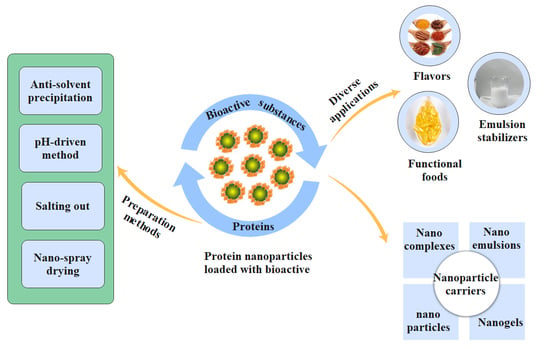
Research Progress of Protein-Based Bioactive Substance Nanoparticles
Abstract
1. Introduction
2. Origin, Classification, and Functional Properties of Bioactive Substances
3. Construction of Protein Nanoparticle Carriers
3.1. Nano-Complexes
3.2. Nano-Emulsions
3.3. Nano-Particles
3.4. Nano-Gels
4. Synthesis Strategies of Protein-Based Nanoparticles
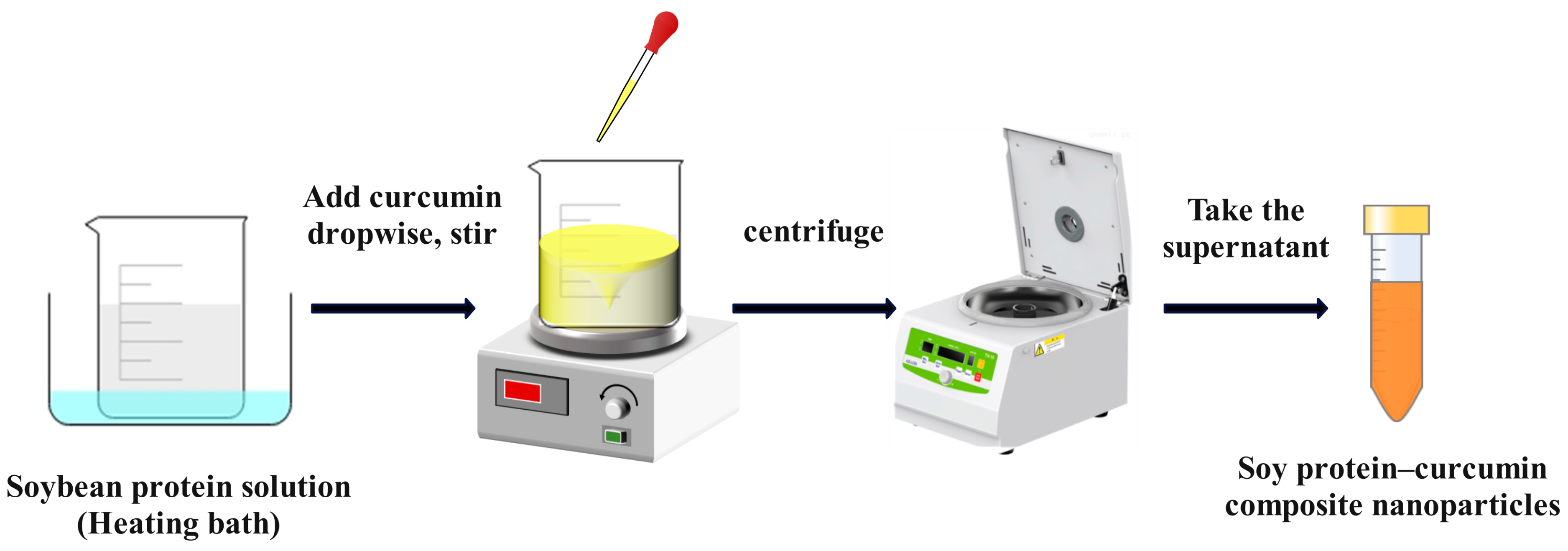
4.1. Anti-Solvent Precipitation
4.2. pH-Driven Method
4.3. Salting Out
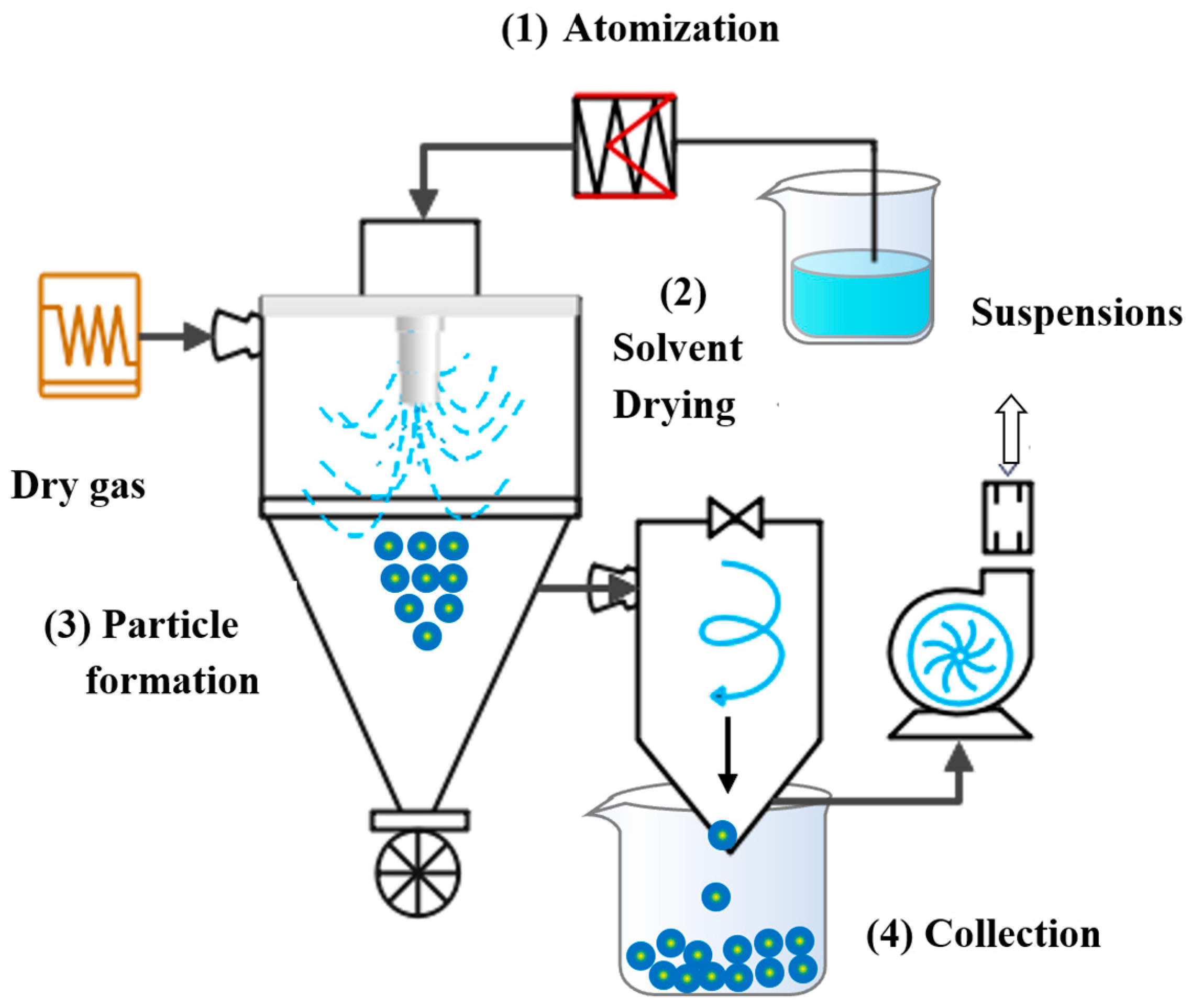
4.4. Nano Spray Drying
5. Application of Protein-Based Nanoparticles in the Food Industry
5.1. Stabilization of Pickering Emulsions
5.2. Production of Functional Foods
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reddy, N.; Rapisarda, M. Properties and Applications of Nanoparticles from Plant Proteins. Materials 2021, 14, 3607. [Google Scholar] [CrossRef]
- Dong, H.; Gao, Y.; Sinko, P.J.; Wu, Z.; Xu, J.; Jia, L. The nanotechnology race between China and the United States. Nano Today 2016, 11, 7–12. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Sandhiutami, N.M.D.; Dewi, R.S.; Khairani, S.; Putri, R.N.A. Enhancement of curcumin level and hepatoprotective effect in rats through antioxidant activity following modification into nanosized particles. Vet. World 2022, 15, 2323–2332. [Google Scholar] [CrossRef]
- Das Gupta, S.; Suh, N. Tocopherols in cancer: An update. Mol. Nutr. Food Res. 2016, 60, 1354–1363. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Bailey, H.H.; Johnson, J.J.; Lozar, T.; Scarlett, C.O.; Wollmer, B.W.; Kim, K.; Havinghurst, T.; Ahmad, N. A randomized, double-blind, dose-ranging, pilot trial of piperine with resveratrol on the effects on serum levels of resveratrol. Eur. J. Cancer Prev. 2021, 30, 285–290. [Google Scholar] [CrossRef]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Agcam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2023, 12, 48. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, M.; Wen, S.; Wang, L.; Zhang, K.; Zhang, C.; Zou, H.; Gu, J.; Liu, X.; Bian, J.; et al. Puerarin alleviates cadmium-induced rat neurocyte injury by alleviating Nrf2-mediated oxidative stress and inhibiting mitochondrial unfolded protein response. Ecotoxicol. Environ. Saf. 2022, 247, 114239. [Google Scholar] [CrossRef]
- Drawbridge, P.C.; Apea-Bah, F.; Beta, T. Bioaccessibility of ferulic acid in hulless barley varieties at stages of simulated in vitro digestion. Cereal Chem. 2023, 358, 129905. [Google Scholar] [CrossRef]
- Tavares, A.G.; Andrade, J.; Assis Silva, R.R.; Marques, C.S.; Ramos da Silva, J.O.; Dantas Vanetti, M.C.; de Melo, N.R.; Ferreira Soares, N.d.F. Carvacrol-loaded liposome suspension: Optimization, characterization and incorporation into poly(vinyl alcohol) films. Food Funct. 2021, 12, 6549–6557. [Google Scholar] [CrossRef]
- Yao, M.; McClements, D.J.; Xiao, H. Improving oral bioavailability of nutraceuticals by engineered nanoparticle-based delivery systems. Curr. Opin. Food Sci. 2015, 2, 14–19. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W. Role of quercetin in the physicochemical properties, antioxidant and antiglycation activities of bread. J. Funct. Foods 2018, 40, 299–306. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.M.A.K.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: A Review on Structural Activity Relationship-Based Studies and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef]
- Ensafi, F.; Fazlyab, M.; Chiniforush, N.; Akhavan, H. Comparative effects of SWEEPS technique and antimicrobial photodynamic therapy by using curcumin and nano-curcumin on Enterococcus faecalis biofilm in root canal treatment. Photodiagnosis Photodyn. Ther. 2022, 40, 103130. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Soltaninejad, H.; Ghorani-Azam, A.; Nafisi-Moghadam, R.; Haddadzadegan, N.; Ansari, M.; Saeed-Banadaki, S.H.; Sobhan, M.R.; Mozafari, S.; Zahedi, M. Slow release curcumin-containing soy protein nanoparticles as anticancer agents for osteosarcoma: Synthesis and characterization. Prog. Biomater. 2022, 11, 311–320. [Google Scholar] [CrossRef]
- Yu, Z.; Hong, Y.; Xie, K.; Fan, Q. Research Progresses on the Physiological and Pharmacological Benefits of Microalgae-Derived Biomolecules. Foods 2022, 11, 2806. [Google Scholar] [CrossRef]
- Tabibiazar, M.; Mohammadifar, M.A.; Roufegarinejad, L.; Ghorbani, M.; Hashemi, M.; Hamishehkar, H. Improvement in dispersibility, stability and antioxidant activity of resveratrol using a colloidal nanodispersion of BSA-resveratrol. Food Biosci. 2019, 27, 46–53. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. A novel and green nanoparticle formation approach to forming low-crystallinity curcumin nanoparticles to improve curcumin’s bioaccessibility. Sci. Rep. 2019, 9, 19112. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Milincic, D.D.; Popovic, D.A.; Levic, S.M.; Kostic, A.Z.; Tesic, Z.L.; Nedovic, V.A.; Pesic, M.B. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef]
- Serpa Guerra, A.M.; Gomez Hoyos, C.; Andres Velasquez-Cock, J.; Velez Acosta, L.; Ganan Rojo, P.; Velasquez Giraldo, A.M.; Zuluaga Gallego, R. The nanotech potential of turmeric (Curcuma longa L.) in food technology: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1842–1854. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert Rev. Anti-Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef]
