw frozen sausage | [61] |
| Clove (Eugenia caryophyllata), cinnamon (Cinnamomum zeylanicum), and synthetic antioxidants. | WOF and non-haem iron release. | Cooked and refrigerated stored meats of three common domestic species (sheep, beef, and pork). | TBHQ, BHA, and PG. | Clove extract showed higher antioxidant activity in the studied matrices during storage than BHA and ascorbic acid | [62] |
| Clove (Eugenia caryophyllata) | TBARS. | Cooked beef patties. | BHT and ascorbic acid. | Clove extract reduced protein and lipid oxidation when compared with BHT and ascorbic acid | [63] |
| Water extracts of 13 spices, including oregano, cloves, and thyme | DPPH. | Processed meats. | Ascorbic acid. | Spices extracts showed greater antioxidant activity than ascorbic acid. | [47] |
| References | Spice | Extraction and/or Analytical Determination | Microorganism, Pathogen | Method for Determining Antimicrobial Activity | Food Matrix | Conclusions |
|---|---|---|---|---|---|---|
| [71] | Ginger (Zingiber officinale Roscoe) | - | HRSV. | ELISA. | - | Estimated CC50 cytotoxic concentration: 1893.8 µg/mL and >3000 µg/mL. |
| [31] | Rosemary (Rosmarinus officinalis), clavo (Syzygium aromaticum), and tomillo (Thymus vulgaris) | Conventional extraction + ultrasound. Solvents water and ethanol. | Bacillus cereus, Staphylococcus aureus, Escherichia coli, Salmonella enteritidis, Vibrio parahaemolyticusy Pseudomonas aeruginosa, and Candida albicans. | Diffusion in agar wells. | - | MIC (% w/v): Clove. 0.313% for BC in water. Rosemary. 1.25% for BC in water. Thyme. 2.5% for SA in water. |
| [46] | Clove (Syzygium aromaticum) | Characterization using Folin–Ciocalteu. | Staphylococcus aureus, Listeria monocytogenes, Salmonella enteritidis, Serratia marcescens, and Escherichia coli. | Mueller–Hinton surface on agar plates. | - | MIC (μg/mL): 50 for G+ and 100 for G−. |
| [72] | Oregano (Origanum vulgare). | Solid–liquid extraction and SFE. | Staphylococcus aureus, Escherichia coli, and Candida albicans. | Mueller–Hinton surface microdilution. | - | MIC (g/mL): 0.147, 0.728 and 0.311, respectively. |
| [50] | Pepper: Piperine and methyl piperate. | NMR, FTIR, UV-vis, fluorescence spectroscopy, and HPLC. | S. aureus (Sa), S pyogenes (Sp) S. thypi (Sth), P. aeruginosa (Pa), and E. coli (Ec). | Dissolution in DMF. | - | MIC (μg/mL) for piperine: 160 0 (Sa), 1200 (Sp), 400 (Ec), 1200 (Sth), 1600 (Pa). MIC (μg/mL) for methyl piperate: 2000 (Sa), 2400 (Sp), 800 (Ec), 2000 (Sty), 2400 (Pa). |
| [40] | Clove (Syzygium aromatium) | Characterization using HPLC. | Urinary tract infections by Proteus mirabilis, Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae. | Mueller–Hinton surface on agar plates. Disk diffusion assay and microwell dilution assay. | - | Pm (19.7 ± 0.6 mm) > Ses (18 mm) > Sa (14.7 ± 0.6 mm) > Eci (12.7 ± 0.6 mm) > Kp (12.3 ± 0.6 mm) |
| [22] | Ginger (Zingiber officinalle L.) | Extraction using UAE. | Salmonella, L. monocytogenes, S. aureus. | - | - | - |
| [55] | Romero (Rosmarinus officialis L.) | UPLC-Orbitrap-MS/MS characterization at room temperature, UV, IR, HRESI-MS, and NMR. | Gram-positive B. subtilis. | - | - | Analysis of the parts of the extract, against B. subtilis. MIC (μg/mL) for M1 16, M2 8, M3 1, M4 128, M5 > 128, M6 > 128, M7 > 128, M8 > 128 |
| [30] | Pepper and rosemary. | Characterization using HPLC and Folin–Ciocalteu. | Salmonella typhimurium (St), Escherichia coli (Ec), Enterococcus faecalis (Ef), Staphylococcus aureus (Sa), and Listeria monocytogenes (Lm). | Mueller–Hinton surface on agar plates. | Curd. | d (inhibitory zone)/mm, respectively: Pepper: 13.3 ± 1.2 (St), 9.3 ± 2.3 (Ec), 15.3 ± 1.2 (Ef), 11.3 ± 1.2 (Sa), n.d (Lm). Rosemary: 12.7 ± 2.3 (St), 13.3 ± 1.2 (Ec), 19.3 ± 1.2 (Ef), 20.7 ± 1.2 (Sa), 14.0 ± 1.0 (Lm). |
| [35] | Clove, thyme, ginger | - | Bacillus cereus (Bc), Staphylococcus aureus (Sa), Escherichia coli (Ec), Pseudomonas aeruginosa (Pa), and Salmonella typhi.(St). | Mueller–Hinton surface on agar plates. | Fish. | d(inhibitory zone)/mm, Clove 14.6 ± 0.37 (Bc), Thyme 17.6 ± 0.31 (Sa), clove 11.9 ± 0.34 (Ec), clove 13.4 ± 0.11 (Pa). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duque-Soto, C.; Ruiz-Vargas, A.; Rueda-Robles, A.; Quirantes-Piné, R.; Borrás-Linares, I.; Lozano-Sánchez, J. Bioactive Potential of Aqueous Phenolic Extracts of Spices for Their Use in the Food Industry—A Systematic Review. Foods 2023, 12, 3031. https://doi.org/10.3390/foods12163031
Duque-Soto C, Ruiz-Vargas A, Rueda-Robles A, Quirantes-Piné R, Borrás-Linares I, Lozano-Sánchez J. Bioactive Potential of Aqueous Phenolic Extracts of Spices for Their Use in the Food Industry—A Systematic Review. Foods. 2023; 12(16):3031. https://doi.org/10.3390/foods12163031
Chicago/Turabian StyleDuque-Soto, Carmen, Ana Ruiz-Vargas, Ascensión Rueda-Robles, Rosa Quirantes-Piné, Isabel Borrás-Linares, and Jesús Lozano-Sánchez. 2023. "Bioactive Potential of Aqueous Phenolic Extracts of Spices for Their Use in the Food Industry—A Systematic Review" Foods 12, no. 16: 3031. https://doi.org/10.3390/foods12163031
APA StyleDuque-Soto, C., Ruiz-Vargas, A., Rueda-Robles, A., Quirantes-Piné, R., Borrás-Linares, I., & Lozano-Sánchez, J. (2023). Bioactive Potential of Aqueous Phenolic Extracts of Spices for Their Use in the Food Industry—A Systematic Review. Foods, 12(16), 3031. https://doi.org/10.3390/foods12163031







