Chayote (Sechium edule (Jacq.) Swartz) Seed as an Unexploited Protein Source: Bio-Functional and Nutritional Quality of Protein Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Material
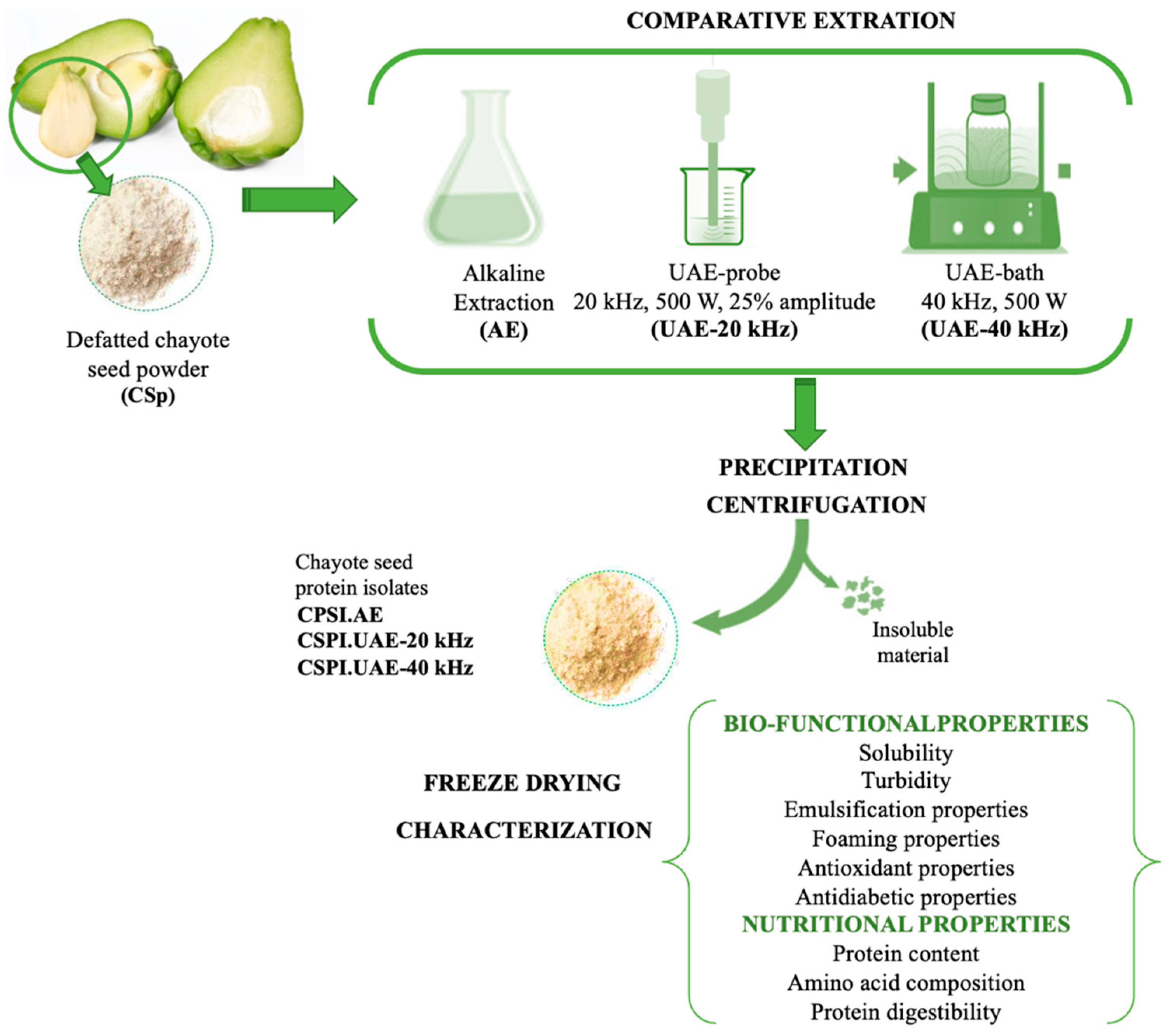
2.3. Preparation of Chayote-Seed Protein Isolates (CSPI)
2.4. Protein Extraction Yield (%) and Protein Purity (%)
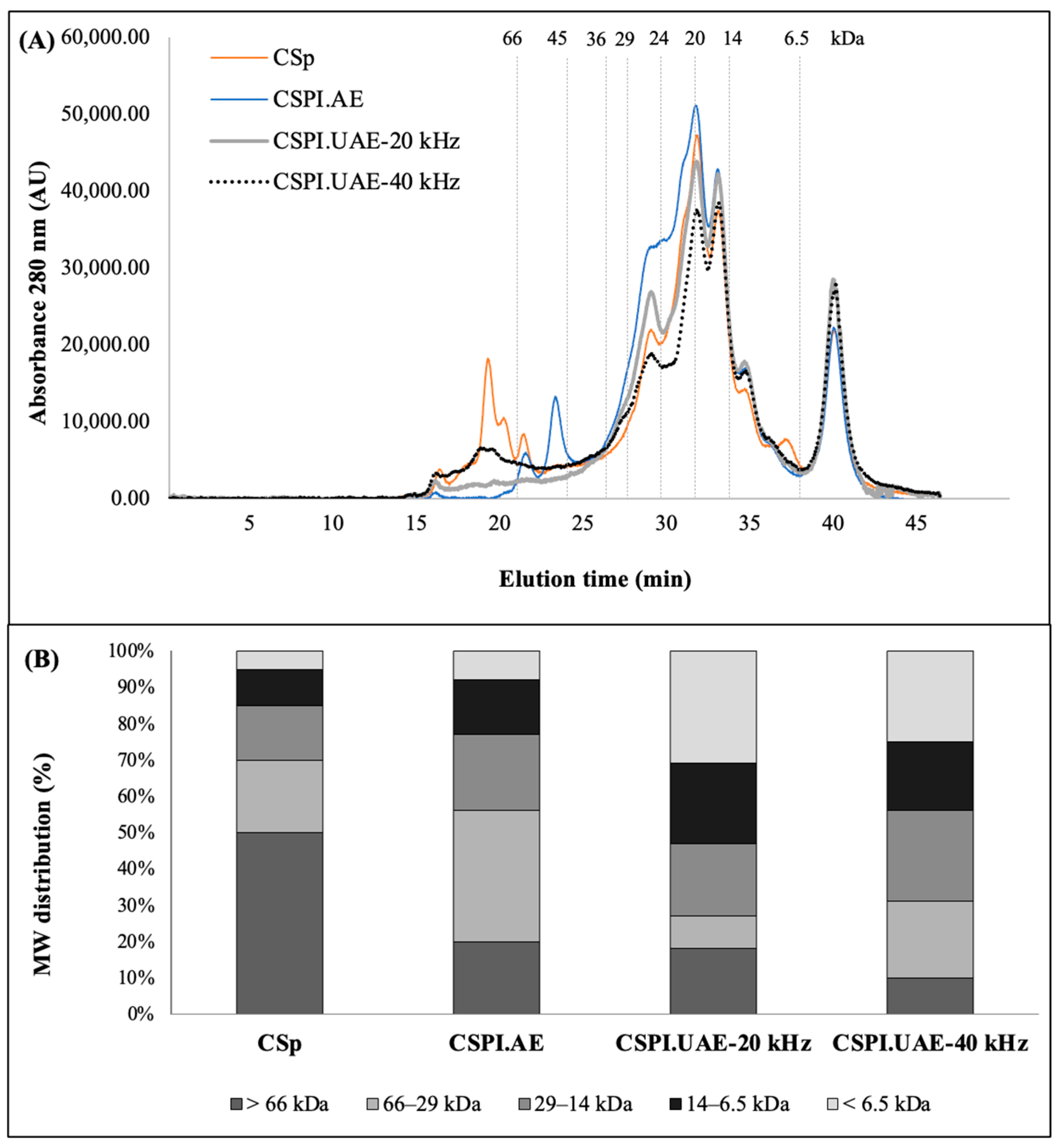
2.5. Size Exclusion Chromatography (SE-HPLC)
2.6. Functional Properties
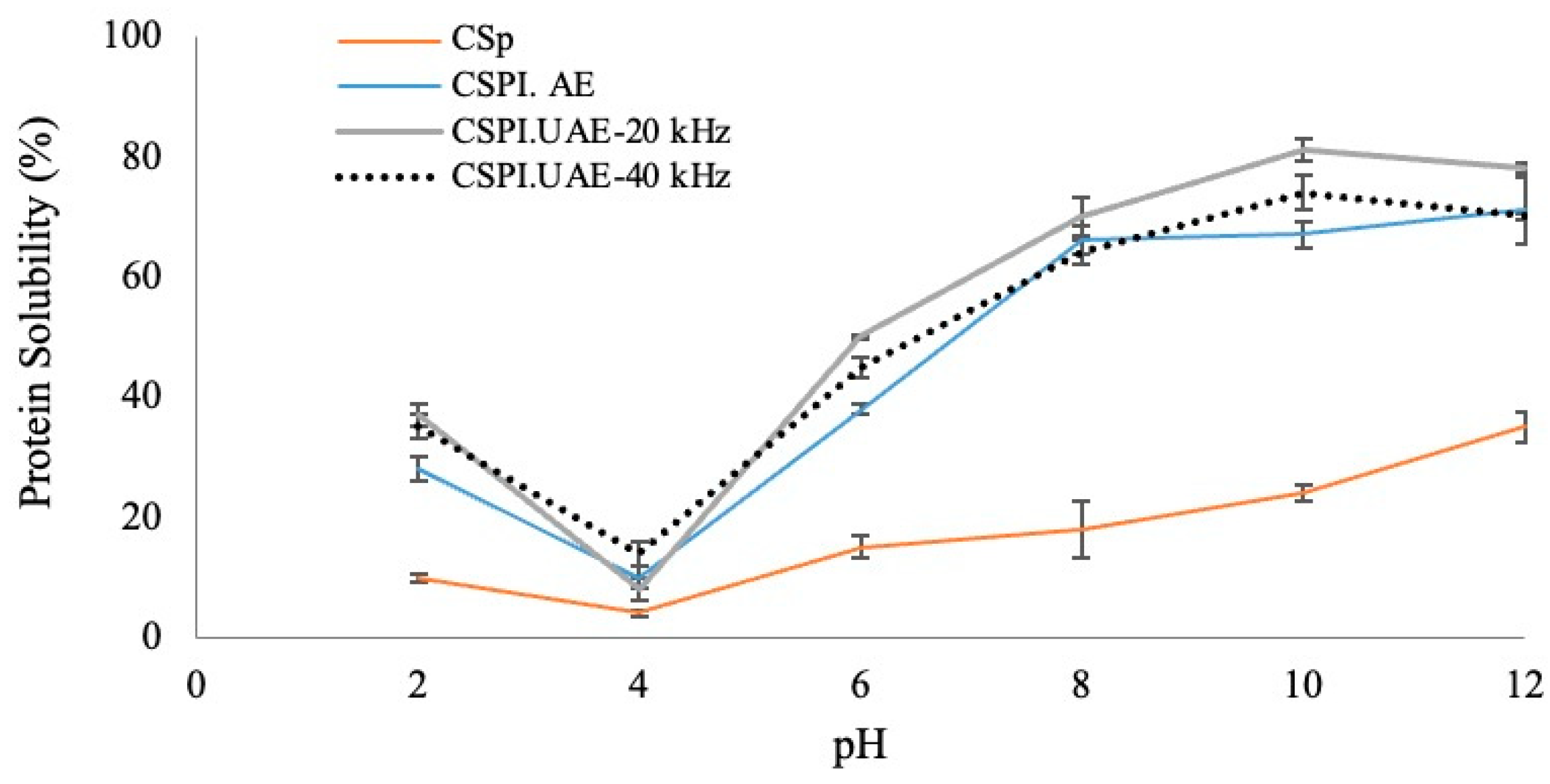
2.6.1. Protein Solubility
2.6.2. Turbidity
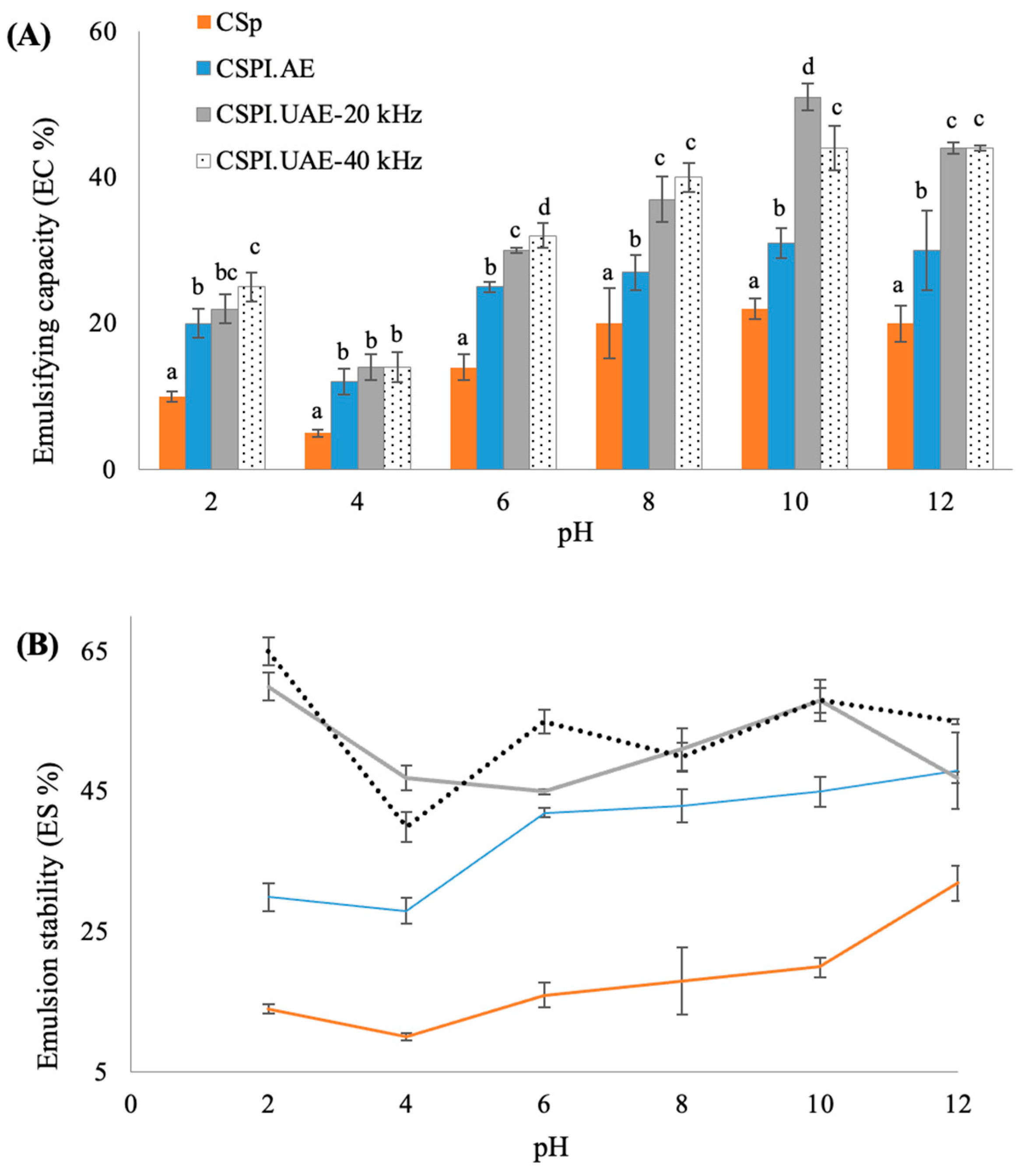
2.6.3. Emulsification Properties
2.6.4. Foaming Properties
2.7. Nutraceutical Potential
2.7.1. Amino Acid Composition