- Alsaba, M.T.; Al Dushaishi, M.F.; Abbas, A.K. A comprehensive review of nanoparticles applications in the oil and gas industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1389–1399. [Google Scholar] [CrossRef]
- Crivelli, B.; Perteghella, S.; Bari, E.; Sorrenti, M.; Tripodo, G.; Chlapanidas, T.; Torre, M.L. Silk nanoparticles: From inert supports to bioactive natural carriers for drug delivery. Soft Matter 2018, 14, 546–557. [Google Scholar] [CrossRef]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm. 2018, 2018, 9285854. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, N.K.; Kim, I.-S. Bioengineered protein-based nanocage for drug delivery. Adv. Drug Del. Rev. 2016, 106, 157–171. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Nie, G. Multifunctional biomolecule nanostructures for cancer therapy. Nat. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef]
- Jin, S.; Li, S.; Wang, C.; Liu, J.; Yang, X.; Wang, P.C.; Zhang, X.; Liang, X.-J. Biosafe Nanoscale Pharmaceutical Adjuvant Materials. J. Biomed. Nanotechnol. 2014, 10, 2393–2419. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Elgohary, M.M.; Kamel, N.M. Implications of Protein- and Peptide-Based Nanoparticles as Potential Vehicles for Anticancer Drugs. Adv. Protein Chem. Struct. Biol. 2015, 98, 169–221. [Google Scholar]
- Zhang, R.; Han, Y.; Xie, W.; Liu, F.; Chen, S. Advances in Protein-Based Nanocarriers of Bioactive Compounds: From Microscopic Molecular Principles to Macroscopical Structural and Functional Attributes. J. Agric. Food Chem. 2022, 70, 6354–6367. [Google Scholar] [CrossRef]
- Nasery, M.M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Hanieh, H.; Ibrahim, H.-I.M.; Mohammed, M.; Alwassil, O.I.; Abukhalil, M.H.; Farhan, M. Activation of aryl hydrocarbon receptor signaling by gallic acid suppresses progression of human breast cancer in vitro and in vivo. Phytomedicine 2022, 96, 153817. [Google Scholar] [CrossRef]
- Senapathy, J.G.; Jayanthi, S.; Viswanathan, P.; Umadeyi, P.; Nalini, N. Effect of gallic acid on xenobiotic metabolizing enzymes in 1,2-dimethyl hydrazine induced colon carcinogenesis in Wistar rats—A chemopreventive approach. Food Chem. Toxicol. 2011, 49, 887–892. [Google Scholar] [CrossRef]
- Soares Alves, A.d.C.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Jiang, H.; Li, J.; Chen, L.; Wang, Z. Adsorption and desorption of chlorogenic acid by macroporous adsorbent resins during extraction of Eucommia ulmoides leaves. Ind. Crops Prod. 2020, 149, 112336. [Google Scholar] [CrossRef]
- Jiao, W.; Li, X.; Wang, X.; Cao, J.; Jiang, W. Chlorogenic acid induces resistance against Penicillium expansum in peach fruit by activating the salicylic acid signaling pathway. Food Chem. 2018, 260, 274–282. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Sharifi, S.; Tavakoli, F.; Hussain, Y.; Forouhandeh, H.; Khatibi, S.M.H.; Memar, M.Y.; Yekani, M.; Khan, H.; Goh, K.W.; et al. Curcumin-Loaded Silica Nanoparticles: Applications in Infectious Disease and Food Industry. Nanomaterials 2022, 12, 2848. [Google Scholar] [CrossRef]
- Gayathri, K.; Bhaskaran, M.; Selvam, C.; Thilagavathi, R. Nano formulation approaches for curcumin delivery—A review. J. Drug Deliv. Sci. Technol. 2023, 82, 104326. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. STAT3 pathway as a molecular target for resveratrol in breast cancer treatment. Cancer Cell Int. 2021, 21, 468. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Z.G.; Xiao, Z.G. Preparation, physicochemical characterization and in vitro release behavior of resveratrol-loaded oxidized gellan gum/resistant starch hydrogel beads. Carbohydr. Polym. 2021, 260, 117794. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. Iubmb Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Amevor, F.K.; Cui, Z.; Ning, Z.; Shu, G.; Du, X.; Jin, N.; Deng, X.; Xu, D.; Tian, Y.; Zhang, Y.; et al. Dietary quercetin and vitamin E supplementation modulates the reproductive performance and antioxidant capacity of aged male breeder chickens. Poult. Sci. 2022, 101, 101851. [Google Scholar] [CrossRef]
- Lee, D.-U.; Park, H.-W.; Lee, S.-C. Comparing the stability of retinol in liposomes with cholesterol, beta-sitosterol, and stigmasterol. Food Sci. Biotechnol. 2021, 30, 389–394. [Google Scholar] [CrossRef]
- Bento, C.; Matos, A.C.; Cordeiro, A.; Ramalho, A. Vitamin A deficiency is associated with body mass index and body adiposity in women with recommended intake of vitamin A. Nutr. Hosp. 2018, 35, 1072–1078. [Google Scholar] [CrossRef]
- Wusigale; Fu, X.; Yin, X.; Ji, C.; Cheng, H.; Liang, L. Effects of Folic Acid and Caffeic Acid on Indirect Photo-oxidation of Proteins and Their Costabilization under Irradiation. J. Agric. Food Chem. 2021, 69, 12505–12516. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Gu, L.; Chang, C.; Su, Y.; Liu, Y.; Yang, Y.; Dong, S. Degradation of 5-methyltetrahydrofolate in model and egg yolk systems and strategies for its stabilization. J. Food Sci. Technol. 2021, 58, 3473–3481. [Google Scholar] [CrossRef]
- Morscher, R.J.; Ducker, G.S.; Li, S.H.-J.; Mayer, J.A.; Gitai, Z.; Sperl, W.; Rabinowitz, J.D. Mitochondrial translation requires folate-dependent tRNA methylation. Nature 2018, 554, 128–132. [Google Scholar] [CrossRef]
- Van der Velden, U. Vitamin C and Its Role in Periodontal Diseases—The Past and the Present: A Narrative Review. Oral Health Prev. Dent. 2020, 18, 115–123. [Google Scholar] [CrossRef]
