Abstract
The need to improve the safety/quality of food and the health of the hosts has resulted in increasing worldwide interest in acidophilic lactic acid bacteria (LAB) for the food, livestock as well as health industries. In addition to the use of acidophilic LAB with probiotic potential for food fermentation and preservation, their application in the natural disposal of acidic wastes polluting the environment is also being investigated. Considering this new benefit that has been assigned to probiotic microorganisms in recent years, the acceleration in efforts to identify new, efficient, promising probiotic acidophilic LAB is not surprising. One of these effots is to determine both the beneficial and harmful compounds synthesized by acidophilic LAB. Moreover, microorganisms are of concern due to their possible hemolytic, DNase, gelatinase and mucinolytic activities, and the presence of virulence/antibiotic genes. Hence, it is argued that acidophilic LAB should be evaluated for these parameters before their use in the health/food/livestock industry. However, this issue has not yet been fully discussed in the literature. Thus, this review pays attention to the less-known aspects of acidophilic LAB and the compounds they release, clarifying critical unanswered questions, and discussing their health benefits and safety.
1. Introduction
The use of living microorganisms for beneficial purposes on the host dates back to ancient times [1]. Among these microorganisms, those that adapt to living in an acidic pH are called low-pH microorganisms [2]. Acidophilic lactic acid bacteria (LAB) are the most commonly used low-pH microorganisms in the healthcare, pharmaceutical, and food industries [3].
As a new benefit of probiotic microorganisms for human health and the food industry has been presented in recent years, the acceleration in the efforts to identify new, efficient, promising probiotic acidophilic LAB is not surprising [4,5,6].
Fermentation, which increases the shelf life and microbiological safety of foods, as well as making some foods more digestible, is a widely applied method [7]. At present, fermentation is completed under controlled conditions with carefully selected strains [8]. Acidophilic microorganisms are generally used in the production of fermented products [9]. In this respect, the identification of new acidophilic–aciduric microorganisms, which are effective and can be safely applied in the fermentation process, can improve food product quality.
The beneficial effects of some acidophilic LAB and traditional fermented foods on human health are mostly attributed to the compounds they release, such as organic acids, some B-group vitamins, gamma-aminobutyric acid (GABA), amylase enzyme, and bacteriocins [10,11,12]. However, the presence of some toxic compounds (such as biogenic amines) released by acidophilic LAB is also known [13,14]. The identification of acidophilic strains that release beneficial compounds and do not release harmful compounds can contribute to the uses of acidophilic LAB, especially in the health and food industry. Thus, it is thought that acidophilic microorganisms, whose beneficial effects and safety have been determined, may be a new strategy for the bio-enrichment of foods and a cost-effective alternative to existing fortification programs.
New application areas of acidophilic LAB are rapidly being discovered and developed. For example, the natural disposal of acidic wastes polluting the environment as a result of many human activities, especially industrial processes, by acidophilic microorganisms is one of the innovative solutions being developed [15,16].
The safety of all microorganisms, including acidophilic LAB, should be evaluated and confirmed before use [5,17]. Non-hemolytic, non-DNase, non-gelatinase, non-mucinolytic activities, and the absence of virulence/antibiotic-resistance genes, are considered safety prerequisites for the selection of probiotic strains [18,19,20,21,22]. The transfer of unwanted genes to pathogenic bacteria is one of the crucial health risks that are emphasized [18,19,20,21]. As the possible health risks of acidophilic LAB are being determined, it is becoming increasingly important to evaluate their safety and reveal safe strains before their use in the food industry, livestock, and health sector.
Although there are some studies examining the usage areas, health benefits, released compounds, and safety concerns of acidophilic LAB in the literature, the absence of a comprehensive review, in which they are evaluated together, reveals the importance of this review. Therefore, the current review focuses on the unknown aspects of acidophilic LAB, clarifies critical unanswered questions, and pioneers the development of new alternative strategies/functional products based on acidophilic probiotic LAB and their metabolites.
2. Overview of Acidophilic Microorganisms
Microorganisms are a group of living creatures that develop or adapt to very different environmental conditions. The conditions under which these creatures optimally develop relate to acidity, high/low temperature, salt concentration, etc. [23]. Some of the microorganisms prefer to live in an acidic environment (acidophilic organisms), while others prefer an alkaline pH (alkaliphilic organisms) [24]. Acidophiles living optimally below the pH-neutral level (7.0) are also divided into different subgroups. Among these, acid-tolerant ones can live above pH 5.0, while optimum conditions are pH 3.0 or below for extreme acidophiles. However, moderate acidophiles reach optimum living conditions between pH 3.0 and 5.0, and hyper-acidophiles reach optimum living conditions below pH 1.0 [2].
Extremophiles are commonly defined as microorganisms that can survive under conditions of severe heat, pH, salt concentration, etc., including extreme acidophiles. These organisms are a significant subject for an innovative study. The idea is inspired by the potential to use these microorganisms in biotechnological and industrial applications, which increases interest in this topic. Very acidic wastes are released into the environment as a result of many human activities, particularly mining, construction, and other industrial processes. The numerous hazardous compounds in these acidic wastes and wastewater are harmful to both people and other organisms. Acidophilic microorganisms are considered good options for the storage of these wastes [15].
Only two species of extreme acidophilic microorganisms had been isolated and characterized by the middle of the 20th century, while, by the beginning of the 21st, more than 50 species had been identified. As they can use different energy sources (solar, organic, and inorganic chemicals), electron-acceptors (oxygen, ferric iron, and sulfur), and carbon sources, acidophiles as a group are now known to be extremely physiologically and metabolically heterogeneous [2].
The main reason why low, acidic conditions are optimal for acidophiles is their cellular adaptation to regulate pH. Many extracellular enzymes derived from acidophiles are known to be functional at a much lower pH than cytoplasmic pH. It is also stated that enzymes such as amylases, proteases, ligases, cellulases, xylanases, α-glucosidases, endoglucanases and esterases, obtained from acidophilic microorganisms, are stable at a low pH [25]. Although they can only grow at pH 2–4, acidophilic bacteria can maintain their cytoplasmic pH at or above 6.0. It is essential to understand the mechanism by which this significant pH difference (ΔpH) is maintained. It is well-known that maintaining this significant transmembrane pH demands energy and that the transmembrane electric potential has to be different from that of neutrophilic bacteria [26]. The highly impermeable cell membrane is one of the many mechanisms that acidophilic microorganisms use to restrict the passage of protons in order to survive in acidic conditions [27]. For instance, both Thiobacillus acidophilus and Bacillus acidocaldarius showed a reverse transmembrane potential. The cytoplasmic pH can remain constant thanks to this transmembrane potential. L. acidophilus has a high cytoplasmic buffering capacity and is responsible for pH homeostasis. When a large influx of H+ occurs, the cytoplasmic buffering capacity prevents drastic changes in pH. The resulting increase in positive membrane potential due to this H+ influx eventually leads to the cessation of further H+ fluxes [26,28].
There are numerous species and subspecies of acidophilic bacteria, as well as many acidophilic microorganisms, surviving under extremely acidic conditions. The pH levels in the food matrix, however, are not as high as those found in harsh natural environments. For this reason, LAB and their subspecies are of great importance as acidophilic microorganisms in the food matrix. Important acidophilic species can mainly be found in the Lactobacillus subgroup.
LAB are rod, cocci and coccobacillus, Gr (+), immobile, catalase (−), microaerophilic or anaerobic microorganisms, with many strains and species. They are also acid-resistant, strongly fermentative, do not reduce nitrates, and need glucose and ammonium as well as some vitamins and amino acids for their growth and development [3,29] Lactobacilli are also a group of Gram-positive, acid-tolerant, non-sporulating, non-respiratory, rod or cocci-shaped bacteria that share common metabolic and physiological characteristics with LAB. These bacteria are commonly found in rotting plants and dairy products, producing lactic acid as the main metabolic product of carbohydrate fermentation [9].
For food fermentation and preservation, LAB are the most frequently used microorganisms. While consuming food, they undergo basically safe metabolic processes that use the available carbohydrates to produce organic acids and other metabolites. They are crucial for the food industry in this regard [3].
LAB, which are frequently used as a starter in foods, follow three main metabolic pathways during fermentation. These are glycolysis (sugar fermentation), lipolysis (degradation of fats) and proteolysis (degradation of proteins). The development of the aroma of dairy products, especially as a result of proteolytic activity, is also important for the food industry [30]. Studies have shown that microorganisms can improve and increase the production of vitamins and flavors, as well as the acidification rate and acid tolerance [31]. Organisms adapted to endure extreme pH conditions have also been shown to be suitable for industrial applications. Many acidophilic microorganisms, some adapted to life at high temperatures, naturally produce enzymes that can degrade polymeric or oligomeric carbon sources [16]. These properties make them preferable for applications in lignocellulosic biorefineries, as well as in the food and textile industries [25].
The proper classification and monitoring of acidophilic metabolic processes also prevents the generation of undesirable metabolic byproducts during food production. For instance, it is vital to know how to control fermentation to prevent the formation of lactic acid and undesirable byproducts. It is also essential to know how LAB work in drinks such as wine and acidic fruit juices [32].
3. Acidophilic Lactic Acid Bacteria in Foods
3.1. Their Utilization in Fermentation Technology as Starter Cultures
The preservation of foods by fermentation is an ancient, widely practiced method. Fermentation increases the shelf life and microbiological safety of a food [33]. Fermentation also yields new products.
The production of these foods in the past was traditional and secretive due to the long history of fermented foods. Today, fermentation is carried out under controlled conditions with strains that have been carefully chosen [32]. Traditionally, fermentation was carried out by inoculation from the previous batch. However, at present, there are starter cultures for fermentation, thus standardizing the process and product quality.
LAB play a role in the fermentation processes of milk, meat, grains and vegetables owing to their metabolic properties [33]. However, the optimum pH for the growth of most LAB is close to neutral. Therefore, it should be noted that most LAB are neutrophilic. However, some bacterial species, such as Lactobacillus and Oenococcus, show more acidophilic behavior [34]. Lactobacilli are usually aciduric or acidophilic. These microorganisms are also strictly fermentative, oxygen-tolerant or anaerobic and have complex nutritional requirements, including carbohydrates, amino acids, peptides, fatty acid esters, salts, nucleic acid derivatives and vitamins [35].
LAB are also significant in animal foods. In the past, straw was used to store grass for use as animal feed. At present, animal feed preserves more nutrients when converted into silage. This process was only made possible when the fermentation of lactic acid bacteria was understood [32]. In this way, acidophilic LAB are used as a starter culture in the production of food.
Lactobacillus delbrueckii subsp. Bulgaricus, used as a starter culture, is an important Lactobacilli. This microorganism is a Gram-positive rod, non-motile, and does not form spores. It is considered either aciduric or acidophilic. It requires a low pH (approximately 5.4–4.6) to grow effectively in the optimum temperature range from 43 to 46 °C [9]. Lactobacillus delbrueckii subsp. bulgaricus is widely used together with Streptococcus thermophilus as a starter culture in yogurt production [36]. However, studies have shown that a wide variety of LAB have been isolated. Aslam and Qazi (2010) isolated Lactobacillus delbrueckii subsp. bulgaricus, Lacticaseibacillus casei, Lactobacillus acidophilus and Ligilactobacillus salivarius from local yogurts in their study and reported that they have high acid tolerance properties [37]. Similarly, Latilactobacillus sakei is a species of microorganism from the same family, which is a facultative heterofermentative that can produce alcohol or lactic acid from sugars. It is used as a starter culture in meat products [38]. For instance, the genus Lactobacillus, which is frequently found in meat and processed food, as well as different fermented food products, includes the species Lactiplantibacillus plantarum. Sauerkraut, pickles, pickled olives, Korean kimchi, Nigerian ogis, sourdough and some cheeses, fermented sausages, and pickled olives contain Lactiplantibacillus plantarum. A homofermentative genus of LAB is called Lactococcus. They can be modified by changing environmental factors such as pH, glucose concentration, and nutrient limitations due to their homofermentative nature. They are a Gram-positive, catalase-negative, dormant cocci in single, double or chain forms [9]. These organisms are widely used in the dairy industry in the production of fermented milk products such as cheese. They can be used in starter cultures with single strains or mixed strains with other LAB, such as Lactobacillus and Streptococcus. Lactococcus lactis and its subspecies lactis and cremoris are important starter cultures in industrial dairy fermentation [39].
Another LAB, the Weissella species, has been isolated from a wide range of habitats, including milk, vegetables, and different fermented foods such as European sourdoughs and traditional Asian and African fermented foods [9]. Important species of acidophilic and aciduric LAB, along with the food sources from which they were obtained, are shown in Table 1.

Table 1.
Important species of acidophilic and aciduric LAB with the food sources (adapted from the references [31,40]).