2.7.2. Protein Digestibility
2.7.3. Nutritional Protein Quality
2.7.4. Total Phenolic Content
2.7.5. Antioxidant Capacity
2.7.6. Anti-Diabetic Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Protein Extraction Yields and Protein Purity
3.2. Molecular Size Distribution of CSPIs
3.3. Functional Properties of CSPIs
3.3.1. Protein Solubility
3.3.2. Turbidity
3.3.3. Emulsifying Property
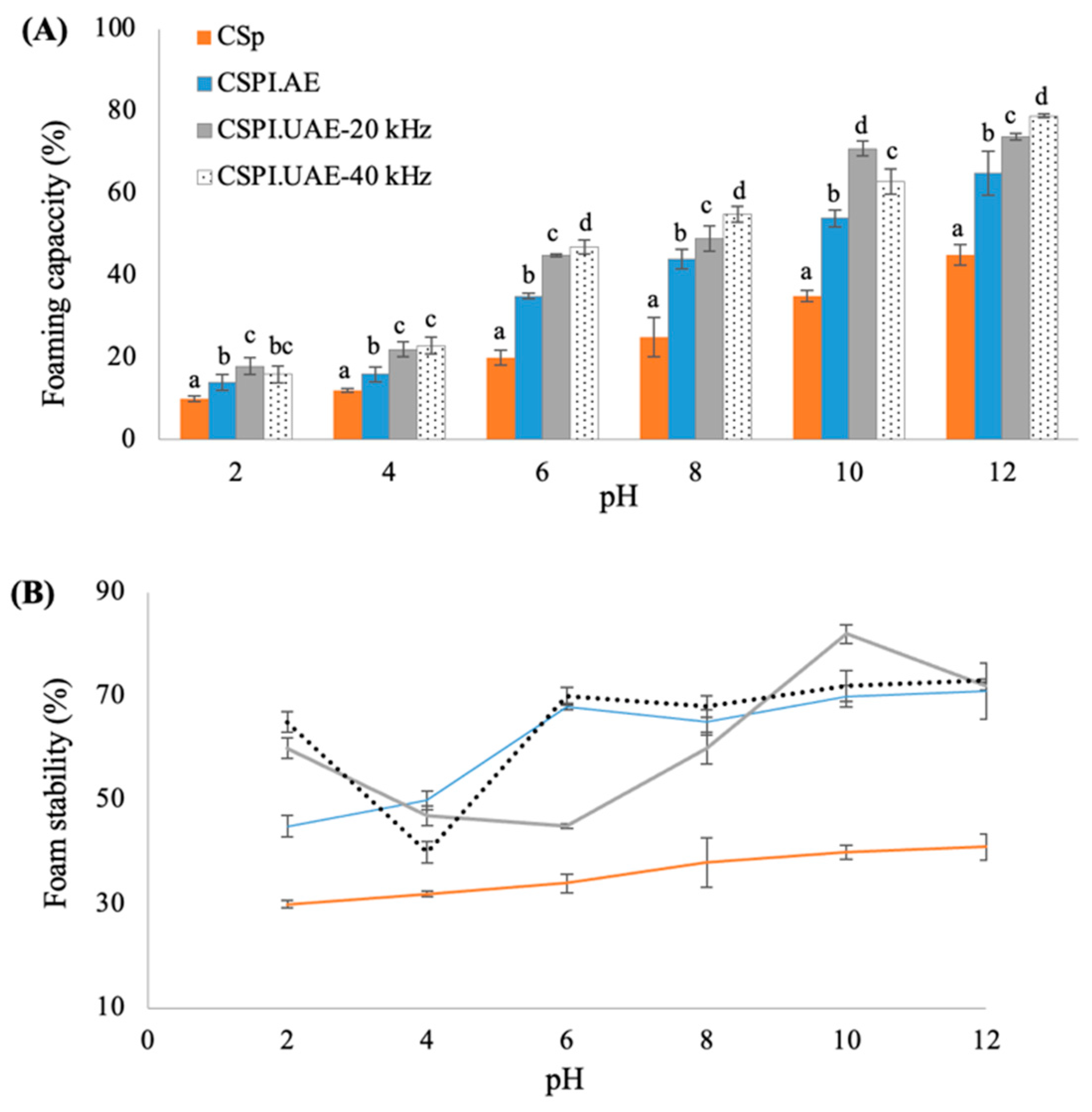
3.3.4. Foaming Properties
3.4. Nutritional Properties of CSPIs
3.4.1. Amino Acid Composition
3.4.2. Protein Quality
3.5. Biological Properties of CSPIs
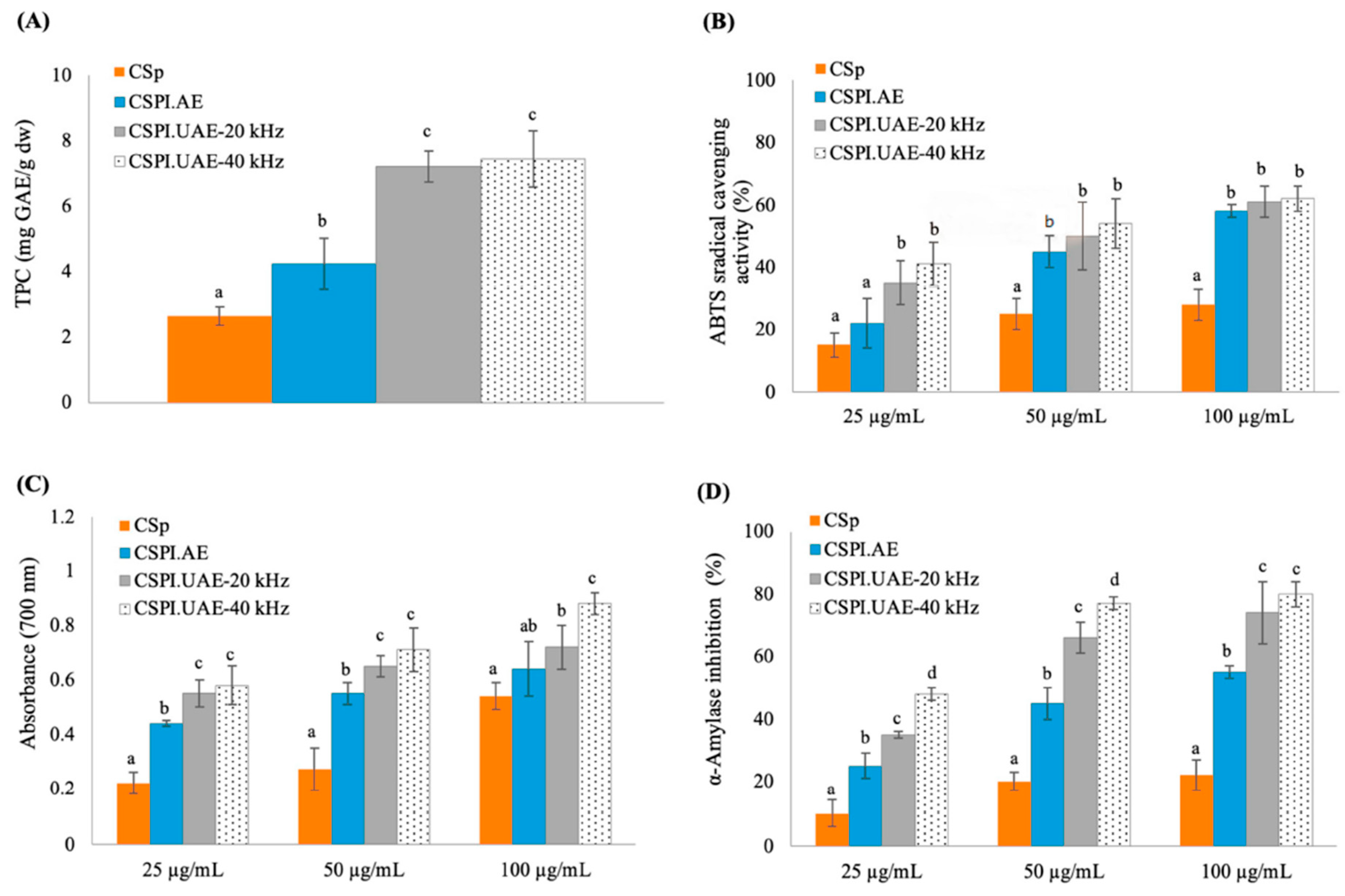
3.5.1. Total Phenolic Content and Antioxidant Activity