- Bedhiafi, T.; Idoudi, S.; Fernandes, Q.; Al-Zaidan, L.; Uddin, S.; Dermime, S.; Billa, N.; Merhi, M. Nano-vitamin C: A promising candidate for therapeutic applications. Biomed. Pharmacother. 2023, 158, 114093. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H. Astaxanthin and beta-carotene in Helicobacter pylori-induced Gastric Inflammation: A Mini-review on Action Mechanisms. J. Cancer Prev. 2017, 22, 57–61. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and extraction of carotenoids produced by microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Dai, Z.; Zhang, Z.; Feng, L.; Nie, M.; Liu, C.; Li, D.; Zhang, M. Study on the relationship between lutein bioaccessibility and in vitro lipid digestion of nanostructured lipid carriers with different interface structures. Food Hydrocoll. 2023, 139, 108569. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, J.; Jiang, L.; Ren, L.; Zhou, J. Encapsulation of Lutein into Starch Nanoparticles to Improve Its Dispersity in Water and Enhance Stability of Chemical Oxidation. Starch-Starke 2019, 71, 1800248. [Google Scholar] [CrossRef]
- Pelissari, J.R.; Souza, V.B.; Pigoso, A.A.; Tulini, F.L.; Thomazini, M.; Rodrigues, C.E.C.; Urbano, A.; Favaro-Trindade, C.S. Production of solid lipid microparticles loaded with lycopene by spray chilling: Structural characteristics of particles and lycopene stability. Food Bioprod. Process. 2016, 98, 86–94. [Google Scholar] [CrossRef]
- Liu, X.; Dilxat, T.; Shi, Q.; Qiu, T.; Lin, J. The combination of nicotinamide mononucleotide and lycopene prevents cognitive impairment and attenuates oxidative damage in D-galactose induced aging models via Keap1-Nrf2 signaling. Gene 2022, 822, 146348. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Z.; Hu, L. Recent technological strategies for enhancing the stability of lycopene in processing and production. Food Chem. 2023, 405, 134799. [Google Scholar] [CrossRef]
- Srimathi Priyanga, K.; Vijayalakshmi, K.J.A.J.P.C.R. Investigation of antioxidant potential of quercetin and hesperidin: An in vitro approach. Asian J. Pharm. Clin. Res. 2017, 10, 83. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Noratto, G.; Layosa, M.A.; Lage, N.N.; Atienza, L.; Ivanov, I.; Mertens-Talcott, S.U.; Chew, B.P. Antitumor potential of dark sweet cherry sweet (Prunus avium) phenolics in suppressing xenograft tumor growth of MDA-MB-453 breast cancer cells. J. Nutr. Biochem. 2020, 84, 108437. [Google Scholar] [CrossRef]
- Husain, A.; Chanana, H.; Khan, S.A.; Dhanalekshmi, U.M.; Ali, M.; Alghamdi, A.A.; Ahmad, A. Chemistry and Pharmacological Actions of Delphinidin, a Dietary Purple Pigment in Anthocyanidin and Anthocyanin Forms. Front. Nutr. 2022, 9, 746881. [Google Scholar] [CrossRef]
- Ye, J.-H.; Augustin, M.A. Nano- and micro-particles for delivery of catechins: Physical and biological performance. Crit. Rev. Food Sci. Nutr. 2019, 59, 1563–1579. [Google Scholar] [CrossRef]
- Yuann, J.-M.P.; Lee, S.-Y.; Yang, M.-J.; Huang, S.-T.; Cheng, C.-W.; Liang, J.-Y. A Study of Catechin Photostability Using Photolytic Processing. Processes 2021, 9, 293. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Y.-Q.; Gu, J.-H.; Song, R.; Cang, P.-H.; Xu, Y.-X.; Shao, X.-x.; Pu, L.-J.; Luo, H.-Y.; Zhou, X.-F. Novel oral edaravone attenuates diastolic dysfunction of diabetic cardiomyopathy by activating the Nrf2 signaling pathway. Eur. J. Pharmacol. 2022, 920, 174846. [Google Scholar] [CrossRef]
- Toupchian, O.; Abdollahi, S.; Salehi-Abargouei, A.; Heshmati, J.; Clark, C.C.T.; Sheikhha, M.H.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The effects of resveratrol supplementation on PPAR alpha, p16, p53, p21 gene expressions, and sCD163/sTWEAK ratio in patients with type 2 diabetes mellitus: A double-blind controlled randomized trial. Phytother. Res. 2021, 35, 3205–3213. [Google Scholar] [CrossRef]
- Venkatas, J.; Daniels, A.; Singh, M. The Potential of Curcumin-Capped Nanoparticle Synthesis in Cancer Therapy: A Green Synthesis Approach. Nanomaterials 2022, 12, 3201. [Google Scholar] [CrossRef]
- Samsamikor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2016, 47, 304–309. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Horvath, O.; Eros, K.; Sandor, B.; Urban, P.; Soos, S.; Marton, Z.; Sumegi, B.; Toth, K.; et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants 2020, 9, 1108. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Wang, Z.; Li, W. Ginsenoside Rg3 and Rh2 protect trimethyltin-induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation. Phytother. Res. 2018, 32, 2531–2540. [Google Scholar] [CrossRef]
- Ahsan, R.; Arshad, M.; Khushtar, M.; Ahmad, M.A.; Muazzam, M.; Akhter, M.S.; Gupta, G.; Muzahid, M. A Comprehensive Review on Physiological Effects of Curcumin. Drug Res. 2020, 70, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Reolon, J.B.; Brustolin, M.; Accarini, T.; Vicozzi, G.P.; Marcondes Sari, M.H.; Bender, E.A.; Haas, S.E.; Sperrotto Brum, M.C.; Gundel, A.; Colome, L.M. Co-encapsulation of acyclovir and curcumin into microparticles improves the physicochemical characteristics and potentiates in vitro antiviral action: Influence of the polymeric composition. Eur. J. Pharm. Sci. 2019, 131, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, A.; Gamrani, H. Neuronal, astroglial and locomotor injuries in subchronic copper intoxicated rats are repaired by curcumin: A possible link with Parkinson’s disease. Acta Histochem. 2018, 120, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Thi Thanh, N.; My Dung, V.; Man Anh, H.; Masamitsu, Y.; Linh Thuoc, T.; Thi Phuong Thao, D. Curcumin Effectively Rescued Parkinson’s Disease-Like Phenotypes in a Novel Drosophila melanogaster Model with dUCH Knockdown. Oxid. Med. Cell. Longev. 