3.2. Food Safety and Stability Issues
In traditional fermented food production, the food is produced by inoculating the fermented food that is produced beforehand. However, this situation is mostly valid for home-type food production at present. One of the important steps to ensure food safety and stabilization in large-scale, standard fermented food production is the use of starter cultures. Acidophilic–aciduric microorganisms are also involved in various production processes for fermented foods. In this situation, acidophilic LAB are used as a starter culture for several purposes. Producing fermented dairy products such as meat, fish, fruit, vegetable, and cereal products is the major objective of the usage of acidophilic LAB. Another objective is to enhance the nutritional value, flavor, and texture of fermented foods by producing flavor compounds. In this regard, the maturation process is very significant. The most prevalent acidophilic LAB identified in ripened cheeses are Pediococcus acidilactici and Pediococcus pentosaceus [41]. Despite not being a starter, acidophilic LAB can be isolated from many foods. For example, nonstarter Lacticaseibacillus casei, which has a wide pH and temperature range, can be found in ripe cheddar cheese and Sicilian green olives [9].
In addition, another purpose of the use of acidophilic LAB, which is used as a starter culture, is the support of food hygiene. The majority of acidophilic LAB live independently of any host species, are not pathogenic, and produce no products that are toxic or unpleasant to humans. Therefore, people can consume fermented foods formed by the growth of acidophilic LAB. The reducing conditions provided by these bacteria and low-pH habitats with high concentrations of fermented acids can inhibit the growth of many bacteria [32,42]. For example, they have been reported to inhibit the growth of Listeria monocytogenes and Escherichia coli O157:H7 in the processed meat products from which Latilactobacillus sakei is isolated [38].
It is clear that the fermentation process used by acidophilic LAB improves food flavor, aroma, and hygiene. It should be noted that some acidophilic and aciduric microorganisms contribute to food spoilage. Bacillus coagulans, a microaerophilic, slightly acidophilic, and heat-tolerant species, was isolated from spoiled canned milk and first described in 1915. This microorganism is highly suitable for development in acidic food due to its acidophilic nature. Moreover, it has been isolated from this type of food and is usually identified as the cause of spoilage in dairy products, vegetables, and fruits. High levels of lactic acid are produced as a result of this type of degradation [43]. Bacillus coagulans is also a thermophile, but differs from Geobacillus stearothermophilus (previously Bacillus stearothermophilus) as it can grow at pH values below 4.0 [44]. During deterioration, it causes the product to become tasteless and sour. Generally speaking, Bacillus coagulans has caused significant economic losses for the food industry due to flat-sour spoilage in canned foods [45]. Similarly, Alicyclobacillus acidoterrestris is an obligate acidophilic that grows optimally at pH 3.5–4.0 and has a pH of 2.5–5.5 for growth. Alicyclobacillus is the only spore-producing acidophilic genus that has been described as a cause of spoilage at these pH values to date [9]. Alicyclobacillus acidoterrestris spores are generally more heat resistant than other acidophilic spore formers and cause the spoilage of processed fruit and vegetable juices and concentrates [46,47]. Some species in the genus Alicyclobacillus have greater perishability as they can produce large amounts of guaiacol, which negatively affects the odor of the product [47]. In addition, butyric anaerobes, Clostridium butyricum, Clostridium beijerinckii and Clostridium pasteurianum, which cause spoilage in low-acid canned foods, are generally associated with the spoilage of products with pH values between 3.9 and 4.5 [9].
In conclusion, acidophilic microorganisms can often result in losses, food deterioration, and changes in food stability. These different acidophilic and aciduric microorganism states occupy different places on the benefit–harm axis. The importance of using acidophilic LAB as a starter culture in the production of fermented foods, in the development of new foods, and in the formation of the texture, taste, aroma and odor of products, is indisputable. Moreover, they improve food hygiene by limiting other microbial species’ ability to reproduce by balancing the pH of the food. In acidic food production lines, where production hygiene and processes are taken into consideration, the hazards produced by acidophilic LAB can be minimized, ensuring that the negative impacts of these microorganisms on food safety and stability do not occur.
3.3. Effects of Acidophilic Lactic Acid Bacteria on the Nutritional Value of Foods
It is known that acidophilic LAB are generally used in the production of fermented products. In this regard, the expected benefits and effects of the fermentation process can often be evaluated as the benefits provided by acidophilic LAB.
The most important output of the fermentation process is the production of organic acids. These organic acids (lactic acid, acetic acid, formic acid, propionic acid) are important food preservatives [48]. The antagonistic activity of acidophilic LAB against other microorganisms is due to the metabolites they produce, such as bacteriocin [49]. Thus, acidophilic LAB provide hygienic conditions by preventing and even eliminating food pathogens [40], since acidophilic LAB are used as bioprotective cultures [50]. Acidophilic LAB provide hygienic and organoleptic benefits, especially in fermented foods such as yogurt, wine and cheese [28]. In addition to its known benefits, acidophilic LAB can bind heavy metals in the water and environmental matrix. This is especially useful in the aquaculture industry [48].
Moreover, acidophilic LAB are used in the production of probiotic foods, which plays a critical role in maintaining human health. Due to their potential benefits as probiotics, the acidophilic Lactobacillus species are the most commonly used group of microorganisms within the LAB group [51]. Acidophilic LAB are also important because of the antimicrobial and antifungal substances they produce. Thus, they also reduce the formation of mycotoxins. In addition, it is possible to increase the bioavailability of grain-based products by fermentation with the fermentation activities of LAB [52]. It is possible to obtain healthier, more delicious and innovative products with the lactic acid fermentation of legumes [53]. The benefits of the fermented product produced by acidophilic LAB fermentation of soy milk, whose consumption has increased in recent years, are also emphasized. Various enhanced health benefits of this fermented product have also been reported, including bioactive compounds, enhanced nutritional values, and antihypertensive, antioxidant, antidiabetic, anticancer, and hypocholesterolemic effects [54]. It was also reported that the soluble dietary fiber, total polyphenol content and organic acid levels of potatoes increased at the end of the fermentation process of sweet potatoes using acidophilic LAB [55]. It is also known that many intestinal and urinary pathogenic bacteria are inhibited by the antagonistic activity of these microorganisms [37]. Furthermore, certain strains of the important acidophilus Lactobacillus acidophilus have been found to absorb cholesterol in the intestines [51]. It has also been reported that LAB’s dominance in the gut microbiota may be one of the ways to treat obesity [56].
In general, acidophilic–aciduric microorganisms affect the nutritional value of foods, both by preserving the food and its positive effects on human health. Specifically, with the use of acidophilic LAB as a starter culture in foods, it is stated that aroma, taste, texture, odor, and beneficial microorganism growth increase, while preservatives, artificial sweeteners, sucrose, lactose, oil and contaminants decrease in food [31]. Although many food-preserving processes and substances have been developed in advanced food production techniques, the conditions provided by fermentation are essential for ensuring the shelf life and microbiological safety of products [33]. Figure 1 shows the effects of low-pH microorganisms on the nutritional values and safety of foods.
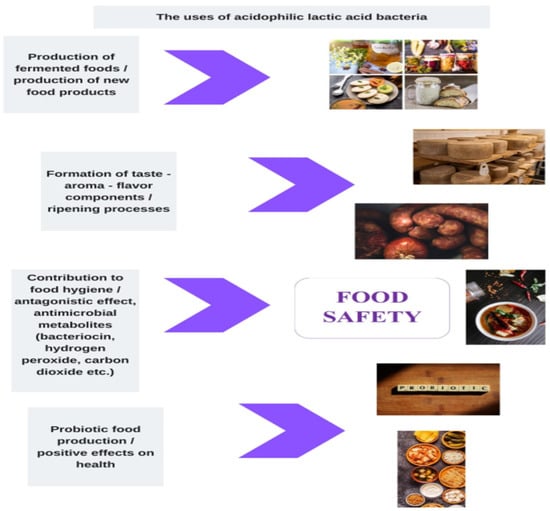
Figure 1.
The effects of low-pH microorganisms on the nutritional value and safety of foods.
4. Acidophilic Lactic Acid Bacteria’s Role in the Human Diet and Health
4.1. Their Probiotic Potentials
Live microorganisms that, when provided to a host in sufficient quantities, confer a health benefit to the host are known as probiotics [57]. Relationships with the health benefits of probiotics, which have been in human’s lives since fermented dairy products were first consumed, only date to the beginning of the last century. Over the years, the understanding of the health benefits of probiotics and the identification of microorganisms with probiotic potential have encouraged consumers and the food industry to use these microorganisms worldwide [58,59]. The global market size for probiotics was estimated to be USD 54 billion in 2020 and will reach USD 111.21 billion by 2030 [60].
Inhibition of the growth of pathogenic organisms such as Salmonella, Shigella, and Helicobacter, prevention/reduction of diarrhea and lactose intolerance symptoms, support of immunity, modulation of gut microbiota, immunomodulation, reduction in serum ammonia and cholesterol levels, anticarcinogenic and antimutagenic activities, and alleviation of allergies and atopic diseases are among the health benefits of low-pH probiotics [4,5,6]. As a result, the probiotic industries are exerting a significant amount of effort to find new probiotic strains that are effective, promising, and efficient, and that have a positive impact on the health of consumers [61].
For microorganisms to possess probiotic properties, they must be able to traverse the gastrointestinal tract, endure the acidic conditions of the stomach, and be resistant to bile salts [5,62]. They need to stick to the intestinal mucosa and establish a short-term colony in the colon [5,63,64]. Simultaneously, probiotic food products must contain an adequate amount of live probiotic microorganisms [5].
The most common probiotics microorganisms belong to the genus Lactobacillus, Leuconostoc, Pediococcus, and Bifidobacterium [65,66]. Since LAB are native inhabitants of the healthy human gut and are also present in many dairy products and conventional fermented foods, they are thought to be the most suitable acidophilic candidates for probiotics, aside from pathogenic strains [61]. LAB are generally regarded as potential probiotic bacteria that are prevalent in nature and have a wide range of applications in the food industry [6]. The main types of LAB with probiotic properties are Lactobacillus acidophilus, Lacticaseibacillus casei, Lactobacillus johnsonii, Lacticaseibacillus rhamnosus, Limosilactobacillus reuteri, Lactiplantibacillus plantarum, Limosilactobacillus fermentum and Lactiplantibacillus pentosus [5,67]. Being resistant to a low pH, bile salt, pepsin, and pancreatin, Pediococcus pentosaceus OZF is one of the acidophilic forms of LAB that can survive in the upper part of the gastrointestinal tract, and thus has a potential probiotic effect on the host organism [68]. Although LAB are mainly isolated from dairy products, they can also be obtained from different fermented foods, such as fermented meats and vegetables [5]. The isolation, identification, and characterization of the new acidophilic LAB has two main benefits, identifying the characteristic taxonomy of these microorganisms and revealing promising useful and functional new probiotic microorganisms [6,69].
Plessas et al. (2017) evaluated the probiotic potential of 45 LAB strains isolated from feta-type cheese by subjecting them to a series of tests, including resistance to low pH, resistance to pepsin and pancreatin, and tolerance of bile salts. It was determined that the strain defined as Lacticaseibacillus paracasei K5 has a similar or even better/more desirable probiotic properties than the reference strain Lactiplantibacillus plantarum ATCC 14917 [5]. Enterococcus durum LAB18S is another acidophilic LAB isolated from cheese that shows probiotic properties [70]. The Lactococcus hircilactis strain CH4, Lactobacillus delbrueckii strain GRIPUMSK, Lactobacillus johnsonii strain PUMSKGRI, and Lactobacillus leichmannii strain SKGRIPUM were determined as acidophilic LAB with the highest probiotic properties among 41 bacterial isolates obtained from homemade fermented food products (cheese, curd, fermented rice water, yogurt, and buttermilk) [71]. Falfán-Cortés et al. (2022) found that Lacticaseibacillus paracasei has the highest probiotic potential among the acidophilic LAB isolated from tenate cheese [72]. It is also reported that yogurt made from Yak milk contains higher amounts of probiotic LAB than yogurt made from other bovine milk, and acidophilic Limosilactobacillus fermentum (31%) and Lacticaseibacillus casei (28%), noted to be probiotics, are predominant [73,74].
The Lactobacillus gasseri MA-4 strain was observed to have a high tolerance to a low pH, bile salts, artificial gastric and small intestinal fluids, and was isolated from human breast milk. This strain was found to be a suitable candidate for use as a probiotic according to the findings of a study that evaluated the probiotic potential of this acidophilic strain [10]. Lactobacillus gasseri, one of the dominant species of the Lactobacillus group, is one of the low-pH probiotic microorganisms with a Generally Recognized As Safe (GRAS) status [75]. Another study concluded that 17 LAB isolated from yak milk also showed excellent probiotic properties, along with desirable health benefits [61]. In one of two different studies, Lactiplantibacillus plantarum, Lactococcus lactis, and Enterococcus lactis were found to be effective probiotics among all LAB obtained from camel milk [76], while in the other, Bifidobacterium longum obtained from camel milk was concluded to be the best potential probiotic [77]. In Nigerian goat milk, only six (Weissella cibaria GM 93m3, Weissella confusa GM 92m1, Pediococcus acidilactici GM 18a, Pediococcus pentosaceus GM 23d, Lactiplantibacillus pentosus GM 102s4 and Limosilactobacillus fermentum GM 30m1) of the 27 evaluated LAB were found to survive in pH 2.5, 0.3% bile salt, stimulated gastrointestinal conditions, and had auto-aggregative and hydrophobic properties [78].