3.5.2. Inhibition of α-Amylase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngo, N.T.T.; Shahidi, F. Functional properties of protein isolates from camelina (Camelina sativa (L.) Crantz) and flixweed (sophia, Descurainis sophia L.) seed meals. Food Prod. Process Nutr. 2021, 3, 31. [Google Scholar] [CrossRef]
- Vinayashree, S.; Vasu, P. Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var. Kashi Harit) seeds. Food Chem. 2021, 340, 128177. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.; Natarajan, V.; Modupalli, N.; Thangaraj, S.; Rawson, A. Pulsed ultrasound assisted extraction of protein from defatted Bitter melon seeds (Momardica charantia L.) meal: Kinetics and quality measurements. LWT 2022, 155, 112997. [Google Scholar] [CrossRef]
- Franca-Oliveira, G.; Fornari, T.; Hernández-Ledesma, B. A review on the extraction and processing of natural source-derived proteins through eco-innovative approaches. Processes 2021, 9, 1626. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrason. Sonochem. 2017, 39, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Das, R.S.; Tiwari, B.K.; Chemat, F.; Garcia-Vaquero, M. Impact of ultrasound processing in alternative protein systems: Protein extraction, nutritional effects and associated challenges. Ultrason. Sonochem. 2022, 91, 106234. [Google Scholar] [CrossRef]
- Dabbour, M.; He, R.; Ma, H.; Musa, A. Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Process Eng. 2018, 41, e12799. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef]