2018, 2018, 2038267. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Choi, W.; Ju Son, D.; Sung Kim, Y.; Kim, M.-K.; Yoon, B.-E.; Pyee, J.; Tae Hong, J.; Go, Y.-M.; et al. Antiatherogenic Effect of Resveratrol Attributed to Decreased Expression of ICAM-1 (Intercellular Adhesion Molecule-1) Mechanistic Link From Focal Adhesion to Monocyte Adhesion. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Gligorijevic, N.; Stanic-Vucinic, D.; Radomirovic, M.; Stojadinovic, M.; Khulal, U.; Nedic, O.; Cirkovic Velickovic, T. Role of Resveratrol in Prevention and Control of Cardiovascular Disorders and Cardiovascular Complications Related to COVID-19 Disease: Mode of Action and Approaches Explored to Increase Its Bioavailability. Molecules 2021, 26, 2834. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.S.; Javed Shaikh, M.A.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Almalki, W.H.; Singh, S.K.; Kumar, D.; Kumar, A.P.; Dua, K.; et al. Role of Flavonoids in Management of Various Biological Targets in Alzheimer’s Disease: Evidence from Preclinical to Clinical Studies. Curr. Med. Chem. 2023, 30, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.S. A study from structural insight to the antiamyloidogenic and antioxidant activities of flavonoids: Scaffold for future therapeutics of Alzheimer’s disease. Med. Chem. Res. 2023, 32, 15–31. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H.-m. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S. Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety. Glob. Chall. 2023, 7, 2200209. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Jones, O.G. Assembled protein nanoparticles in food or nutrition applications. Adv. Food Nutr. Res. 2019, 88, 47–84. [Google Scholar] [CrossRef]
- Han, L.; Lu, K.; Zhou, S.; Zhang, S.; Xie, F.; Qi, B.; Li, Y. Development of an oil-in-water emulsion stabilized by a black bean protein-based nanocomplex for co-delivery of quercetin and perilla oil. LWT Food Sci. Technol. 2021, 138, 110644. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Wu, G.; Li, P.; Zhang, H.; Qi, X.; Wang, L.; Qian, H. The characterization and stability of the soy protein isolate/1-Octacosanol nanocomplex. Food Chem. 2019, 297, 124766. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadli, F.; Nikzad, M.; Ghoreyshi, A.A.; Mohammadi, M.; Poureini, F. Preparation and Characterization of Zein/Sodium Caseinate/Xanthan Gum Complex for Encapsulation of Piperine and its In Vitro Release Study. Food Biophys. 2021, 16, 254–269. [Google Scholar] [CrossRef]
- Yang, S.; Dai, L.; Sun, C.; Gao, Y. Characterization of curcumin loaded gliadin-lecithin composite nanoparticles fabricated by antisolvent precipitation in different blending sequences. Food Hydrocoll. 2018, 85, 185–194. [Google Scholar] [CrossRef]
- Fan, Y.; Zeng, X.; Yi, J.; Zhang, Y. Fabrication of pea protein nanoparticles with calcium-induced cross-linking for the stabilization and delivery of antioxidative resveratrol. Int. J. Biol. Macromol. 2020, 152, 189–198. [Google Scholar] [CrossRef]
- Yi, J.; Peng, G.; Zheng, S.; Wen, Z.; Gan, C.; Fan, Y. Fabrication of whey protein isolate-sodium alginate nanocomplex for curcumin solubilization and stabilization in a model fat-free beverage. Food Chem. 2021, 348, 129102. [Google Scholar] [CrossRef]
- Carmelo-Luna, F.J.; Mendoza-Wilson, A.M.; Montfort, G.R.-C.; Lizardi-Mendoza, J.; Madera-Santana, T.; Gutierrez, D.L.; Quintana-Owen, P. Synthesis and experimental/computational characterization of sorghum procyanidins-gelatin nanoparticles. Bioorg. Med. Chem. 2021, 42, 116240. [Google Scholar] [CrossRef]
- Wang, C.; Ren, J.; Song, H.; Chen, X.; Qi, H. Characterization of whey protein-based nanocomplex to load fucoxanthin and the mechanism of action on glial cells PC12. LWT Food Sci. Technol. 2021, 151, 112208. [Google Scholar] [CrossRef]
- Penalva, R.; Esparza, I.; Agueros, M.; Gonzalez-Navarro, C.J.; Gonzalez-Ferrero, C.; Irache, J.M. Casein nanoparticles as carriers for the oral delivery of folic acid. Food Hydrocoll. 2015, 44, 399–406. [Google Scholar] [CrossRef]
- Zhao, C.; Wei, L.; Yin, B.; Liu, F.; Li, J.; Liu, X.; Wang, J.; Wang, Y. Encapsulation of lycopene within oil-in-water nanoemulsions using lactoferrin: Impact of carrier oils on physicochemical stability and bioaccessibility. Int. J. Biol. Macromol. 2020, 153, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef]
- Feng, J.; Xu, H.; Zhang, L.; Wang, H.; Liu, S.; Liu, Y.; Hou, W.; Li, C. Development of Nanocomplexes for Curcumin Vehiculization Using Ovalbumin and Sodium Alginate as Building Blocks: Improved Stability, Bioaccessibility, and Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Sonawane, S.; Potoroko, I.; Sonawane, S.H. Recent Advances in Ultrasound-Assisted Synthesis of Nano-Emulsions and their Industrial Applications. Curr. Pharm. Biotechnol. 2021, 22, 1748–1758. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of Oil Type on Nanoemulsion Formation and Ostwald Ripening Stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef]
- Prastuty; Kaur, G.; Singh, A. Shelf life extension of muffins coated with cinnamon and clove oil nanoemulsions. J. Food Sci. Technol. 2022, 59, 1878–1888. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, R.; Li, L. The interaction between anionic polysaccharides and legume protein and their influence mechanism on emulsion stability. Food Hydrocoll. 2022, 131, 107814. [Google Scholar] [CrossRef]