The increasing global interest in products with functional properties has encouraged the search for new acidophilic microorganisms, especially the acidophilic LAB found in natural sources such as traditional fermented foods [6,11]. Many traditional fermented foods, especially fermented milk products, are accepted as vital sources of acidophilic LAB that are beneficial to human health [79]. LAB are food-fermentation agents used in the production of numerous foods, including yogurt, cheese, cultured butter, sour cream, sausages, cucumber pickles, olives, sauerkraut, and cocoa [80]. Revealing the probiotic potential of acidophilic LAB will help to determine which of these microorganisms can be used for probiotic purposes, especially in the health and food industry. Studies investigating the probiotic potentials of acidophilic and aciduric LAB isolated from some traditional fermented foods are demonstrated in Table 2.

Table 2.
Overview of the probiotic properties of acidophilic and aciduric LAB in some traditional fermented foods.
4.2. Beneficial and Toxic Compounds Released by Acidophilic Lactic Acid Bacteria
Although some acidophilic lactic acid bacteria and traditional fermented foods are associated with many beneficial effects on human health, the presence of some toxic compounds released by these microorganisms is also known. The beneficial effects mentioned in the literature are mostly attributed to the beneficial compounds released by these acidophilic LAB (Figure 2) [10,11,12]. Of these compounds, organic acids, some B group vitamins, GABA, amylase enzyme, and bacteriocins have important therapeutic-beneficial effects on host health [10,11,12], while biogenic amines (BA) are not desirable compounds (Figure 2) [13,14].
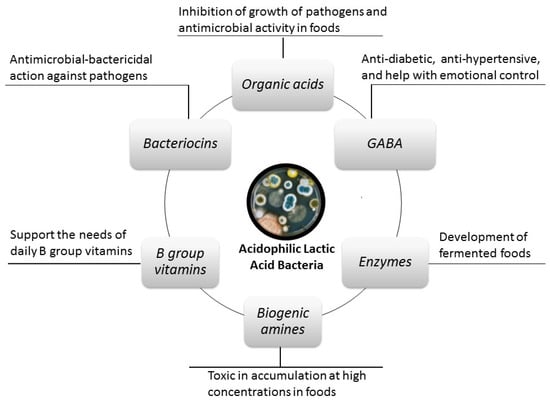
Figure 2.
Some compounds released by acidophilic lactic acid bacteria and their health effects.
4.2.1. Organic Acids
Organic acids released from acidophilic LAB show antimicrobial activity [90]. LAB produce a wide variety of organic acids, such as acetic acid, lactic acid, benzoic acid, sorbic acid, formic acid, citric acid, succinic acid, and propionic acid, as the end product of the fermentation of carbohydrates [90,91]. Organic acids, predominantly synthesized from acidophilic LAB, such as lactic acid, benzoic acid, and sorbic acid, create an unfavorable environment for the growth of pathogenic microorganisms [91]. Wang et al. discovered that 0.5% lactic acid inhibits the growth of pathogens, including Salmonella enteritidis, Escherichia coli, and Listeria monocytogenes [92]. Benzoic acid alone inhibits the growth of Enterobacter agglomerans by 10 to 15%; when combined with lactic acid, it can inhibit growth by up to 100% [90]. Salomskiene et al. (2019) determined that the LAB strains produced organic acids, which are natural antimicrobial compounds such as ethanol, lactic, citric, benzoic, and sorbic acids. They also reported that Lactobacillus helveticus was the best producer of benzoic acid [90]. LAB such as Lactobacillus, Lactococcus, Leuconostoc, Streptococcus, and Pediococcus are known as starter cultures frequently used for the fermentation of milk, meat, and vegetable products that produce organic acids as the final products [91]. In light of these, it is possible to hypothesize that LAB can maintain antimicrobial activity by producing organic acids like sorbic and benzoic acids in significant quantities that inhibit food pathogens, particularly in fermented foods. New industrial-based studies should be planned, though, as the effective production of organic acids on an industrial scale has not yet been accomplished.
4.2.2. B Group Vitamins
For every living cell, vitamins are essential micronutrients. They perform as precursors or as participants in a variety of key enzymatic processes, including electron transport chains. Humans are unable to produce the B-group vitamins and must therefore rely on external sources to meet their daily needs, in contrast to microorganisms, which can typically biosynthesize them according to their needs [93]. Vitamin deficiencies can still occur in many countries, despite the fact that the majority of vitamins can be found in a wide variety of foods. This is primarily caused by insufficient food intake and/or an unbalanced diet [94,95]. In recent years, the growing interest in fortifying foods with vitamins of microbial origin has led scientists to focus on identifying LAB strains that are GRAS and capable of synthesizing essential vitamins and other biomolecules [93,96]. It is known that some LAB strains can produce/release and/or increase some B group vitamins such as thiamine (B1), riboflavin (B2), pyridoxine (B6), folate (B9), and cobalamin (B12) [95,97]. Numerous acidophilic LAB species, including Lactococcus lactis, Lactobacillus gasseri, and Limosilactobacillus reuteri, have been suggested to play a part in the production of vitamins [93]. Hati et al. (2019) found out that Lactiplantibacillus plantarum produced the highest B2 and Limosilactobacillus fermentum produced the highest B12 and folate among the acidophilic Lactobacillus isolates they isolated from traditional Indian fermented foods [93]. In another study, it was determined that Limosilactobacillus reuteri JCM1112 produces vitamins B12 and folate [98]. Two strains of Latilactobacillus sakei and Lactiplantibacillus plantarum were found to produce high levels of folate in a study that evaluated the folate, vitamin B12 and B1 production of LAB isolated from nukazuke, a traditional Japanese pickle. However, the study did not find any strains of LAB that produced high levels of vitamin B12 and B1 [99]. Increased serum B2 and B9 levels were observed in mouse models that consumed pasta made with quinoa sourdough fermented with Lactiplantibacillus plantarum strains, a acidophilic LAB that produces vitamins B2, B9, and phytase [100]. In the food industries, LAB can be used to increase the concentrations of group B vitamins (especially folate) in yogurts [101,102]. Laiño et al. (2013) discovered that yogurt prepared with folate-producing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus strains had a statistically significantly higher folate content [101]. Soybean-based functional food production using acidophilic LAB capable of producing compounds with B12 activity is also seen as an alternative method to prevent vitamin deficiency [103]. Moreover, there are genetic engineering efforts to increase the production of B group vitamins or to create new vitamin-producing strains [95,104]. However, the use of genetic engineering for this purpose should be discussed in a broader perspective. As a result, it can be thought that the bio-enrichment of foods (such as milk, cereals, and derivatives) using acidophilic LAB with proven effectiveness may be a new strategy to increase the bioavailability of vitamins in foods. In addition, it is predicted that the use of vitamin-producing LAB in the food industry can be a cost-effective alternative to existing vitamin supplementation programs.
4.2.3. Gamma-Aminobutyric Acid (GABA)
Some acidophilic LAB can produce GABA bioactive compounds. GABA is produced by the conversion of glutamic acid with the enzyme glutamate decarboxylase. GABA has functions such as primary neurotransmission inhibitor, diabetic suppressor and antihypertensive, and helps with emotional control in the sympathetic nervous system [105]. In addition, it is thought that the use of GABA-enriched foods may provide health benefits due to the regulation of depression, insomnia, and autonomic disorders, and their anti-diabetic effects [106]. Many GABA-enriched functional foods are reported to be developed, including fermented seaweed drinks, black raspberry juice, and dairy products [106,107,108]. Increasing GABA production in a seaweed beverage fermented with acidophilic Lactiplantibacillus plantarum DW12 as a starter culture [107], and developing yogurt containing high levels of GABA, free amino acids, and isoflavones using LAB and germinated soybean extract, are examples of these enrichments [108]. In a study in which Lactobacillus namurensis NH2 and Pediococcus pentosaceus HN8 were used to increase the GABA content in Thai fermented pork sausage, it was found that the GABA content of the sausages increased eight times after the intervention, and these GABA-enriched sausages had a better taste and lower fat, carbohydrate, and energy contents than controls [109]. Jitpakdee et al. (2021) reported Pediococcus pentosaceus ENM104 and Lactiplantibacillus plantarum SPS109 isolated from Thai fermented foods as GABA-producing low-pH probiotic candidates [105]. Lozano et al. (2022) found that, among 101 Lactobacillus strains isolated from natural whey starters used by Uruguayan artisans in cheese production, 15 strains from the Lactiplantibacillus group had a significantly higher GABA production than the others [110]. Lactobacillus and Lactococcus spp. are also acidophilic LAB that were previously reported to produce GABA [106,108,111]. Considering the potential health benefits, it is crucial to define the complete list of acidophilic LAB that produce high levels of GABA, with new studies to be carried out. The fact that functional foods are increasingly preferred by consumers over the years suggests that the use of these GABA-producing microorganisms in the food industry will become widespread.
4.2.4. Enzymes
Some LAB also produce enzymes such as proteases, peptidases, polysaccharide degrading enzymes, lipases, amylases, esterases, and phenoloxidases [12]. One of the acidophilic LAB, Lactobacillus acidophilus SAM1, produces lipase enzymes, whereas Lactiplantabacillus plantarum SAM2 produces amylase and protease enzymes [63]. Some acidophilic LAB may also help reduce the risk of lactose intolerance by producing the lactase enzyme, which breaks down lactose into simple sugars [1,112]. Amylolytic LAB mainly belongs to the genera Lactobacillus, Lactococcus, Streptococcus, Pediococcus, Carnobacterium, and Weissella [113]. Tallapragada et al. (2018) came to the conclusion that the acidophilic–aciduric Lactobacillus species Limosilactobacillus fermentum and Lactobacillus sp G3_4_1TO2 produce amylase enzyme, and that Lactobacillus spp. G3_4_1TO2 from these two bacteria is a potential probiotic bacterium that produces maximum amylase enzyme [12]. Another study showed that 132 of the LAB found in fermented grain products in China produced amylase, and the Lactiplantibacillus plantarum strain produced the most [114]. Other acidophilic LAB with amylolytic activity (by making the enzyme amylase) include Lactobacillus amylovorus and Lacticaseibacillus manihotivorans [12,113]. Amylases catalyze the initial hydrolysis of starch to short oligosaccharides [115]. The use of amylolactic acid bacteria in conjunction with starch results in an improved fermentation process that is more efficient and cost-effective [12]. Because microbial amylases are more stable than plant and animal amylases, and because they are simpler and more affordable to manipulate, they can be used to produce enzymes with the desired properties in large quantities [116,117]. In light of these studies, the use of amylolactic acid bacteria in the development of grain-based foods and fermented foods/beverages can be recommended.
4.2.5. Bacteriocins
Bacteriocins are ribosomally synthesized antimicrobial peptides produced by certain microorganisms, including LAB, that are active against closely related microorganism, mostly Gram-positive bacteria, to obtain a competitive advantage for nutrients in the environment [118,119]. They usually have bactericidal and/or bacteriostatic activity targeting the bacterial cytoplasmic membrane [120]. Bacteriocins are divided into four main classes [118]. The first group of bacteriocins is generally known as lantibiotics. Nisin, one of the most commonly used and studied bacteriocins, can be provided as an example of this group of bacteriocins. Examples of bacteriocins in the second group are Pediocin PA1, Lactococcin A and B, Leucocin A, Sakacins A and P, Curvacin A, and Bavaricin MN, which are heat-resistant and hydrophobic, and inhibit Listeria monocytogenes [1,118]. The third group of bacteriocins is larger than 30 kDa and are heat-stable peptides (for instance, helveticin J), while the fourth group of bacteriocins is classified as bacteriolysins, that is, hydrolytic polypeptides [1,118].
The use of antibiotics as a growth-promoter in animal husbandry and aquaculture has been banned in various countries due to the increase in antibiotic resistance worldwide [121]. The use of antimicrobial growth-promoters (AGPs) in animal production has been discouraged in some nations, including the United States, Canada, and Japan [121,122]. However, the livestock industry is negatively impacted by the ban on using antibiotics as AGPs owing to various, uncontrolled bacterial diseases [121,123]. Because of this, it has become necessary to find new non-antibiotic growth-promoters in order to enhance animal growth and reproduction, as well as control multiple bacterial infections. Probiotics are one of the potential non-antibiotic growth-promoters being researched for this purpose [121]. Acidophilic LAB are potential probiotics being evaluated as an alternative to antibiotics during food–animal production [121]. Bacteriocins synthesized from LAB ribosomes are the antimicrobial peptides primarily responsible for this effect [124]. A large number of the bacteriocins made by LAB are secure and effective against a specific or broad range of bacteria. They may also possess sporostatic or sporicidal properties toward bacterial spores [124,125]. For instance, laparaxin is an antibacterial polypeptide secreted by Lacticaseibacillus paracasei NRRL B-50314, which has antibacterial activity against numerous Gram-positive bacteria [124,126]. It can be concluded that some bacteriocins released by LAB show potential as antibacterial agents and as new non-antibiotic growth-promoters. In addition, the rapidly rising antibiotic resistance worldwide suggests that LAB and bacteriocins may be the only treatment for some clinical cases in future and/or may need to be combined with low-dose antibiotics. However, to reach definite conclusions, it is necessary to determine from which bacteria antibacterial bacteriocins are produced and to evaluate the bactericidal effect of these bacteriocins with new studies.