- Du, H.; Zhang, J.; Wang, S.; Manyande, A.; Wang, J. Effect of high-intensity ultrasonic treatment on the physicochemical, structural, rheological, behavioral, and foaming properties of pumpkin (Cucurbita moschata Duch.)-seed protein isolates. LWT 2022, 155, 112952. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, G. Chayote—A potential vegetable crop for Florida: HS1454. EDIS 2022. [Google Scholar] [CrossRef]
- Vieira, E.F.; Pinho, O.; Ferreira, I.M.P.L.V.O.; Delerue-Matos, C. Chayote (Sechium edule): A review of nutritional composition, bioactivities and potential applications. Food Chem. 2019, 275, 557–568. [Google Scholar] [CrossRef]
- Communities AoA. Official Methods of Analysis of AOAC International, 7th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Vieira, E.F.; Pinho, O.; Ferreira, I.M.P.L.V.O. Bio-functional properties of sardine protein hydrolysates obtained by brewer’s spent yeast and commercial proteases. J. Sci. Food Agric. 2017, 97, 5414–5422. [Google Scholar] [CrossRef] [PubMed]
- Achouri, A.; Nail, V.; Boye, J.I. Sesame protein isolate: Fractionation, secondary structure and functional properties. Food Res. Int. 2012, 46, 360–369. [Google Scholar] [CrossRef]
- Bradford Protein Assay. Bio-Protocol Bio101. e45. 2011. Available online: http://wwwbio-protocolorg/e45 (accessed on 19 May 2023).