- Niknam, R.; Mousavi, M.; Kiani, H. Comprehensive evaluation of emulsifying and foaming properties of Gleditsia caspica seed galactomannan as a new source of hydrocolloid: Effect of extraction method. Food Hydrocoll. 2022, 131, 107758. [Google Scholar] [CrossRef]
- Wei, R.; Zhao, S.; Zhang, L.; Feng, L.; Zhao, C.; An, Q.; Bao, Y.; Zhang, L.; Zheng, J. Upper digestion fate of citrus pectin-stabilized emulsion: An interfacial behavior perspective. Carbohydr. Polym. 2021, 264, 118040. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, W.; Zhang, H.; Wang, H.; He, X. The oil/water interfacial behavior of microgels used for enhancing oil recovery: A comparative study on microgel powder and microgel emulsion. Colloids Surf. A 2022, 632, 127731. [Google Scholar] [CrossRef]
- Pandey, P.; Gulati, N.; Makhija, M.; Purohit, D.; Dureja, H. Nanoemulsion: A Novel Drug Delivery Approach for Enhancement of Bioavailability. Recent Pat. Nanotechnol. 2020, 14, 276–293. [Google Scholar] [CrossRef]
- Sabjan, K.B.; Munawar, S.M.; Rajendiran, D.; Vinoji, S.K.; Kasinathan, K. Nanoemulsion as Oral Drug Delivery—A Review. Curr. Drug Res. Rev. 2020, 12, 4–15. [Google Scholar] [CrossRef]
- Lyu, Z.; Wang, F.; Liu, P.; Zhang, K.; Sun, Q.; Bai, X.; Li, A.; Song, X. One-pot preparation of lutein block methoxy polyethylene glycol copolymer-coated lutein nanoemulsion. Colloid Polym. Sci. 2021, 299, 1055–1062. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, L.; Tan, L. Effectiveness of nanoscale delivery systems on improving the bioavailability of lutein in rodent models: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2375–2390. [Google Scholar] [CrossRef]
- Adriany, A.; Jessica, S.; Ana, O.; Raimunda, S.; Andreanne, V.; Luan, S.; Thiago, A.; Wanessa, C.; Maria, S.; Ana, M.; et al. Anti-inflammatory and antioxidant activity improvement of lycopene from guava on nanoemulsifying system. J. Dispers. Sci. Technol. 2021, 42, 760–770. [Google Scholar] [CrossRef]
- Zou, B.; Shao, C.; Shao, L.; Zhao, Y.; Dai, R.; Liu, Y. Preparation of lemon essential oil nanoemulsion and its effect on the microbial community of pork patties. J. Food Sci. 2023, 88, 2286–2300. [Google Scholar] [CrossRef]
- Li, J.; Guo, R.; Hu, H.; Wu, X.; Ai, L.; Wu, Y. Preparation optimisation and storage stability of nanoemulsion-based lutein delivery systems. J. Microencaps. 2018, 35, 570–583. [Google Scholar] [CrossRef]
- Salatin, S.; Jelvehgari, M.; Maleki-Dizaj, S.; Adibkia, K. A sight on protein-based nanoparticles as drug/gene delivery systems. Ther. Deliv. 2015, 6, 1017–1029. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y. Nanoparticles derived from plant proteins for controlled release and targeted delivery of therapeutics. Nanomedicine 2015, 10, 2001–2004. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zou, Y.; Peng, Y.; McClements, D.J.; Hu, K. Resveratrol-loaded biopolymer core-shell nanoparticles: Bioavailability and anti-inflammatory effects. Food Funct. 2020, 11, 4014–4025. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, X.; Tang, X.; Zhen, N.; Wang, Y.; Luo, Z.; Zhang, H.; Liu, J.; Zhou, D.; Huang, K. In vitro antioxidant and antitumor study of zein/SHA nanoparticles loaded with resveratrol. Food Sci. Nutr. 2021, 9, 3530–3537. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, Y.; Ouyang, X.-k.; Ling, J. Fabrication of soy protein isolate/cellulose nanocrystal composite nanoparticles for curcumin delivery. Int. J. Biol. Macromol. 2020, 165, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Yang, L. Nano-Hydrogel for the Treatment of Depression and Epilepsy. J. Biomed. Nanotechnol. 2022, 18, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.; Vinogradov, S.V.; Morrissey, P.; Chernin, M.; Ahmed, M.M. Curcumin-encapsulating Nanogels as an Effective Anticancer Formulation for Intracellular Uptake. Mol. Cell. Pharmacol. 2015, 7, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Mohsenabadi, N.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A. Physical and antimicrobial properties of starch-carboxy methyl cellulose film containing rosemary essential oils encapsulated in chitosan nanogel. Int. J. Biol. Macromol. 2018, 112, 148–155. [Google Scholar] [CrossRef]
- Singhal, P.; Vashisht, H.; Nisar, S.; Mehra, S.; Rattan, S. Stimulus responsive soy-protein based hydrogels through grafting HEMA for biomedical applications. Ind. Crops Prod. 2022, 178, 114621. [Google Scholar] [CrossRef]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Beaulieu, L.; Savoie, L.; Paquin, P.; Subirade, M. Elaboration and characterization of whey protein beads by an emulsification/cold gelation process: Application for the protection of retinol. Biomacromolecules 2002, 3, 239–248. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.X.; Zhang, C.; Dai, C.; Ju, X.; He, R. Fabrication of Stable and Self-Assembling Rapeseed Protein Nanogel for Hydrophobic Curcumin Delivery. J. Agric. Food Chem. 2019, 67, 887–894. [Google Scholar] [CrossRef]
- Aslzad, S.; Heydari, P.; Abdolahinia, E.D.; Amiryaghoubi, N.; Safary, A.; Fathi, M.; Erfan-Niya, H. Chitosan/gelatin hybrid nanogel containing doxorubicin as enzyme-responsive drug delivery system for breast cancer treatment. Colloid Polym. Sci. 2023, 301, 273–281. [Google Scholar] [CrossRef]