Bacteriocins released by some acidophilic LAB also attract attention by their potential to reduce the growth of pathogenic microorganisms in foods, as well as being seen as potential non-antibiotic new growth-promoters in livestock [127,128]. The fact that most bacteriocins are generally colorless, odorless, and tasteless makes them usable in the food industry [129]. Lactococcus lactis bacteria from acidophilic LAB create the polycyclic antibacterial peptide known as lantibiotic nisin. This bacteriocin, the first antimicrobial peptide approved for use as a food preservative, is applied as a broad-spectrum antibacterial agent against many bacteria that are food-spoilage pathogens [124,125,130]. Nisin and pediocin are two bacteriocins approved by the Food and Drug Administration (FDA) for use in the food industry [118]. In order to produce high-quality fermentation products, the dairy industry frequently uses Lactobacillus acidophilus, another acidophilic LAB that produces the antimicrobial compound bacteriocin [63]. Additionally, Pediococcus pentosaceus, an acidophilic microorganism, creates anti-Listerial bacteriocin [68,127]. Todorov et al. demonstrated that an anti-listerial bacteriocin, Pediocin ST18, is produced by Pediococcus pentosaceus isolated from boza, a traditional fermented beverage [127]. Different bacteriocins, such as Leucocin OZ and Leucocin F10, are produced by Leuconostoc carnosum, a lactic acid bacterium isolated from fermented meat products [131]. These bacteriocins, produced by acidophilic LAB, show antimicrobial–bactericidal activity against some foodborne pathogenic bacteria, such as Listeria Monocytogenes and Listeria innocua [127]. In line with the data obtained from the literature, it can be concluded that the bacteriocins released by these acidophilic LAB generally have a bactericidal effect against the bacteria responsible for food spoilage and food-borne pathogens, and their use in the food industry may become widespread in the coming years, along with a clearer understanding of their effects.
The failure of current therapies for Clostridium difficile infections and the rise in recurrence has compelled the creation of new ones [130,132]. The use of probiotic bacteria that generate antimicrobial molecules, such as bacteriocins, has recently emerged as a promising alternative for the prevention and treatment of diseases linked to Clostridium difficile [130]. Even though numerous bacteriocins, including Nisin, Microbisporicin, Lacticin 3147, and thuricin CD, are effective against Clostridium difficile, only Nisin has been recognized as a natural food additive by the FDA, WHO, and the European Union to date [130,133]. It is stated that nisin released by the acidophilic Lactococcus lactis can be used to control Clostridium difficile infections. [133,134]. However, Le Lay et al. (2015) discovered that, in a human colon model, nisin Z produced by Lactococcus lactis UL719 did not inhibit Clostridium difficile [130]. The use of these acidophilic LAB, which positively affects some diseases such as Clostridium difficile infections, and the bacteriocins they produce, such as nisin, can be considered in the treatment process. However, there are very few and contradictory study results in the literature, making them difficult to interpret.
Bacteriocins produced by acidophilic LAB are being researched as a promising antiviral alternative to traditional antiviral agents, in addition to their antibacterial activities. The antiviral activity of bacteriocins, which has previously received much less attention than their antibacterial activity, is currently the subject of extensive research [129]. Bacteriocins, which are mainly produced from probiotic bacteria, can reduce the viral load of the host and improve the immunomodulatory mechanism against viral infections [1,129]. It is reported that some bacteriocins produced by some acidophilic LAB hace anti-influenza virus activity [135]. Maeda et al. also determined that Lactiplantibacillus plantarum L-137 from LAB presented with activity against the influenza virus [136]. The Enterococcus faecium-produced Enterocin ST5HA, Enterococcus faecalis-produced Enterocin AAR-74 and Enterococcus AAR-71, and Enterococcus mundtii-produced Enterocin CRL35 and Enterococcus ST4V from acidophilic LAB are additional examples of known antiviral bacteriocins [1,129]. Enterocin ST4V and enterocin CRL35 are reported to inhibit herpes simplex virus (HSV) types 1 and 2 in a dose-dependent manner, mainly by inhibiting late glycoprotein synthesis [129]. These data indicate that bacteriocins released from acidophilic LAB with antiviral activities can also be used as antiviral agents in the prevention and treatment of viral infections. Although the detailed mechanism(s) for the antiviral activities of acidophilic LAB has not yet been fully elucidated, the scientific community is increasingly focusing on the benefits of these microorganisms and their metabolites in the fight against viruses.
4.2.6. Biogenic Amines
One of the undesirable compounds released from acidophilic LAB is BAs. BAs are low-molecular-weight nitrogenous organic bases that can be accumulated in foods at high concentrations due to their microbial activity and have toxic effects on consumers [14]. LAB are believed to be the primary producers of BAs in fermented foods [13]. In the meantime, the leading producers of BAs in dairy products are mostly LAB belonging to the Enterococcus, Lactobacillus, Leuconostoc, Lactococcus, and Streptococcus genera [137]. In a study testing the biogenic amine production of fifteen LAB strains, Lacticaseibacillus casei (TISTR 389) and Lactobacillus delbrueckii subsp. bulgaricus (TISTR 895) were found to be potentially acidophilic microorganisms for the production of histamine and tyramine. In another study, the TISTR 389 strain produced the highest levels of histamine and tyramine [138]. Foods likely to contain high levels of biogenic amines include fish, fish products, and fermented foods (meat, dairy products, some vegetables, beers, and wines) [14]. The consumption of foods or beverages containing high concentrations of BA is a risk factor for consumer health because it may cause symptoms including flushing, headache, heart palpitations, vomiting, diarrhea, and an increase or decrease in blood pressure [13,14]. Additionally, they can cause the organoleptic properties of the food they are in to depreciate [14]. However, their toxic effects depend on the type of BA, individual susceptibility, allergy, and consumption of monoamine oxidase inhibitor drugs or ethanol, which interact with the amino oxidase enzymatic systems responsible for the detoxification process of exogenous BAs [13].
Histamine, tyramine, putrescine, cadaverine, and phenylethylamine are the most important BAs found in food and are generated by the decarboxylation histidine, tyrosine, ornithine, lysine, and phenylalanine, respectively [14]. Histamine and tyramine are regarded as the most dangerous BAs due to the severity of the symptoms they can cause [13]. Putrescine, cadaverine, phenylethylamine, agmatine, spermine, and spermidine may be toxic, but they may also make histamine and tyramine toxicity worse by inhibiting the enzymes that break down these substances down [13,139]. Since tyramine is mostly found in cheese, tyramine poisoning is known as the “cheese reaction”. Tyramine poisoning can cause diet-induced migraine, increased cardiac output, nausea, vomiting, respiratory disorders, and high blood sugar [13,140]. There is no specific legislation regarding the presence of BAs in food, with the exception of fishery products, where the maximum acceptable level of histamine is set, even though eating foods high in BA can have toxicological effects [13]. However, a qualitative risk assessment of BA in fermented foods by EFSA has revealed concentrations that could have negative effects on consumers. According to this report, in order to lower the risk of BA toxicity in foods, hygienic precautions, additional microbial controls, and the use of starter cultures that do not produce BA are crucial [141]. Moreover, to prevent BA toxicity, the potential risk can be evaluated by analyzing the BA-forming ability of the bacteria for use in fermentation [140]. However, more in vitro and in vivo research on BA and its toxicities is needed.
4.3. Safety Assessments of Acidophilic LAB with Probiotic Potentials
Microorganisms with probiotic potential can be used for the improvement of dysbiosis, which is an imbalance in the enteric microbial population of the intestinal microbiota, with many different health effects [10]. However, before all microorganisms, including acidophilic LAB, can be used for their probiotic potential, their safety must be evaluated and verified for the food and human health industries with some activity tests (Figure 3) [5,17].
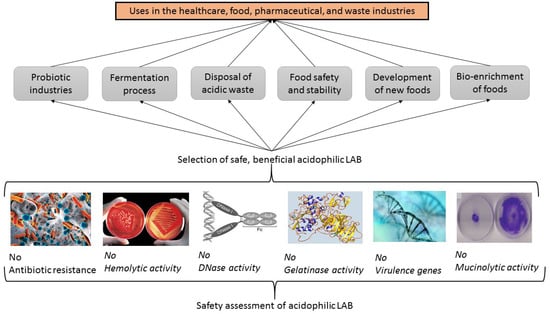
Figure 3.
Safety assessments of acidophilic lactic acid bacteria (LAB) with probiotic potential.
4.3.1. Antibiotic Resistance
Microorganisms, especially probiotics, should not spread antibiotic-resistance genes since they may interact with the normal microbiota of the human gastrointestinal tract after consumption [19]. Although the majority of probiotic bacteria have been granted GRAS status, there is still cause for concern regarding the potential transfer of antibiotic-resistance genes from used probiotics, including some LAB [5,6,17]. Over the years, the development of resistance to antibiotics has turned into a global public health problem [142]. Although the development of resistance to antibiotics has mostly been investigated on pathogenic microorganisms, some recent studies examine the ability of LAB to develop resistance to antibiotics such as tetracycline, erythromycin, and vancomycin [142]. In a study evaluating the antibiotic resistance of Lactobacillus leichmannii, Lacticaseibacillus casei, Lactobacillus delbrueckii, Levilactobacillus brevis, Limosilactobacillus fermentum, Lactobacillus coagulans, Lactobacillus acidophilus, Lactobacillus lactis, and Lacticaseibacillus rhamnosus strains, it was found that Lacticaseibacillus casei, Lactobacillus delbrueckii, and Levilactobacillus brevis strains had a higher resistance to antibiotics [18]. It has also been reported that some strains of Enterococcus faecium, one of the acidophilic LAB, can transfer vancomycin-resistance genes to Lactobacillus acidophilus [142]. Many people who suffer from common bacterial infections that were once easily treated with antibiotics may experience serious health issues as a result of possible antibiotic resistance [17]. EFSA has advised that screening for antibiotic resistance in potential probiotics should become mandatory [143]. For this reason, it can be said that acidophilic LAB, whose probiotic properties are mentioned in this review, should be screened for antibiotic resistance before using them for probiotic purposes.
4.3.2. Hemolytic Activity
It is important that acidophilic LAB with probiotic qualities should not show hemolytic activity in order to be used safely [5,63,64,144]. Non-hemolytic activity is considered a safety prerequisite for the selection of probiotic strains [18]. Even if bacteria have GRAS or Quality Presumption of Safety (QPS) status, EFSA strongly advises investigating their hemolytic activities [77]. Following incubation on Columbia agar plates, the hemolytic activity of the isolated strains is evaluated and categorized in accordance with the lysis of red blood cells in the medium surrounding the colonies [77]. Strains forming green zones on agar plates are classified as α-hemolysis (partial hemolytic), clean zones as β-hemolysis (complete hemolytic), and no zones as γ-hemolysis (non-hemolytic) [145,146]. Only strains with γ-hemolysis are considered safe [145]. In a study evaluating nine probiotic LAB (Lactobacillus leichmannii, Lacticaseibacillus casei, Lactobacillus delbrueckii, Levilactobacillus brevis, Limosilactobacillus fermentum, Lactobacillus coagulans, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. lactis, and Lacticaseibacillus rhamnosus) strains, it was reported that none of them showed β-hemolytic activity. It was also revealed that most of them were γ-hemolytic (without hemolysis), and only two strains (Lactobacillus coagulans and Lacticaseibacillus rhamnosus) showed α-hemolytic activity [18]. Oh et al. (2015) found that six strains of seven acidophilic LAB (Lactiplantibacillus pentosus SW02, Lactiplantibacillus plantarum subsp. Plantarum SW03, Latilactobacillus sakei subsp. Sakeii SW04, Lactiplantibacillus plantarum subsp. Plantarum SW06, Lactiplantibacillus plantarum subsp. Plantarum SW07, Pedeococcus acidococcus SW01) had γ-hemolytic (no hemolytic activity), and Pediococcus pentosaceus SW01 also showed α-hemolytic activity, which is partial hemolysis [147]. In another study evaluating the hemolytic activity of 71 LAB with probiotic potential isolated from fermented olives, none of the strains showed β-hemolytic activity, while four strains (Lactiplantibacillus pentosus B278, B279, B281, and B285) exhibited α-haemolysis [148]. Ismael et al. (2022) reported that only Enterococcus faecium out of 56 LAB isolated from fermented milk products showed α-hemolytic activity, while other strains did not [149]. On the contrary, another study found that Enterococcus faecium and Pediococcus acidilactici strains did not show hemolytic activity [150]. Motey et al. (2021) also observed β-hemolysis in none of the LAB isolated from fermented milk products, while α-hemolysis was observed in 38% of the strains [151]. In conclusion, while acidophilic LAB with probiotic potential generally do not show hemolytic activity (γ-hemolytic), there are some strains that show hemolytic activity (especially α-hemolytic activity). Therefore, it is recommended to evaluate the hemolytic activities of these microorganisms before they are used in the food industry, livestock, and health sector.