- Lawal, O.S.; Adebowale, K.O.; Adebowale, Y.A. Functional properties of native and chemically modified protein concentrates from bambarra groundnut. Food Res. Int. 2007, 40, 1003–1011. [Google Scholar] [CrossRef]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-Teles, M.T.; Carvalho, A.P.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.O.R.S.T.E.N.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Friedman, M. Nutritional value of proteins from different food sources. A review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- FAO; WHO; UNU. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef]
- Vieira, E.F.; Ferreira, I.M.P.L.V.O. Antioxidant and antihypertensive hydrolysates obtained from by-products of cannery sardine and brewing industries. Int. J. Food Prop. 2017, 20, 662–673. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
- Esfandi, R.; Seidu, I.; Willmore, W.; Tsopmo, A. Antioxidant, pancreatic lipase, and α-amylase inhibitory properties of oat bran hydrolyzed proteins and peptides. J. Food Biochem. 2022, 46, e1376. [Google Scholar] [CrossRef]
- Wu, T.H.; Chow, L.P.; Lin, J.Y. Sechiumin, a ribosome-inactivating protein from the edible gourd, Sechium edule Swartz: Purification, characterization, molecular cloning and expression. Eur. J. Biochem. 1998, 255, 400–408. [Google Scholar] [CrossRef]
- Espíndola Mateos, S.; Cervantes, C.A.M.; Zenteno, E.; Slomianny, M.C.; Alpuche, J.; Hernández-Cruz, P.; Martínez-Cruz, R.; Canseco, M.D.S.P.; Pérez-Campos, E.; Rubio, M.S.; et al. Purification and partial characterization of β-glucosidase in chayote (Sechium edule). Molecules 2015, 20, 19372–19392. [Google Scholar] [CrossRef] [PubMed]
- Vladova, R.; Tsanev, V.; Petcolicheva, K. Seed storage proteins in Solanaceae and Cucurbitaceae species. Biol. Plant 2004, 48, 601–603. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Guo, L.-L.; Wei, F.; Li, J.-F.; Jiang, M.-L.; Li, G.-M.; Zhao, Y.-D.; Chen, H. Some characteristics and functional properties of rapeseed protein prepared by ultrasonication, ultrafiltration and isoelectric precipitation. J. Sci. Food Agric. 2011, 91, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Zhang, Q.-T.; Tu, Z.-C.; Xiao, H.; Wang, H.; Huang, X.-Q.; Liu, G.-X.; Liu, C.-M.; Shi, Y.; Fan, L.-L.; Lin, D.-R. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Bioprod. Process 2014, 92, 30–37. [Google Scholar] [CrossRef]
- Morales, R.; Martínez, K.D.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M. Modification of foaming properties of soy protein isolate by high ultrasound intensity: Particle size effect. Ultrason. Sonochem. 2015, 26, 48–55. [Google Scholar] [CrossRef]
- Flick, G.J., Jr.; Burnette, F.S.; Aung, L.H.; Ory, R.L.; St. Angelo, A.J. Chemical composition and biochemical properties of mirlitons (Sechium edule) and purple, green, and white eggplants (Solanum melongena). J. Agric. Food Chem. 1978, 26, 1000–1005. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J. Arginine nutrition and cardiovascular function. J. Nutr. 2000, 130, 2626–2629. [Google Scholar] [CrossRef]
- Wani, A.A.; Sogi, D.S.; Singh, P.; Wani, I.A.; Shivhare, U.S. Characterisation and functional properties of watermelon (Citrullus lanatus) seed proteins. J. Sci. Food Agric. 2011, 91, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, A.A.L.; Gómez, J.D.; Cudmani, N.M.; Vattuone, M.A.; Isla, M.I. Antimicrobial activity of nine extracts of Sechium edule (Jacq.) Swartz. Microb. Ecol. Health Dis. 2003, 15, 33–39. [Google Scholar] [CrossRef]
- Guzmán-Lorite, M.; Marina, M.L.; García, M.C. Pressurized liquids vs. high intensity focused ultrasounds for the extraction of proteins from a pomegranate seed waste. Innov. Food Sci. Emerg. Technol. 2022, 77, 102958. [Google Scholar] [CrossRef]