- Ding, X.; Yao, P. Soy Protein/Soy Polysaccharide Complex Nanogels: Folic Acid Loading, Protection, and Controlled Delivery. Langmuir 2013, 29, 8636–8644. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, M. Development of a nanoparticle delivery system based on zein/polysaccharide complexes. J. Food Sci. 2020, 85, 4108–4117. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.A.d.A.; Silva Neto, A.F.; Noronha, M.C.S.; de Lima, M.F.; Cavalcanti, I.M.F.; Santos-Magalhaes, N.S. Zein nanoparticles for drug delivery: Preparation methods and biological applications. Int. J. Pharm. 2023, 635, 122754. [Google Scholar] [CrossRef] [PubMed]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Kharanaghi, E.A.; Arough, J.M.; Zandieh-Doulabi, B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 383–400. [Google Scholar] [CrossRef]
- Martinez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodriguez, S.A.; Alvarez Roman, R.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci. Technol. 2013, 34, 109–123. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Wang, Q. Nanoparticles Synthesized from Soy Protein: Preparation, Characterization, and Application for Nutraceutical Encapsulation. J. Agric. Food Chem. 2012, 60, 2712–2720. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Jiang, X.; Gui, Z.; Zhang, L. Development of Hydrophilic Drug Encapsulation and Controlled Release Using a Modified Nanoprecipitation Method. Processes 2019, 7, 331. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y. Heat-induced self-assembly of zein nanoparticles: Fabrication, stabilization and potential application as oral drug delivery. Food Hydrocoll. 2019, 90, 403–412. [Google Scholar] [CrossRef]
- Chen, F.-P.; Li, B.-S.; Tang, C.-H. Nanocomplexation between Curcumin and Soy Protein Isolate: Influence on Curcumin Stability/Bioaccessibility and in Vitro Protein Digestibility. J. Agric. Food Chem. 2015, 63, 3559–3569. [Google Scholar] [CrossRef] [PubMed]
- Ebert, S.; Koo, C.K.W.; Weiss, J.; McClements, D.J. Continuous production of core-shell protein nanoparticles by antisolvent precipitation using dual-channel microfluidization: Caseinate-coated zein nanoparticles. Food Res. Int. 2017, 92, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Li, J.; Lai, Z.; Gao, F.; Zeng, Z.; Zhao, X.; Liu, G.; Cui, H. Emamectin benzoate-loaded zein nanoparticles produced by antisolvent precipitation method. Polym. Test. 2021, 94, 107020. [Google Scholar] [CrossRef]
- Zada, M.H.; Rottenberg, Y.; Domb, A.J. Peptide loaded polymeric nanoparticles by non-aqueous nanoprecipitation. J. Colloid Interface Sci. 2022, 622, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; Joye, I.J.; McClements, D.J. Food-Grade Protein-Based Nanoparticles and Microparticles for Bioactive Delivery: Fabrication, Characterization, and Utilization. Adv. Protein Chem. Struct. Biol. 2015, 98, 293–325. [Google Scholar] [PubMed]
- Dai, L.; Zhou, H.; Wei, Y.; Gao, Y.; McClements, D.J. Curcumin encapsulation in zein-rhamnolipid composite nanoparticles using a pH-driven method. Food Hydrocoll. 2019, 93, 342–350. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Zhang, J.; Zhang, M.; Cheng, J.; Guo, M. Physicochemical and in vitro digestion properties of soy isoflavones loaded whey protein nanoparticles using a pH-driven method. Innov. Food Sci. Emerg. Technol. 2022, 82, 103209. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, L.; Cai, Q.; Zou, L.; Liu, C.; Liu, W.; McClements, D.J. Utilization of biopolymers to stabilize curcumin nanoparticles prepared by the pH-shift method: Caseinate, whey protein, soy protein and gum Arabic. Food Hydrocoll. 2020, 107, 105963. [Google Scholar] [CrossRef]
- Xu, G.; Li, L.; Bao, X.; Yao, P. Curcumin, casein and soy polysaccharide ternary complex nanoparticles for enhanced dispersibility, stability and oral bioavailability of curcumin. Food Biosci. 2020, 35, 100569. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- Korkut, O.C.; Ozdemir, G. Encapsulation of resveratrol in rhamnolipid-zein nanoparticles using a pH-driven method: Kinetic modeling on controlled release from nanoparticles. J. Dispers. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, P.; Xu, X.; Zhou, G. pH-shifting encapsulation of curcumin in egg white protein isolate for improved dispersity, antioxidant capacity and thermal stability. Food Res. Int. 2020, 137, 109366. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, P.K.M.; Nidamanuri, B.S.S.; Balan, A.P.; Venkatachalam, S.; Jawahar, N. A review on structure, preparation and applications of silk fibroin-based nano-drug delivery systems. J. Nanopart. Res. 2022, 24, 141. [Google Scholar] [CrossRef]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- Lammel, A.S.; Hu, X.; Park, S.-H.; Kaplan, D.L.; Scheibel, T.R. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010, 31, 4583–4591. [Google Scholar] [CrossRef]
- Farrell, L.-L.; Pepin, J.; Kucharski, C.; Lin, X.; Xu, Z.; Uludag, H. A comparison of the effectiveness of cationic polymers poly-L-lysine (PLL) and polyethylenimine (PEI) for non-viral delivery of plasmid DNA to bone marrow stromal cells (BMSC). Eur. J. Pharm. Biopharm. 2007, 65, 388–397. [Google Scholar] [CrossRef]
- Geranpour, M.; Assadpour, E.; Jafari, S.M. Recent advances in the spray drying encapsulation of essential fatty acids and functional oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Shyu, S.-S.; Su, F.-H.; Yu, P.-C. Development of biodegradable co-poly(d,l-lactic/glycolic acid) microspheres for the controlled release of 5-FU by the spray drying method. Colloids Surf. B. Biointerfaces 2002, 25, 269–279. [Google Scholar] [CrossRef]