4.3.3. DNase Activity
Apart from the hemolytic activity and antibiotic resistance, another undesirable feature in acidophilic LAB is DNase activity [152]. For this reason, DNase activity should be considered in the safety assessment made before the acidophilic LAB are used [152,153]. In the study by Rodrigues et al. (2021), in which they tested the safety of 93 isolates identified as LAB isolated from fruits, it was concluded that 14 isolates were not safe for use because they had DNase activity [20]. However, Somashekaraiah et al. (2019) found that none of the 75 LAB strains isolated from the traditional Indian fermented beverage Neera showed direct DNase activity [154]. Likewise, in three different studies evaluating the safety of LAB isolated from dairy systems [155], fruit processing residues [153], and fermented grain products [156], it was determined that none of these acidophilic LAB showed DNase activity. It was also reported that Levilactobacillus brevis and Lacticaseibacillus paracasei acidophilic bacteria isolated from goat milk do not show DNase activity [157]. The DNase activity was not reported in strains belonging to Lactiplantibacillus plantarum and Enterococcus faecalis [158]. Furthermore, DNase activity was not observed in any of the LAB strains isolated from breast milk [159]. In most of the studies investigating the DNase activity of LAB, it was concluded that these acidophilic bacteria do not show DNase activity and are safe in this regard [155,156,157,158,159].
4.3.4. Gelatinase Activity
Gelatinase activity is accepted as a detrimental factor as it can hydrolyze collagen, initiating an inflammatory response [21]. In addition, gelatinase is expressed as a zinc metalloprotease, a lethal factor that hydrolyzes casein, hemoglobin, and other bioactive compounds in bacteria [160]. Because of these possible harmful effects, it is also important to determine the gelatinase activity of acidophilic LAB [161]. Rodrigues et al. (2021) found that 18 of 93 isolates identified as LAB isolated from fruits had positive gelatinase activity [20]. Muñoz-Atienza et al. (2013) found that Enterococcus faecalis and Enterococcus faecium (71% and 11%, respectively) had gelatinase activity among 99 examined LAB [161]. On the contrary, Sakoui et al. (2022) reported that strains of Enterococcus genus (Enterococcus faecium V6-112, Enterococcus faecalis JM102, Enterococcus mundtii MAV6B, Enterococcus gallinarum HBUAS52471, Enterococcus casseflavus APHG2) did not show gelatinase activity [21]. Ben Farhat et al. (2022) also did not observe gelatinase activity in any of the LAB strains of Limosilactobacillus fermentum (4 strains), Lacticaseibacillus paracasei (1 strain), Lacticaseibacillus rhamnosus (1 strain) [162]. In another study, it was revealed that strains belonging to the Lactiplantibacillus plantarum and Enterococcus faecalis groups did not show gelatinase activities [158]. Limosilactobacillus fermentum KL4 and Lactiplantibacillus plantarum MOBL1 strains, which are acidophilic LAB, also do not show gelatinase activity [163]. Kaktcham et al. (2018) reported that none of the seven LAB strains (Lactococcus lactis subsp. lactis 1FT, 1FW, and 3FT; Lactiplantibacillus plantarum 1MTK, 4BC, and 13BC and Levilactobacillus brevis 1BT) that they investigated for safety showed gelatinase activity [164]. Based on all these studies, there are concerns about whether some strains from LAB (especially strains belonging to the Enterococcus genus) have gelatinase activity. It is becoming increasingly crucial to evaluate the safety of acidophilic LAB that are considered for application in the food industry before use. In recent years, gelatinase activity has been seen as a remarkable determinant of the safety of low-pH microorganisms with known probiotic potential.
4.3.5. Presence of Virulence Genes
For microorganisms to be considered probiotics, they must not contain virulence genes [22]. For this reason, investigating virulence characteristics in the selection of LAB that are to be used in the food industry is another important safety consideration. Some of the virulence genes were investigated in the following strains: gelatinase (gelE), aggregation substance (asa1), enterococcal surface protein (esp), cytolysin (cylA), endocarditis antigen (efaA), adhesion of collagen (ace) [165]. Ribeiro et al. (2014) found that Enterococci from LAB isolated from Pico cheese, a traditional cow’s milk cheese, was positive for the presence of some virulence genes [166]. In another study by Domingos-Lopes et al. (2017), investigating the safety of LAB isolated from traditional Pico cheese, it was found that all Enterococcus isolates exhibited at least one virulence gene. In the same study, one Leuconostoc mesenteroides (L3C21R7) strain and five Lacticaseibacillus paracasei subsp. paracasei (L2A1K8, L2B21R1a, L2B21R3, L3B1M2, L3C21M6) strains have no tested virulence genes and these low-pH strains are good candidates to be used safely as starter/helper cultures in food fermentation [165]. Similarly, in a study reporting on safety concerns regarding the presence of virulence genes in the Enterococcus genus in 99 LAB, it was concluded that most of the Enterococcus faecalis strains (20 strains, 95%) harbor at least one relevant virulence factor (efaAfs (95%), gelE (71%), or agg (67%) genes) [161]. However, in another study investigating the presence of virulence genes (agg, gelE, esp, efaAfs, efaAfm and cylA, cylB, cylM, cylLL, and cylLS) in 280 LAB strains, it was reported that none of the strains harbored virulence genes [22]. Considering the health risks of strains with known virulence genes and the risk of transferring these genes to pathogenic microorganisms, it can be concluded that their application in the pharmaceutical and food industries raises concerns. Therefore, even if acidophilic LAB have GRAS or QPS status, whether they harbor virulence genes should be verified in vivo.
4.3.6. Mucinolytic Activity
The mucus that lines the intestinal epithelium and serves as a home for the commensal flora is composed primarily of mucins [167]. Thus, mucin, a glycosylated protein, provides the first line of defense and prevents the translocation of bacteria [168]. Because the production of mucin-degrading enzymes is a virulence determinant and affects the intestinal mucosal barrier, mucinolytic activity is also considered an undesirable property for microorganisms with probiotic potential (including acidophilic LAB). Another way that pathogens and toxins can enter the host is if an invading organism has mucin-degrading activity [169]. For this reason, the mucinolytic activities of acidophilic LAB are also investigated before they are used for probiotic purposes. Rabaoui et al. (2022) revealed that 15% of 47 analyzed LAB strains showed mucinolytic activity, while mucin degradation was dependent on glucose in 21% of the strains. In the same study, it was observed that 32% of Levilactobacillus brevis species and 37% of Enterococcus species were able to degrade mucin [167]. It has been reported that the mucin degradation of some LAB strains is nutrient-dependent. These microorganisms do not show mucinolytic activity in the presence of ready-to-use carbohydrates as an energy source [167]. In another study, 11 isolates of 93 LAB isolates were found to have mucinolytic activity [20]. However, Le et al. (2019) reported that Lactiplantibacillus plantarum MJM60383, Lactococcus lactis MJM60392, Limosilactobacillus fermentum MJM60393, and Lacticaseibacillus paracasei MJM60396 strains isolated from fermented foods did not show mucinolytic activity [170]. Coimbra-Gomes et al. (2022) also suggested that all strains of acidophilic LAB isolated from fermented Cobrançosa table olives were safe for human consumption, with negative results for mucin degradation, hemolytic activity, and DNase activity [85]. In another study, none of the strains of 59 enterococci and 40 non-enterococci showed mucinolytic activity [161]. Considering the health risks, in vivo animal model experiments can be planned in the future to determine the mucinolytic activities of acidophilic LAB with probiotic potential.
5. Conclusions
Microorganisms are a group of living creatures that adapt or develop in very different environmental conditions. Acidophilic LAB (such as Lactobacillus and Oenococcus, which show more acidophilic behavior), which are among the low-pH microorganisms that are attributed as microorganisms that adapt to living in an acidic pH, are the most frequently used microorganisms for food fermentation and preservation. At present, LAB are often preferred as starter cultures due to their unique metabolic properties in the fermentation process under controlled conditions with carefully selected strains. However, it should not be ignored that some acidophilic microorganisms may cause food spoilage and strains with proven safety in the food industry should be preferred. In addition, it is predicted that the use of acidophilic LAB in the natural disposal of wastes generated as a result of industrial applications will become increasingly widespread.
The search for novel, safe, efficient, and promising acidophilic LAB with beneficial effects on human health and the food industry is gaining momentum around the world. Considering the health benefits and antimicrobial and bactericidal activities in foods of acidophilic LAB, which produce compounds that have been shown to have beneficial effects, such as organic acids, B group vitamins, GABA, some enzymes, and bacteriocins, their use is likely to become widespread in the coming years. However, it should not be ignored that some LAB with a low pH can produce BAs, a harmful compound. Antibiotic-resistance gene transfer should also be taken into account when selecting isolates, as there is a risk of the horizontal transfer of these genes through acidophilic LAB. Moreover, the presence of hemolytic, DNase, gelatinase, mucinolytic activities, and virulence genes of these acidophilic microorganisms should be evaluated before they are used in the health and food industry, and it should be demonstrated that their use is safe. For all these reasons, more inclusive studies involving in vitro and in vivo analyses, animal and human subjects should be conducted to investigate the health benefits and safety of acidophilic LAB and the compounds they release. Consequently, this review may direct attention toward the unknown aspects of acidophilic LAB, clarify crucial unanswered questions, and ultimately lead to the development of novel alternative therapies/functional products based on acidophilic probiotic LAB and their metabolites.
Author Contributions
Conceptualization, D.A.; writing—original draft preparation, M.A.I., S.Ö. and D.A.; writing—review and editing, B.K., E.B., J.M.F.R. and F.O.; visualization, M.A.I., S.Ö. and B.K.; supervision, D.A., J.M.R., E.B. and F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would also like to acknowledge the COST Action 18101 SOUR-DOMICS-Sourdough biotechnology net-work towards novel, healthier and sustainable food and bioprocesses (https://sourdomics.com/; https://www.cost.eu/actions/CA18101/, accessed on 28 July 2023), where the author F.Ö. is the leader of the working group 8 “Food safety, health-promoting, sensorial perception and consumers’ behavior”, the author E.B. is the Vice Chair and leader of the working group 6 “Project design and development innovative prototypes of products and small-scale processing technologies” and the author J.M.R. is the Chair and Grant Holder Scientific Representative and is supported by COST (European Co-operation in Science and Technology) (https://www.cost.eu/, accessed on 28 July 2023). COST is a funding agency for research and in-novation networks. Author J.M.R. also acknowledges the Universidade Católica Portuguesa, CBQF—Centro de Biotecnologia e Química Fina—Laboratório Associado, Escola Superior de Biotecnologia, Porto, Portugal, as well as the support made by LA/P/0045/2020 (ALiCE) and UIDB/00511/2020-UIDP/00511/2020 (LEPABE) funded by national funds through FCT/MCTES (PIDDAC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.D.; Ibrahim, S.A. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Johnson, D.B.; Quatrini, R. Acidophile microbiology in space and time. Curr. Issues Mol. Biol. 2020, 39, 63–76. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, B.K.; Claes, I.J.; Lebeer, S. Functional mechanisms of probiotics. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 321–327. [Google Scholar]