- Ozuna, C.; León-Galván, M.F. Cucurbitaceae seed protein hydrolysates as a potential source of bioactive peptides with functional properties. BioMed Res. Int. 2017, 2017, 2121878. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, B.A.; Sharma, P. Recently isolated antidiabetic hydrolysates and peptides from multiple food sources: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 322–340. [Google Scholar] [CrossRef]
- Horax, R.; Hettiarachchy, N.; Over, K.; Chen, P.; Gbur, E. Extraction, fractionation and characterization of bitter melon seed proteins. J. Agric. Food Chem. 2010, 58, 1892–1897. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Gani, A. Characterization of apple (Malus domestica) seed flour for its structural and nutraceutical potential. LWT 2021, 151, 112138. [Google Scholar] [CrossRef]
| Protein (% dw) | Protein Yield Recovery (%) | Protein Purity (%) | |
|---|---|---|---|
| CSp 1 | 5.5 ± 0.4 a | - | - |
| CSPI.AE 2 | 6.6 ± 0.6 b | 58.0 ± 2.2 a | 47.9 ± 1.8 a |
| CSPI.UAE-20 kHz 3 | 8.2 ± 0.9 d | 88.0 ± 3.2 c | 66.7 ± 2.5 b |
| CSPI.UAE-40 kHz 4 | 7.7 ± 1.3 c | 72.4 ± 5.6 b | 64.4 ± 5.1 b |
| AA | CSp | CSPI.AE | CSPI.UAE-20 kHz | CSPI.UAE-40 kHz | FAO/WHO/UNU (2007) [20] |
|---|---|---|---|---|---|
| Asp | 34.70 | 45.34 | 57.83 | 54.32 | |
| Glu | 65.70 | 65.50 | 87.37 | 83.37 | |
| Ser | 31.10 | 41.88 | 66.33 | 66.33 | |
| Thr | 23.50 (1.0) | 25.39 (1.1) | 32.84 (1.4) | 31.14 (1.4) | 23 |
| His | 15.15 (1.0) | 15.44 (1.0) | 17.90 (1.2) | 15.52 (1.0) | 15 |
| Gly | 12.50 | 16.80 | 27.82 | 28.21 | |
| Gln | 17.40 | 21.93 | 37.43 | 33.08 | |
| Asn | 8.70 | 19.50 | 21.47 | 21.46 | |
| Arg | 135.40 | 147.27 | 167.46 | 166.95 | |
| Ala | 25.40 | 29.73 | 38.79 | 30.91 | |
| Tyr | 18.10 | 20.30 | 32.55 | 31.85 | |
| Lys | 46.80 (1.0) | 47.62 (1.1) | 48.97 (1.1) | 48.70 (1.1) | 45 |
| Val | 40.80 (1.0) | 43.70 (1.1) | 47.85 (1.2) | 47.42 (1.2) | 39 |
| Met | 7.57 | 8.00 | 8.50 | 8.70 | |
| Trp | 5.60 (0.9) | 6.01 (1.0) | 6.15 (1.0) | 6.05 (1.0) | 6 |
| Cys | 25.02 | 28.02 | 31.25 | 30.25 | |
| Phe | 20.60 | 31.35 | 44.71 | 40.13 | |
| Ile | 31.20 (1.0) | 33.70 (1.1) | 49.80 (1.7) | 47.76 (1.6) | 30 |
| Leu | 30.10 (0.5) | 32.56 (0.6) | 58.91 (0.9) | 54.06 (0.9) | 59 |
| Pro | 20.65 | 28.86 | 21.20 | 20.90 | |
| ∑ AA 1 | 615.97 | 708.91 | 905.14 | 867.09 | |
| ∑ EAA 2 | 221.31 | 243.77 | 315.63 | 299.46 | |
| ∑ AAA 3 | 38.70 | 51.65 | 77.26 | 71.98 | |
| ∑ SAA 4 | 32.57 | 36.02 | 39.75 | 38.95 | |
| ∑ FAA 5 | 138.30 | 157.37 | 211.82 | 196.81 | |
| % EAA | 35.93 | 34.39 | 34.87 | 34.54 | |
| % FAA | 22.45 | 22.20 | 23.40 | 22.70 | |
| EAAI (%) 6 | 103.03 | 153.58 | 424.25 | 339.29 |
| Protein Digestibility (%) | PDCAAS 5 | |
|---|---|---|
| CSp 1 | 47.2 ± 3.1 a | 24.1 ± 2.8 a |
| CSPI.AE 2 | 76.8 ± 3.1 b | 42.4 ± 1.7 b |
| CSPI.UAE-20 kHz 3 | 80.3 ± 4.5 bc | 73.6 ± 3.3 c |
| CSPI.UAE-40 kHz 4 | 86.1 ± 1.4 c | 86.1 ± 3.6 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, E.F.; Fontoura, A.Q.; Delerue-Matos, C. Chayote (Sechium edule (Jacq.) Swartz) Seed as an Unexploited Protein Source: Bio-Functional and Nutritional Quality of Protein Isolates. Foods 2023, 12, 2949. https://doi.org/10.3390/foods12152949
Vieira EF, Fontoura AQ, Delerue-Matos C. Chayote (Sechium edule (Jacq.) Swartz) Seed as an Unexploited Protein Source: Bio-Functional and Nutritional Quality of Protein Isolates. Foods. 2023; 12(15):2949. https://doi.org/10.3390/foods12152949
Chicago/Turabian StyleVieira, Elsa F., Ana Q. Fontoura, and Cristina Delerue-Matos. 2023. "Chayote (Sechium edule (Jacq.) Swartz) Seed as an Unexploited Protein Source: Bio-Functional and Nutritional Quality of Protein Isolates" Foods 12, no. 15: 2949. https://doi.org/10.3390/foods12152949
APA StyleVieira, E. F., Fontoura, A. Q., & Delerue-Matos, C. (2023). Chayote (Sechium edule (Jacq.) Swartz) Seed as an Unexploited Protein Source: Bio-Functional and Nutritional Quality of Protein Isolates. Foods, 12(15), 2949. https://doi.org/10.3390/foods12152949