- Schafroth, N.; Arpagaus, C.; Jadhav, U.Y.; Makne, S.; Douroumis, D. Nano and microparticle engineering of water insoluble drugs using a novel spray-drying process. Colloids Surf. B Biointerfaces 2012, 90, 8–15. [Google Scholar] [CrossRef]
- Bohr, A.; Boetker, J.P.; Rades, T.; Rantanen, J.; Yang, M. Application of spray-drying and electrospraying/electospinning for poorly water-soluble drugs: A particle engineering approach. Curr. Pharm. Des. 2014, 20, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, L.M.; Araguez-Fortes, Y.; Pinoa, J.A. Microencapsulation of vegetable oils by spray drying. Afinidad 2022, 79, 326–337. [Google Scholar]
- Weng, Y.; Li, Y.; Chen, X.; Song, H.; Zhao, C.-X. Encapsulation of enzymes in food industry using spray drying: Recent advances and process scale-ups. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.A.; Arpagaus, C. Nano Spray-Dried Drugs for Oral Administration: A Review. Assay Drug Dev. Technol. 2021, 19, 412–441. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-O.; Kim, D.; Lim, J.D.; Ko, S.; Hong, G.-P.; Lee, S. Functional enhancement of ultrafine Angelica gigas powder by spray-drying microencapsulation. LWT Food Sci. Technol. 2019, 101, 161–166. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.J.; Wang, D.Q. Dual effects of Tween 80 on protein stability. Int. J. Pharm. 2008, 347, 31–38. [Google Scholar] [CrossRef]
- Salama, R.O.; Traini, D.; Chan, H.-K.; Sung, A.; Ammit, A.J.; Young, P.M. Preparation and Evaluation of Controlled Release Microparticles for Respiratory Protein Therapy. J. Pharm. Sci. 2009, 98, 2709–2717. [Google Scholar] [CrossRef]
- Park, C.-W.; Song-Yi, H.; Hyun-Woo, N.; PureunnaraeSeo; Seung-Hwan, L. Effect of Spray-drying Condition and Surfactant Addition on Morphological Characteristics of Spray-dried Nanocellulose. J. For. Environ. Sci. 2017, 33, 33–38. [Google Scholar] [CrossRef]
- Lee, S.H.; Heng, D.; Ng, W.K.; Chan, H.-K.; Tan, R.B.H. Nano spray drying: A novel method for preparing protein nanoparticles for protein therapy. Int. J. Pharm. 2011, 403, 192–200. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Perez-Masia, R.; Lopez-Nicolas, R.; Jesus Periago, M.; Ros, G.; Lagaron, J.M.; Lopez-Rubio, A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015, 168, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Benetti, J.V.M.; do Prado Silva, J.T.; Nicoletti, V.R. SPI microgels applied to Pickering stabilization of O/W emulsions by ultrasound and high-pressure homogenization: Rheology and spray drying. Food Res. Int. 2019, 122, 383–391. [Google Scholar] [CrossRef]
- Zhou, F.-Z.; Yan, L.; Yin, S.-W.; Tang, C.-H.; Yang, X.-Q. Development of Pickering Emulsions Stabilized by Gliadin/Proanthocyanidins Hybrid Particles (GPHPs) and the Fate of Lipid Oxidation and Digestion. J. Agric. Food Chem. 2018, 66, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Zhu, G.; Huang, G.; Shen, X.; Zhang, Y.; Jiang, L.; Sui, X. A novel pickering emulsion produced using soy protein-anthocyanin complex nanoparticles. Food Hydrocoll. 2020, 99, 105329. [Google Scholar] [CrossRef]
- Feng, X.; Sun, Y.; Yang, Y.; Zhou, X.; Cen, K.; Yu, C.; Xu, T.; Tang, X. Zein nanoparticle stabilized Pickering emulsion enriched with cinnamon oil and its effects on pound cakes. LWT Food Sci. Technol. 2020, 122, 109025. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. The potential of zein nanoparticles to protect entrapped β-carotene in the presence of milk under simulated gastrointestinal (GI) conditions. LWT Food Sci. Technol. 2016, 72, 302–309. [Google Scholar] [CrossRef]
- Sneharani, A.H. Curcumin-sunflower protein nanoparticles-A potential antiinflammatory agent. J. Food Biochem. 2019, 43, e12909. [Google Scholar] [CrossRef]


| Classification | Designation | Primary Sources | Functional Properties | Restrictions | Ref. |
|---|---|---|---|---|---|
| Polyphenols | gallic acid | Fruit, green tea, nuts, red wine | Antibacterial, anti-viral, anticancer, anti-ulcer, anti-allergy | Poor bioavailability and rapid metabolism in vivo | [33,34,35] |
| chlorogenic acid | Coffee beans, apples, pears, honeysuckle | Antioxidant, anti-inflammatory, hypotensive, anti-diabetic, intestinal regulator | Easy to hydrolyze under alkaline and high-temperature environment, likely to oxidize when exposed to light and heat | [36,37] | |
| Curcumin | Turmeric, curry | Antibacterial, anti-inflammatory, anti-tumor, anti-liver fibrosis, hypolipidemic, anti-coagulant | Poor water solubility, low solubility, fast metabolism, easy inactivation under acid and alkaline conditions | [38,39] | |
| Resveratrol | Grapes, peanuts, knotweed, red wine | Antioxidant, anti-inflammatory, anticancer, heart and nervous system protection | Low water solubility, poor stability, easy to degrade under acidic or light conditions | [40,41] | |
| Vitamins | Tocopherol | Soybean, corn, alfalfa, wheat germ oil | Antioxidant, anti-inflammatory, maintain fertility and nervous system function, improve immunity | Insoluble in water, sensitive to oxygen, unstable under alkaline conditions | [42,43] |
| Retinol | Carrots, spinach, pumpkin, animal liver | Maintains visual function, regulates cell differentiation and apoptosis, and preserves epithelial tissue cell health | Poor water solubility, sensitive to light, heat, oxygen, metal ions and acidic environment, susceptible to oxidative degradation | [44,45] | |