- Plessas, S.; Nouska, C.; Karapetsas, A.; Kazakos, S.; Alexopoulos, A.; Mantzourani, I.; Chondrou, P.; Fournomiti, M.; Galanis, A.; Bezirtzoglou, E. Isolation, characterization and evaluation of the probiotic potential of a novel Lactobacillus strain isolated from Feta-type cheese. Food Chem. 2017, 226, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bin Masalam, M.S.; Bahieldin, A.; Alharbi, M.G.; Al-Masaudi, S.; Al-Jaouni, S.K.; Harakeh, S.M.; Al-Hindi, R.R. Isolation, molecular characterization and probiotic potential of lactic acid bacteria in Saudi raw and fermented milk. Evid.-Based Complement. Altern. Med. 2018, 2018, 7970463. [Google Scholar] [CrossRef] [PubMed]
- Obafemi, Y.D.; Oranusi, S.U.; Ajanaku, K.O.; Akinduti, P.A.; Leech, J.; Cotter, P.D. African fermented foods: Overview, emerging benefits, and novel approaches to microbiome profiling. NPJ Sci. Food 2022, 6, 15. [Google Scholar] [CrossRef]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main groups of microorganisms of relevance for food safety and stability: General aspects and overall description. In Innovative Technologies for Food Preservation; Academic Press: Cambridge, MA, USA, 2018; pp. 53–107. [Google Scholar]
- Gunyakti, A.; Asan-Ozusaglam, M. Lactobacillus gasseri from human milk with probiotic potential and some technological properties. LWT 2019, 109, 261–269. [Google Scholar] [CrossRef]
- Margalho, L.P.; Jorge, G.P.; Noleto, D.A.; Silva, C.E.; Abreu, J.S.; Piran, M.V.; Brocchi, M.; Sant’Ana, A.S. Biopreservation and probiotic potential of a large set of lactic acid bacteria isolated from Brazilian artisanal cheeses: From screening to in product approach. Microbiol. Res. 2021, 242, 126622. [Google Scholar] [CrossRef]
- Padmavathi, T.P.; Bhargavi, R.; Priyanka, P.R.; Niranjan, N.R.; Pavitra, P.V. Screening of potential probiotic lactic acid bacteria and production of amylase and its partial purification. J. Genet. Eng. Biotechnol. 2018, 16, 357–362. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Razia, S.; Hadibarata, T.; Lau, S.Y. Acidophilic microorganisms in remediation of contaminants present in extremely acidic conditions. Bioprocess Biosyst. Eng. 2023, 46, 341–358. [Google Scholar] [CrossRef]
- Montaño López, J.; Duran, L.; Avalos, J.L. Physiological limitations and opportunities in microbial metabolic engineering. Nat. Rev. Microbiol. 2022, 20, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Singh, R. Antibiotic resistance in food lactic acid bacteria—A review. Int. J. Food Microbiol. 2005, 105, 281–295. [Google Scholar] [CrossRef]
- Hawaz, E. Isolation and identification of probiotic lactic acid bacteria from curd and in vitro evaluation of its growth inhibition activities against pathogenic bacteria. Afr. J. Microbiol. Res. 2014, 8, 1419–1425. [Google Scholar]
- Cervantes-Elizarrarás, A.; Cruz-Cansino, N.d.S.; Ramírez-Moreno, E.; Vega-Sánchez, V.; Velázquez-Guadarrama, N.; Zafra-Rojas, Q.Y.; Piloni-Martini, J. In vitro probiotic potential of lactic acid bacteria isolated from aguamiel and pulque and antibacterial activity against pathogens. Appl. Sci. 2019, 9, 601. [Google Scholar] [CrossRef]
- Rodrigues, N.P.A.; Garcia, E.F.; de Souza, E.L. Selection of lactic acid bacteria with promising probiotic aptitudes from fruit and ability to survive in different food matrices. Braz. J. Microbiol. 2021, 52, 2257–2269. [Google Scholar] [CrossRef]
- Sakoui, S.; Derdak, R.; Addoum, B.; Pop, O.L.; Vodnar, D.C.; Suharoschi, R.; Soukri, A.; El Khalfi, B. The first study of probiotic properties and biological activities of lactic acid bacteria isolated from Bat guano from Er-rachidia, Morocco. LWT 2022, 159, 113224. [Google Scholar] [CrossRef]
- Pinto, A.; Barbosa, J.; Albano, H.; Isidro, J.; Teixeira, P. Screening of bacteriocinogenic lactic acid bacteria and their characterization as potential probiotics. Microorganisms 2020, 8, 393. [Google Scholar] [CrossRef]
- Irwin, J.A. Overview of extremophiles and their food and medical applications. In Physiological and Biotechnological Aspects of Extremophiles; Academic Press: Cambridge, MA, USA, 2020; pp. 65–87. [Google Scholar]
- Souza, T.S.P.d.; de Andrade, C.J.; Koblitz, M.G.B.; Fai, A.E.C. Microbial Peptidase in Food Processing: Current State of the Art and Future Trends. Catal. Lett. 2022, 153, 114–137. [Google Scholar] [CrossRef]
- Sharma, A.; Kawarabayasi, Y.; Satyanarayana, T. Acidophilic bacteria and archaea: Acid stable biocatalysts and their potential applications. Extremophiles 2012, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Moat, A.G.; Foster, J.W.; Spector, M.P. Microbial physiology; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Chen, X. Thriving at Low pH: Adaptation Mechanisms of Acidophiles. In Acidophiles—Fundamentals and Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Xu, J.; Guo, L.; Zhao, N.; Meng, X.; Zhang, J.; Wang, T.; Wei, X.; Fan, M. Response mechanisms to acid stress of acid-resistant bacteria and biotechnological applications in the food industry. Crit. Rev. Biotechnol. 2022, 43, 258–274. [Google Scholar] [CrossRef] [PubMed]
- YÖRÜK, G.; GÜNER, A. Laktik asit bakterilerinin sınıflandırılması ve Weissella türlerinin gıda mikrobiyolojisinde önemi. Atatürk Üniversitesi Vet. Bilim. Derg. 2011, 6, 163–176. [Google Scholar]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Johansen, E. Use of natural selection and evolution to develop new starter cultures for fermented foods. Annu. Rev. Food Sci. Technol. 2018, 9, 411–428. [Google Scholar] [CrossRef]
- Garvie, E.I. Bacterial lactate dehydrogenases. Microbiol. Rev. 1980, 44, 106–139. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Han, G.; Seo, Y.S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The next generation of malolactic fermentation starter cultures—An overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Gürsoy, O.; kınık, Ö. Lactobacıllı and theır usage potentıal ın probıotıc cheese productıon. J. Eng. Sci. 2005, 11, 361–371. [Google Scholar]
- Courtin, P.; Rul, F. Interactions between microorganisms in a simple ecosystem: Yogurt bacteria as a study model. Le Lait 2004, 84, 125–134. [Google Scholar] [CrossRef]
- Aslam, S.; Qazi, J.I. Isolation of acidophilic lactic acid bacteria antagonistic to microbial contaminants. Pak. J. Zool. 2010, 42, 567–573. [Google Scholar]
- Laranjo, M.; Potes, M.E.; Elias, M. Role of Starter Cultures on the Safety of Fermented Meat Products. Front. Microbiol. 2019, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Bachmann, H.; van Pelt-KleinJan, E.; Douwenga, S.; Smid, E.J.; Teusink, B.; van Mastrigt, O. Lifestyle, metabolism and environmental adaptation in Lactococcus lactis. FEMS Microbiol. Rev. 2020, 44, 804–820. [Google Scholar] [CrossRef] [PubMed]
- Bourdichon, F.; Boyaval, P.; Casaregola, S.; Dupont, J.; Farrokh, C.; Frisvad, J.; Hammes, W.; Huys, G.; Jany, J.; Laulund, S. 3 The 2012 Inventory of Microbial Species with technological beneficial role in fermented food products. Bull. Int. Dairy Fed. 2012, 455, 22–61. [Google Scholar]
- Tzora, A.; Nelli, A.; Voidarou, C.; Fthenakis, G.; Rozos, G.; Theodorides, G.; Bonos, E.; Skoufos, I. Microbiota “Fingerprint” of Greek Feta Cheese through Ripening. Appl. Sci. 2021, 11, 5631. [Google Scholar] [CrossRef]
- Russell, J.B.; Diez-Gonzalez, F. The effects of fermentation acids on bacterial growth. Adv. Microb. Physiol. 1997, 39, 205–234. [Google Scholar]
- De Clerck, E.; Rodriguez-Diaz, M.; Forsyth, G.; Lebbe, L.; Logan, N.A.; DeVos, P. Polyphasic characterization of Bacillus coagulans strains, illustrating heterogeneity within this species, and emended description of the species. Syst. Appl. Microbiol. 2004, 27, 50–60. [Google Scholar] [CrossRef]
- Haberbeck, L.U.; da Silva Riehl, C.A.; Salomão, B.d.C.M.; De Aragao, G.M.F. Bacillus coagulans spore inactivation through the application of oregano essential oil and heat. LWT-Food Sci. Technol. 2012, 46, 267–273. [Google Scholar] [CrossRef][Green Version]
- Lucas, R.; Grande, M.J.; Abriouel, H.; Maqueda, M.; Omar, N.B.; Valdivia, E.; Martínez-Cañamero, M.; Gálvez, A. Application of the broad-spectrum bacteriocin enterocin AS-48 to inhibit Bacillus coagulans in canned fruit and vegetable foods. Food Chem. Toxicol. 2006, 44, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.; Gibbs, P. Target selection in designing pasteurization processes for shelf-stable high-acid fruit products. Crit. Rev. Food Sci. Nutr. 2004, 44, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Danyluk, M.D.; Friedrich, L.M.; Jouquand, C.; Goodrich-Schneider, R.; Parish, M.E.; Rouseff, R. Prevalence, concentration, spoilage, and mitigation of Alicyclobacillus spp. in tropical and subtropical fruit juice concentrates. Food Microbiol. 2011, 28, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Chizhayeva, A.; Amangeldi, A.; Oleinikova, Y.; Alybaeva, A.; Sadanov, A. Lactic acid bacteria as probiotics in sustainable development of aquaculture. Aquat. Living Resour. 2022, 35, 10. [Google Scholar] [CrossRef]
- Sharma, H.; Ozogul, F.; Bartkiene, E.; Rocha, J.M. Impact of lactic acid bacteria and their metabolites on the techno-functional properties and health benefits of fermented dairy products. Crit. Rev. Food Sci. Nutr. 2023, 63, 4819–4841. [Google Scholar] [CrossRef] [PubMed]
- Grattepanche, F.; Miescher-Schwenninger, S.; Meile, L.; Lacroix, C. Recent developments in cheese cultures with protective and probiotic functionalities. Dairy Sci. Technol. 2008, 88, 421–444. [Google Scholar] [CrossRef]
- Hammes, W.P.; Tichaczek, P.S. The potential of lactic acid bacteria for the production of safe and wholesome food. Z. Lebensm.-Unters. Und-Forsch. 1994, 198, 193–201. [Google Scholar] [CrossRef]
- Wafula, E.N.; Muhonja, C.N.; Kuja, J.O.; Owaga, E.E.; Makonde, H.M.; Mathara, J.M.; Kimani, V.W. Lactic Acid Bacteria from African Fermented Cereal-Based Products: Potential Biological Control Agents for Mycotoxins in Kenya. J. Toxicol. 2022, 2022, 2397767. [Google Scholar] [CrossRef]
- Emkani, M.; Oliete, B.; Saurel, R. Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review. Fermentation 2022, 8, 244. [Google Scholar] [CrossRef]
- Kumari, M.; Kokkiligadda, A.; Dasriya, V.; Naithani, H. Functional relevance and health benefits of soymilk fermented by lactic acid bacteria. J. Appl. Microbiol. 2022, 133, 104–119. [Google Scholar] [CrossRef]
- Zhu, L.; Mu, T.; Ma, M.; Sun, H.; Zhao, G. Nutritional composition, antioxidant activity, volatile compounds, and stability properties of sweet potato residues fermented with selected lactic acid bacteria and bifidobacteria. Food Chem. 2022, 374, 131500. [Google Scholar] [CrossRef]
- Burakova, I.; Smirnova, Y.; Gryaznova, M.; Syromyatnikov, M.; Chizhkov, P.; Popov, E.; Popov, V. The Effect of Short-Term Consumption of Lactic Acid Bacteria on the Gut Microbiota in Obese People. Nutrients 2022, 14, 3384. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food, Report of a Joint FAO/WHO Working Group on Drafting Guideline for the Evaluation of Probiotic in Food; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Prabhurajeshwar, C.; Chandrakanth, R.K. Probiotic potential of Lactobacilli with antagonistic activity against pathogenic strains: An in vitro validation for the production of inhibitory substances. Biomed. J. 2017, 40, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Ahire, J.J.; Pawar, S.P.; Chaudhari, B.L.; Chincholkar, S.B. Comparative accounts of probiotic characteristics of Bacillus spp. isolated from food wastes. Food Res. Int. 2009, 42, 505–510. [Google Scholar] [CrossRef]
- Lee, H.-W.; Park, Y.-S.; Jung, J.-S.; Shin, W.-S.J.A. Chitosan oligosaccharides, dp 2–8, have prebiotic effect on the Bifidobacterium bifidium and Lactobacillus sp. Anaerobe 2002, 8, 319–324. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, H.; Jangra, M.; Kaur, L.; Jaswal, P.; Dureja, C.; Nandanwar, H.; Chaudhuri, S.R.; Raje, M.; Mishra, S. Lactic acid bacteria isolated from yak milk show probiotic potential. Appl. Microbiol. Biotechnol. 2017, 101, 7635–7652. [Google Scholar] [CrossRef]
- Saxami, G.; Ypsilantis, P.; Sidira, M.; Simopoulos, C.; Kourkoutas, Y.; Galanis, A. Distinct adhesion of probiotic strain Lactobacillus casei ATCC 393 to rat intestinal mucosa. Anaerobe 2012, 18, 417–420. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.I.; El-Borai, A.M.; Akl, S.H.; El-Aassar, S.A.; Abdel-Latif, M.S. Identification of Lactobacillus strains from human mother milk and cottage cheese revealed potential probiotic properties with enzymatic activity. Sci. Rep. 2022, 12, 22522. [Google Scholar] [CrossRef]
- Vemuri, R.; Shinde, T.; Shastri, M.D.; Perera, A.P.; Tristram, S.; Martoni, C.J.; Gundamaraju, R.; Ahuja, K.D.; Ball, M.; Eri, R. A human origin strain Lactobacillus acidophilus DDS-1 exhibits superior in vitro probiotic efficacy in comparison to plant or dairy origin probiotics. Int. J. Med. Sci. 2018, 15, 840. [Google Scholar] [CrossRef]
- Byakika, S.; Mukisa, I.M.; Byaruhanga, Y.B.; Muyanja, C. A review of criteria and methods for evaluating the probiotic potential of microorganisms. Food Rev. Int. 2019, 35, 427–466. [Google Scholar] [CrossRef]
- Lee, K.W.; Shim, J.M.; Park, S.-K.; Heo, H.-J.; Kim, H.-J.; Ham, K.-S.; Kim, J.H. Isolation of lactic acid bacteria with probiotic potentials from kimchi, traditional Korean fermented vegetable. LWT-Food Sci. Technol. 2016, 71, 130–137. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, S.; Dong, X.; Wang, Y.; Zhang, H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Osmanagaoglu, O.; Kiran, F.; Ataoglu, H. Evaluation of in vitro probiotic potential of Pediococcus pentosaceus OZF isolated from human breast milk. Probiotics Antimicrob. Proteins 2010, 2, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Bujnakova, D.; Strakova, E.; Kmet, V. In vitro evaluation of the safety and probiotic properties of Lactobacilli isolated from chicken and calves. Anaerobe 2014, 29, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Comerlato, C.B.; Prichula, J.; Siqueira, F.M.; Ritter, A.C.; Varela, A.P.M.; Mayer, F.Q.; Brandelli, A. Genomic analysis of Enterococcus durans LAB18S, a potential probiotic strain isolated from cheese. Genet. Mol. Biol. 2022, 45, e20210201. [Google Scholar] [CrossRef]
- Srinivash, M.; Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B.; Keerthivasan, M. Probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from homemade fermented food products. J. Agric. Food Res. 2023, 11, 100517. [Google Scholar] [CrossRef]
- Falfán-Cortés, R.N.; Mora-Peñaflor, N.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Acevedo-Sandoval, O.A.; Franco-Fernández, M.J.; Castro-Rosas, J. Characterization and evaluation of the probiotic potential in vitro and in situ of Lacticaseibacillus paracasei isolated from tenate cheese. J. Food Prot. 2022, 85, 112–121. [Google Scholar] [CrossRef]
- Guo, X.; Long, R.; Kreuzer, M.; Ding, L.; Shang, Z.; Zhang, Y.; Yang, Y.; Cui, G. Importance of functional ingredients in yak milk-derived food on health of Tibetan nomads living under high-altitude stress: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 292–302. [Google Scholar] [CrossRef]
- Chen, X.; Du, X.; Wang, W.; Zhang, J.; Sun, Z.; Lıu, W.; Li, L.; Sun, T.; Zhang, H. Isolation and identification of cultivable lactic acid bacteria in traditional fermented milk of Tibet in China. Int. J. Dairy Technol. 2010, 63, 437–444. [Google Scholar]
- Stoeker, L.; Nordone, S.; Gunderson, S.; Zhang, L.; Kajikawa, A.; LaVoy, A.; Miller, M.; Klaenhammer, T.R.; Dean, G.A. Assessment of Lactobacillus gasseri as a candidate oral vaccine vector. Clin. Vaccine Immunol. 2011, 18, 1834–1844. [Google Scholar] [CrossRef]
- Sharma, A.; Lavania, M.; Singh, R.; Lal, B. Identification and probiotic potential of lactic acid bacteria from camel milk. Saudi J. Biol. Sci. 2021, 28, 1622–1632. [Google Scholar] [CrossRef]
- Yasmin, I.; Saeed, M.; Khan, W.A.; Khaliq, A.; Chughtai, M.F.J.; Iqbal, R.; Tehseen, S.; Naz, S.; Liaqat, A.; Mehmood, T. In vitro probiotic potential and safety evaluation (hemolytic, cytotoxic activity) of Bifidobacterium strains isolated from raw camel milk. Microorganisms 2020, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, M.O.; Ogunremi, O.R.; Adeleke, R.A.; Ezekiel, C.N. Probiotic Potentials of Lactic Acid Bacteria and Yeasts from Raw Goat Milk in Nigeria. Probiotics Antimicrob. Proteins 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Uddin, M.E.; Rahman, M.T.; Islam, M.A.; Harun-ur-Rashid, M. Isolation and characterization of dominant lactic acid bacteria from raw goat milk: Assessment of probiotic potential and technological properties. Small Rumin. Res. 2021, 205, 106532. [Google Scholar] [CrossRef]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and disadvantages of non-starter lactic acid bacteria from traditional fermented foods: Potential use as starters or probiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1537–1567. [Google Scholar] [CrossRef]
- Sadeghi, M.; Panahi, B.; Mazlumi, A.; Hejazi, M.A.; Komi, D.E.A.; Nami, Y. Screening of potential probiotic lactic acid bacteria with antimicrobial properties and selection of superior bacteria for application as biocontrol using machine learning models. LWT 2022, 162, 113471. [Google Scholar] [CrossRef]
- Khan, I.; Kang, S.C. Probiotic potential of nutritionally improved Lactobacillus plantarum DGK-17 isolated from Kimchi–A traditional Korean fermented food. Food Control 2016, 60, 88–94. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Yapar, N.; Yetim, H. Diversity and probiotic potentials of lactic acid bacteria isolated from gilaburu, a traditional Turkish fermented European cranberrybush (Viburnum opulus L.) fruit drink. Food Res. Int. 2014, 64, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Metrouh, R.; Fares, R.; Mechai, A.; Debabza, M.; Menasria, T. Technological properties and probiotic potential of Lactiplantibacillus plantarum SJ14 isolated from Algerian traditional cheese “Jben”. J. Food Process. Preserv. 2022, 46, e16482. [Google Scholar] [CrossRef]
- Coimbra-Gomes, J.; Reis, P.J.; Tavares, T.G.; Malcata, F.X.; Macedo, A.C. Study of lactic acid bacteria biodiversity in fermented Cobrançosa table olives to determine their probiotic potential. Foods 2022, 11, 3050. [Google Scholar] [CrossRef]
- Liu, C.; Xue, W.-j.; Ding, H.; An, C.; Ma, S.-j.; Liu, Y. Probiotic Potential of Lactobacillus Strains Isolated From Fermented Vegetables in Shaanxi, China. Front. Microbiol. 2022, 12, 4168. [Google Scholar] [CrossRef]
- Akmal, U.; Ghori, I.; Elasbali, A.M.; Alharbi, B.; Farid, A.; Alamri, A.S.; Muzammal, M.; Asdaq, S.M.B.; Naiel, M.A.; Ghazanfar, S. Probiotic and antioxidant potential of the Lactobacillus spp. isolated from artisanal fermented pickles. Fermentation 2022, 8, 328. [Google Scholar] [CrossRef]
- Leite, A.M.; Miguel, M.; Peixoto, R.; Ruas-Madiedo, P.; Paschoalin, V.; Mayo, B.; Delgado, S. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J. Dairy Sci. 2015, 98, 3622–3632. [Google Scholar] [CrossRef]
- Olajugbagbe, T.E.; Elugbadebo, O.E.; Omafuvbe, B.O. Probiotic potentials of Pediococuss acidilactici isolated from wara; A Nigerian unripened soft cheese. Heliyon 2020, 6, e04889. [Google Scholar] [CrossRef] [PubMed]
- Salomskiene, J.; Jonkuviene, D.; Macioniene, I.; Abraitiene, A.; Zeime, J.; Repeckiene, J.; Vaiciulyte-Funk, L. Differences in the occurence and efficiency of antimicrobial compounds produced by lactic acid bacteria. Eur. Food Res. Technol. 2019, 245, 569–579. [Google Scholar] [CrossRef]
- Bangar, S.P.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Hati, S.; Patel, M.; Mishra, B.K.; Das, S. Short-chain fatty acid and vitamin production potentials of Lactobacillus isolated from fermented foods of Khasi Tribes, Meghalaya, India. Ann. Microbiol. 2019, 69, 1191–1199. [Google Scholar] [CrossRef]
- Gonmei, Z.; Toteja, G. Micronutrient status of Indian population. Indian J. Med. Res. 2018, 148, 511. [Google Scholar]
- LeBlanc, J.; Laiño, J.E.; Del Valle, M.J.; Vannini, V.v.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B-Group vitamin production by lactic acid bacteria–current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef]
- Goswami, G.; Bora, S.S.; Parveen, A.; Boro, R.C.; Barooah, M. Identification and functional properties of dominant lactic acid bacteria isolated from Kahudi, a traditional rapeseed fermented food product of Assam, India. J. Ethn. Foods 2017, 4, 187–197. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A. Application of vitamin-producing lactic acid bacteria to treat intestinal inflammatory diseases. Appl. Microbiol. Biotechnol. 2020, 104, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Wegkamp, A.; de Vos, W.M.; Smid, E.J.; Hugenholtz, J. High-level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Appl. Environ. Microbiol. 2008, 74, 3291–3294. [Google Scholar] [CrossRef]
- Masuda, M.; Ide, M.; Utsumi, H.; Niiro, T.; Shimamura, Y.; Murata, M. Production potency of folate, vitamin B12, and thiamine by lactic acid bacteria isolated from Japanese pickles. Biosci. Biotechnol. Biochem. 2012, 76, 2061–2067. [Google Scholar] [CrossRef]
- Carrizo, S.L.; de LeBlanc, A.d.M.; LeBlanc, J.G.; Rollán, G.C. Quinoa pasta fermented with lactic acid bacteria prevents nutritional deficiencies in mice. Food Res. Int. 2020, 127, 108735. [Google Scholar] [CrossRef] [PubMed]
- Laiño, J.E.; del Valle, M.J.; de Giori, G.S.; LeBlanc, J.G.J. Development of a high folate concentration yogurt naturally bio-enriched using selected lactic acid bacteria. LWT-Food Sci. Technol. 2013, 54, 1–5. [Google Scholar] [CrossRef]
- Laiño, J.E.; LeBlanc, J.G.; Savoy de Giori, G. Production of natural folates by lactic acid bacteria starter cultures isolated from artisanal Argentinean yogurts. Can. J. Microbiol. 2012, 58, 581–588. [Google Scholar] [CrossRef]
- Molina, V.; Médici, M.; de Valdez, G.F.; Taranto, M.P. Soybean-based functional food with vitamin B12-producing lactic acid bacteria. J. Funct. Foods 2012, 4, 831–836. [Google Scholar] [CrossRef]
- Thakur, K.; Tomar, S.K.; De, S. Lactic acid bacteria as a cell factory for riboflavin production. Microb. Biotechnol. 2016, 9, 441–451. [Google Scholar] [CrossRef]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Selected probiotic lactic acid bacteria isolated from fermented foods for functional milk production: Lower cholesterol with more beneficial compounds. LWT 2021, 135, 110061. [Google Scholar] [CrossRef]
- Ratanaburee, A.; Kantachote, D.; Charernjiratrakul, W.; Sukhoom, A. Selection of γ-aminobutyric acid-producing lactic acid bacteria and their potential as probiotics for use as starter cultures in T hai fermented sausages (N ham). Int. J. Food Sci. Technol. 2013, 48, 1371–1382. [Google Scholar] [CrossRef]
- Ratanaburee, A.; Kantachote, D.; Charernjiratrakul, W.; Penjamras, P.; Chaiyasut, C. Enhancement of γ-aminobutyric acid in a fermented red seaweed beverage by starter culture Lactobacillus plantarum DW12. Electron. J. Biotechnol. 2011, 14, 1. [Google Scholar]
- Park, K.-B.; Oh, S.-H. Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour. Technol. 2007, 98, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Ratanaburee, A.; Kantachote, D.; Charernjiratrakul, W.; Sukhoom, A. Enhancement of γ-aminobutyric acid (GABA) in Nham (Thai fermented pork sausage) using starter cultures of Lactobacillus namurensis NH2 and Pediococcus pentosaceus HN8. Int. J. Food Microbiol. 2013, 167, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Fernández-Ciganda, S.; González Revello, Á.; Hirigoyen, D.; Martínez, M.; Scorza, C.; Zunino, P. Probiotic potential of GABA-producing lactobacilli isolated from Uruguayan artisanal cheese starter cultures. J. Appl. Microbiol. 2022, 133, 1610–1619. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, M.Y.; Ji, G.E.; Lee, Y.S.; Hwang, K.T. Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int. J. Food Microbiol. 2009, 130, 12–16. [Google Scholar] [CrossRef]
- Kolev, P.; Rocha-Mendoza, D.; Ruiz-Ramírez, S.; Ortega-Anaya, J.; Jiménez-Flores, R.; García-Cano, I. Screening and characterization of β-galactosidase activity in lactic acid bacteria for the valorization of acid whey. JDS Commun. 2022, 3, 1–6. [Google Scholar] [CrossRef]
- Panda, S.H.; Ray, R.C. Amylolytic Lactic Acid Bacteria. İn Fermented Foods, Part I: Biochemistry and Biotechnology; CRC Press: Boca Raton, FL, USA, 2016; p. 133. [Google Scholar]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control 2020, 111, 107057. [Google Scholar] [CrossRef]
- Paul, J.S.; Gupta, N.; Beliya, E.; Tiwari, S.; Jadhav, S.K. Aspects and recent trends in microbial α-amylase: A review. Appl. Biochem. Biotechnol. 2021, 193, 2649–2698. [Google Scholar] [CrossRef]
- Pasin, T.M.; dos Anjos Moreira, E.; de Lucas, R.C.; Benassi, V.M.; Ziotti, L.S.; Cereia, M.; Polizeli, M.d.L.T.d.M. Novel amylase-producing fungus hydrolyzing wheat and brewing residues, Aspergillus carbonarius, discovered in tropical forest remnant. Folia Microbiol. 2020, 65, 173–184. [Google Scholar] [CrossRef]
- Niyonzima, F.N.; Veena, S.; More, S.S. Industrial production and optimization of microbial enzymes. In Microbial Enzymes: Roles and Applications in İndustries; Springer: Singapore, 2020; pp. 115–135. [Google Scholar]