| Folic acid | Green vegetables, fruits, legumes, eggs, fish | Participates in amino acid conversion and provides nutrients for cell division, a deficiency of which leads to increased risk of megaloblastic anemia, atherosclerosis and central nervous system diseases | Unstable under light (UV), heat, acidic environment, easy oxidative degradation, limited bioavailability | [46,47,48] | |
| Vitamin C | Fresh vegetables (potatoes, tomatoes), fruits (oranges, apples, pineapples) | Anti-oxidation, anti-scurvy, anticancer, improve human immunity, prevent and treat anemia, and participate in collagen synthesis | Unstable in aqueous solution and air, easily oxidized and degraded, vulnerable to destruction under high temperature | [49,50] | |
| Natural pigments | β-carotene | Carrots, spinach, broccoli, soybeans, goji berries | Antioxidant, anti-inflammatory, anti-tumor, immune system, heart disease prevention | Poor water solubility, easy chemical degradation under oxygen, high temperature, and sufficient light | [51,52] |
| Lutein | Corn, pumpkin, kale, orange, algae | Antioxidant, anti-inflammatory, anti-mutagenic, retinal protection, cataract retardation | Insoluble in water, sensitive to light, oxygen, and high temperature, easily degraded by oxidation | [53,54] | |
| Lycopene | Tomato, watermelon, grapefruit, papaya | Antioxidant, anticancer, scavenging free radicals, slowing down aging, preventing cardiovascular diseases, protecting the central nervous system | Poor water solubility and stability, easily degraded by light, heat, oxygen, metal ions and other environmental factors | [55,56,57] | |
| Flavonoids | Quercetin | Vegetables (onions, potatoes), fruits (pomegranates, hawthorn), herbs (ginkgo biloba, mulberry leaves) | Antioxidant, antibacterial, anti-inflammatory, expectorant, cough suppressant, immune system enhancement | Slightly insoluble in water, sensible to heat and alkaline environment, weak bioavailability | [58,59,60] |
| Anthocyanidin | Black wolfberry, blueberry, mulberry, grape, black fungus, black rice | Free radical scavenging, anti-inflammatory, anticancer, antibacterial | Sensitive to light, heat, and oxygen | [61,62] | |
| Catechin | Tea, apples, grapes chocolate | Antioxidant, anti-inflammatory, antibacterial, anti-aging, prevention of cardiovascular disease and diabetes | Unstable in aqueous solutions and neutral and acidic environments, highly susceptible to oxidation | [63,64] |
| Sources | Protein Material | Nanoparticles | Particle Size | Advantages | Refs. |
|---|---|---|---|---|---|
| Plant proteins | Black bean protein | Black bean protein-quercetin nanoemulsion | 278.7 nm | Smaller particle size, lower viscosity, and better emulsification performance in the compound emulsion effectively control the release of quercetin and perilla oil during gastrointestinal digestion. | [83] |
| Soy protein isolate | Soybean protein isolate-1-octacosanol nanocomplex | 70–100 nm | Nanocomplexes are uniformly dispersed in the aqueous phase and have excellent thermal and salt ion stability. | [84] | |
| Zein | Zein-sodium caseinate-xanthan gum nanocomplexes loaded with piperine | 145.9 nm | Improved piperine’s water solubility and stability, significantly enhanced antioxidant activity. | [85] | |
| Gliadin | Curcumin-loaded gliadin-lecithin composite nanoparticles | 250–280 nm | Protection of curcumin in nanoparticles from UV and heat treatment damage | [86] | |
| Pea protein isolate | Pea protein isolate -resveratrol nanoparticles | 191.2 nm | Improves the physicochemical stability and antioxidant capacity of resveratrol | [87] | |
| Animal proteins | Whey protein isolate | Whey-isolated protein-sodium alginate nanocomplexes loaded with curcumin | 209.9 nm | The highest loading amount of curcumin in nanocomplex was 15.26 μg/mg; nanocomplexes exhibit superior stability under high sugar, salt, and high-temperature heat treatments | [88] |
| Gelatin | Gelatin-procyanidin nanogel | 22–138 nm | The antioxidant activity of procyanidin (PC) is protected. PC remains stable in vitro in simulated gastrointestinal digestion. | [89] | |
| Whey protein | Whey protein-based-fucoxanthin nanocomplex | 350 nm | High fucoxanthin (FX) encapsulation rate (96.19%), enhanced FX stability to ultraviolet B, heat, NaCl, and pH, efficient FX delivery to glial cells PC12 | [90] | |
| Casein | Casein-folic acid nanoparticles | 150 nm | Protects the release of folic acid in the intestine, bioavailability of folic acid in nanoparticles is close to 52%, 50% higher than conventional aqueous solutions | [91] | |
| Lactoferrin | Lactoferrin-lycopene nano-emulsion | 200–300 nm | Better stability, slower degradation, superior retention of lycopene, and remarkably improved bioaccessibility | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Liu, K.; Liu, X.; Rashid, M.T.; Zhang, H.; Wang, M. Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods 2023, 12, 2999. https://doi.org/10.3390/foods12162999
Han M, Liu K, Liu X, Rashid MT, Zhang H, Wang M. Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods. 2023; 12(16):2999. https://doi.org/10.3390/foods12162999
Chicago/Turabian StyleHan, Mengqing, Kunlun Liu, Xin Liu, Muhammad Tayyab Rashid, Huiyan Zhang, and Meiyue Wang. 2023. "Research Progress of Protein-Based Bioactive Substance Nanoparticles" Foods 12, no. 16: 2999. https://doi.org/10.3390/foods12162999
APA StyleHan, M., Liu, K., Liu, X., Rashid, M. T., Zhang, H., & Wang, M. (2023). Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods, 12(16), 2999. https://doi.org/10.3390/foods12162999