- Mokoena, M.P. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini-review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. Bacteriocins from lactic acid bacteria: Purification, properties and use as biopreservatives. Braz. Arch. Biol. Technol. 2007, 50, 512–542. [Google Scholar] [CrossRef]
- da Costa, R.J.; Voloski, F.L.; Mondadori, R.G.; Duval, E.H.; Fiorentini, Â.M. Preservation of meat products with bacteriocins produced by lactic acid bacteria isolated from meat. J. Food Qual. 2019, 2019, 4726510. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Uwiera, R.R.; Kalmokoff, M.L.; Brooks, S.P.; Inglis, G.D. Antimicrobial growth promoter use in livestock: A requirement to understand their modes of action to develop effective alternatives. Int. J. Antimicrob. Agents 2017, 49, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Van Boeckel, T.; Teillant, A. The Economic Costs of Withdrawing Antimicrobial Growth Promoters from the Livestock Sector; OECD Food, Agriculture and Fisheries Papers, No. 78; OECD Publishing: Paris, France, 2015. [Google Scholar] [CrossRef]
- Seal, B.S.; Drider, D.; Oakley, B.B.; Brüssow, H.; Bikard, D.; Rich, J.O.; Miller, S.; Devillard, E.; Kwan, J.; Bertin, G. Microbial-derived products as potential new antimicrobials. Vet. Res. 2018, 49, 66. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.; Field, D.; Rea, M.C.; Ross, R.P.; Hill, C.; Cotter, P.D. Bacteriocins: Novel solutions to age old spore-related problems? Front. Microbiol. 2016, 7, 461. [Google Scholar] [CrossRef]
- Liu, S.; Wilkinson, B.J.; Bischoff, K.M.; Hughes, S.R.; Rich, J.O.; Cotta, M.A. Novel antibacterial polypeptide laparaxin produced by Lactobacillus paracasei strain NRRL B-50314 via fermentation. J. Pet. Environ. Biotechnol. 2012, 3. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dicks, L.M. Pediocin ST18, an anti-listerial bacteriocin produced by Pediococcus pentosaceus ST18 isolated from boza, a traditional cereal beverage from Bulgaria. Process Biochem. 2005, 40, 365–370. [Google Scholar] [CrossRef]
- Jiao, D.; Liu, Y.; Zeng, R.; Hou, X.; Nie, G.; Sun, L.; Fang, Z. Preparation of phosphatidylcholine nanovesicles containing bacteriocin CAMT2 and their anti-listerial activity. Food Chem. 2020, 314, 126244. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Dicks, L.M.; Popov, I.V.; Karaseva, A.; Ermakov, A.M.; Suvorov, A.; Tagg, J.R.; Weeks, R.; Chikindas, M.L. Probiotics at war against viruses: What is missing from the picture? Front. Microbiol. 2020, 11, 1877. [Google Scholar] [CrossRef]
- Le Lay, C.; Fernandez, B.; Hammami, R.; Ouellette, M.; Fliss, I. On Lactococcus lactis UL719 competitivity and nisin (Nisaplin®) capacity to inhibit Clostridium difficile in a model of human colon. Front. Microbiol. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Osmanağaoğlu, Ö. Detection and characterization of Leucocin OZ, a new anti-listerial bacteriocin produced by Leuconostoc carnosum with a broad spectrum of activity. Food Control 2007, 18, 118–123. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Polyzos, K.A.; Patouni, K.; Rafailidis, P.I.; Samonis, G.; Falagas, M.E. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: A systematic review of the evidence. Int. J. Antimicrob. Agents 2012, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blay, G.L.; Lacroix, C.; Zihler, A.; Fliss, I. In vitro inhibition activity of nisin A, nisin Z, pediocin PA-1 and antibiotics against common intestinal bacteria. Lett. Appl. Microbiol. 2007, 45, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.L.; Dridi, L.; Bergeron, M.G.; Ouellette, M.; Fliss, I.l. Nisin is an effective inhibitor of Clostridium difficile vegetative cells and spore germination. J. Med. Microbiol. 2016, 65, 169–175. [Google Scholar] [CrossRef]
- Al Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185. [Google Scholar] [CrossRef]
- Maeda, N.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Kase, T.; Yoshikai, Y. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 2009, 9, 1122–1125. [Google Scholar] [CrossRef]
- Fernández, M.; Hudson, J.A.; Korpela, R.; de los Reyes-Gavilán, C.G. Impact on human health of microorganisms present in fermented dairy products: An overview. BioMed Res. Int. 2015, 2015, 412714. [Google Scholar] [CrossRef]
- Deepika Priyadarshani, W.M.; Rakshit, S.K. Screening selected strains of probiotic lactic acid bacteria for their ability to produce biogenic amines (histamine and tyramine). Int. J. Food Sci. Technol. 2011, 46, 2062–2069. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; De Las Rivas, B.; Landete, J.M.; Tabera, L.; Muñoz, R. Tyramine and phenylethylamine biosynthesis by food bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467. [Google Scholar] [CrossRef] [PubMed]
- Hazards, E.P.o.B. Scientific opinion on risk based control of biogenic amine formation in fermented foods. Efsa J. 2011, 9, 2393. [Google Scholar]
- Álvarez-Cisneros, Y.M.; Ponce-Alquicira, E. Antibiotic resistance in lactic acid bacteria. In Antimicrobial Resistance—A Global Threat; IntechOpen: London, UK, 2018. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Wang, X.; Wang, W.; Lv, H.; Zhang, H.; Liu, Y.; Zhang, M.; Wang, Y.; Tan, Z. Probiotic potential and wide-spectrum antimicrobial activity of lactic acid bacteria isolated from infant feces. Probiotics Antimicrob. Proteins 2021, 13, 90–101. [Google Scholar] [CrossRef]
- Mangia, N.P.; Saliba, L.; Deiana, P. Functional and safety characterization of autochthonous Lactobacillus paracasei FS103 isolated from sheep cheese and its survival in sheep and cow fermented milks during cold storage. Ann. Microbiol. 2019, 69, 161–170. [Google Scholar] [CrossRef]
- Abushelaibi, A.; Al-Mahadin, S.; El-Tarabily, K.; Shah, N.P.; Ayyash, M. Characterization of potential probiotic lactic acid bacteria isolated from camel milk. LWT-Food Sci. Technol. 2017, 79, 316–325. [Google Scholar] [CrossRef]
- Oh, Y.J.; Jung, D.S. Evaluation of probiotic properties of Lactobacillus and Pediococcus strains isolated from Omegisool, a traditionally fermented millet alcoholic beverage in Korea. LWT-Food Sci. Technol. 2015, 63, 437–444. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.-A.G.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef]
- Ismael, M.; Gu, Y.; Cui, Y.; Wang, T.; Yue, F.; Yantin, Q.; Lü, X. Lactic acid bacteria isolated from Chinese traditional fermented milk as novel probiotic strains and their potential therapeutic applications. 3 Biotech 2022, 12, 337. [Google Scholar] [CrossRef]
- Singhal, N.; Singh, N.S.; Mohanty, S.; Singh, P.; Virdi, J.S. Evaluation of probiotic characteristics of lactic acid bacteria isolated from two commercial preparations available in Indian market. Indian J. Microbiol. 2019, 59, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Motey, G.A.; Owusu-Kwarteng, J.; Obiri-Danso, K.; Ofori, L.A.; Ellis, W.O.; Jespersen, L. In vitro properties of potential probiotic lactic acid bacteria originating from Ghanaian indigenous fermented milk products. World J. Microbiol. Biotechnol. 2021, 37, 52. [Google Scholar] [CrossRef]
- dos Santos Leandro, E.; Ginani, V.C.; de Alencar, E.R.; Pereira, O.G.; Rose, E.C.P.; do Vale, H.M.M.; Pratesi, R.; Hecht, M.M.; Cavalcanti, M.H.; Tavares, C.S.O. Isolation, identification, and screening of lactic acid bacteria with probiotic potential in silage of different species of forage plants, cocoa beans, and artisanal salami. Probiotics Antimicrob. Proteins 2021, 13, 173–186. [Google Scholar] [CrossRef]
- de Amorim Trindade, D.P.; Barbosa, J.P.; Martins, E.M.F.; Tette, P.A.S. Isolation and identification of lactic acid bacteria in fruit processing residues from the Brazilian Cerrado and its probiotic potential. Food Biosci. 2022, 48, 101739. [Google Scholar] [CrossRef]
- Somashekaraiah, R.; Shruthi, B.; Deepthi, B.; Sreenivasa, M. Probiotic properties of lactic acid bacteria isolated from neera: A naturally fermenting coconut palm nectar. Front. Microbiol. 2019, 10, 1382. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Nero, L.A.; Todorov, S.D. Safety profiles of beneficial lactic acid bacteria isolated from dairy systems. Braz. J. Microbiol. 2020, 51, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.K.; Taneja, N.K.; Jain, D.; Ojha, A.; Kumawat, D.; Mishra, V. In Vitro Assessment of Probiotic and Technological Properties of Lactic Acid Bacteria Isolated from Indigenously Fermented Cereal-Based Food Products. Fermentation 2022, 8, 529. [Google Scholar] [CrossRef]
- da Silva Ferrari, I.; de Souza, J.V.; Ramos, C.L.; da Costa, M.M.; Schwan, R.F.; Dias, F.S. Selection of autochthonous lactic acid bacteria from goat dairies and their addition to evaluate the inhibition of Salmonella typhi in artisanal cheese. Food Microbiol. 2016, 60, 29–38. [Google Scholar] [CrossRef]
- Missaoui, J.; Saidane, D.; Mzoughi, R.; Minervini, F. Fermented seeds (“Zgougou”) from aleppo pine as a novel source of potentially probiotic lactic acid bacteria. Microorganisms 2019, 7, 709. [Google Scholar] [CrossRef]
- Javed, G.; Arshad, N.; Munir, A.; Khan, S.; Rasheed, S.; Hussain, I. Signature probiotic and pharmacological attributes of lactic acid bacteria isolated from human breast milk. Int. Dairy J. 2022, 127, 105297. [Google Scholar] [CrossRef]
- Bindu, A.; Lakshmidevi, N. Identification and in vitro evaluation of probiotic attributes of lactic acid bacteria isolated from fermented food sources. Arch. Microbiol. 2021, 203, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Atienza, E.; Gómez-Sala, B.; Araújo, C.; Campanero, C.; Del Campo, R.; Hernández, P.E.; Herranz, C.; Cintas, L.M. Antimicrobial activity, antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol. 2013, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Ben Farhat, L.; Romeo, F.V.; Foti, P.; Russo, N.; Randazzo, C.L.; Caggia, C.; Abidi, F. Multi-Functional Potential of Lactic Acid Bacteria Strains and Antimicrobial Effects in Minimally Processed Pomegranate (Punica granatum L. cv Jolly Red) Arils. Microorganisms 2022, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Banwo, K.; Asogwa, F.C.; Ogunremi, O.R.; Adesulu-Dahunsi, A.; Sanni, A. Nutritional profile and antioxidant capacities of fermented millet and sorghum gruels using lactic acid bacteria and yeasts. Food Biotechnol. 2021, 35, 199–220. [Google Scholar] [CrossRef]
- Kaktcham, P.M.; Temgoua, J.-B.; Zambou, F.N.; Diaz-Ruiz, G.; Wacher, C.; Pérez-Chabela, M.d.L. In vitro evaluation of the probiotic and safety properties of bacteriocinogenic and non-bacteriocinogenic lactic acid bacteria from the intestines of Nile tilapia and common carp for their use as probiotics in aquaculture. Probiotics Antimicrob. Proteins 2018, 10, 98–109. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.; Stanton, C.; Ross, P.; Dapkevicius, M.; Silva, C. Genetic diversity, safety and technological characterization of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiol. 2017, 63, 178–190. [Google Scholar] [CrossRef]
- Ribeiro, S.; Coelho, M.; Todorov, S.D.; Franco, B.D.G.d.M.; Dapkevicius, M.; Silva, C. Technological properties of bacteriocin-producing lactic acid bacteria isolated from Pico cheese an artisanal cow’s milk cheese. J. Appl. Microbiol. 2014, 116, 573–585. [Google Scholar] [CrossRef]
- Rabaoui, G.; Sánchez-Juanes, F.; Tebini, M.; Naghmouchi, K.; Bellido, J.L.M.; Ben-Mahrez, K.; Réjiba, S. Potential Probiotic Lactic Acid Bacteria with Anti-Penicillium expansum Activity from Different Species of Tunisian Edible Snails. Probiotics Antimicrob. Proteins 2022, 15, 82–106. [Google Scholar] [CrossRef]
- Rastogi, S.; Mittal, V.; Singh, A. Selection of potential probiotic bacteria from exclusively breastfed infant faeces with antagonistic activity against multidrug-resistant ESKAPE pathogens. Probiotics Antimicrob. Proteins 2021, 13, 739–750. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rodríguez-Aparicio, L.; Rúa, J.; Martínez-Blanco, H.; Navasa, N.; García-Armesto, M.R.; Ferrero, M.Á. In vitro evaluation of physiological probiotic properties of different lactic acid bacteria strains of dairy and human origin. J. Funct. Foods 2012, 4, 531–541. [Google Scholar] [CrossRef]
- Le, B.; Anh, P.T.N.; Kim, J.-E.; Cheng, J.; Yang, S.H. Rice bran fermentation by lactic acid bacteria to enhance antioxidant activities and increase the ferulic acid, ρ-coumaric acid, and γ-oryzanol content. J. Appl. Biol. Chem. 2019, 62, 257–264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).