Spectro-Electrochemical Properties of A New Non-Enzymatic Modified Working Electrode Used for Histamine Assessment in the Diagnosis of Food Poisoning
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Composite Solution Preparation
2.3. Electrochemical Polymerization of Graphene-Thiophene Composite
2.4. Preparation of Histamine Solutions
2.5. Preparation of the Interfering Solution
2.6. Electrochemical Characterization
3. Results and Discussions
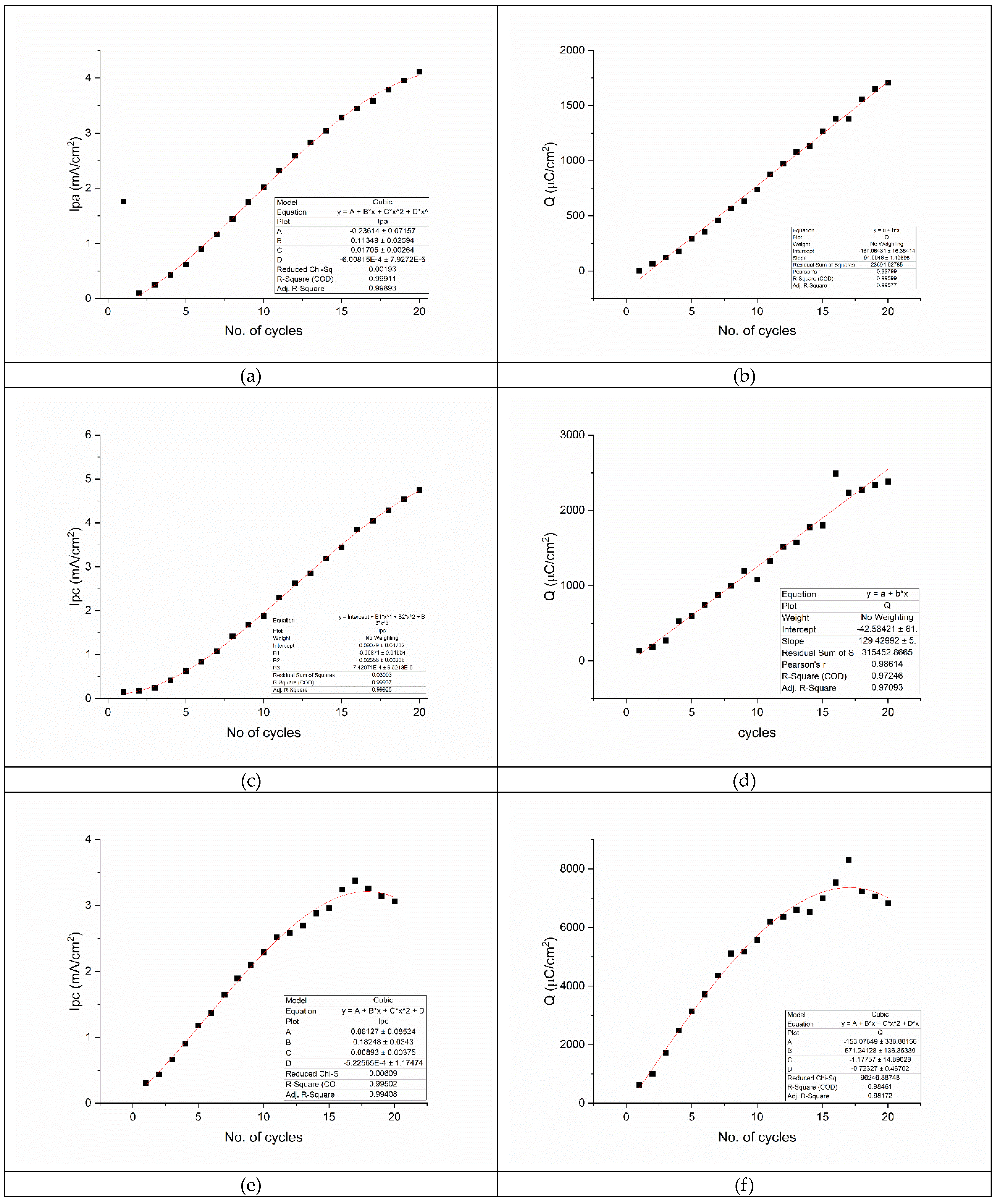
3.1. Electropolymerization of the Graphene-Thiophene Sensitive Layer
3.2. Raman Spectroscopy of the Sensitive Layer
3.3. Detection of Histamine in Phosphate Buffer Solution (PBS)
3.4. Determination of the Appropriate TCA Concentration
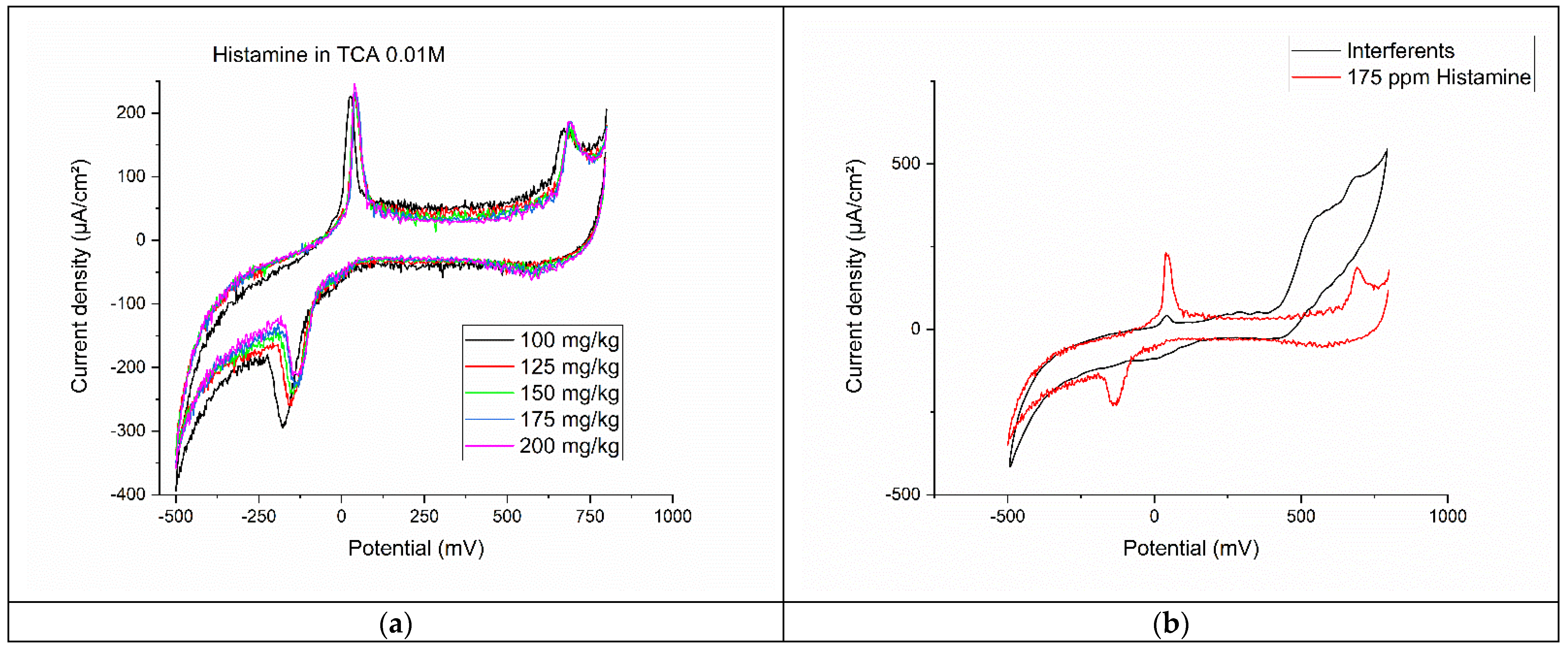
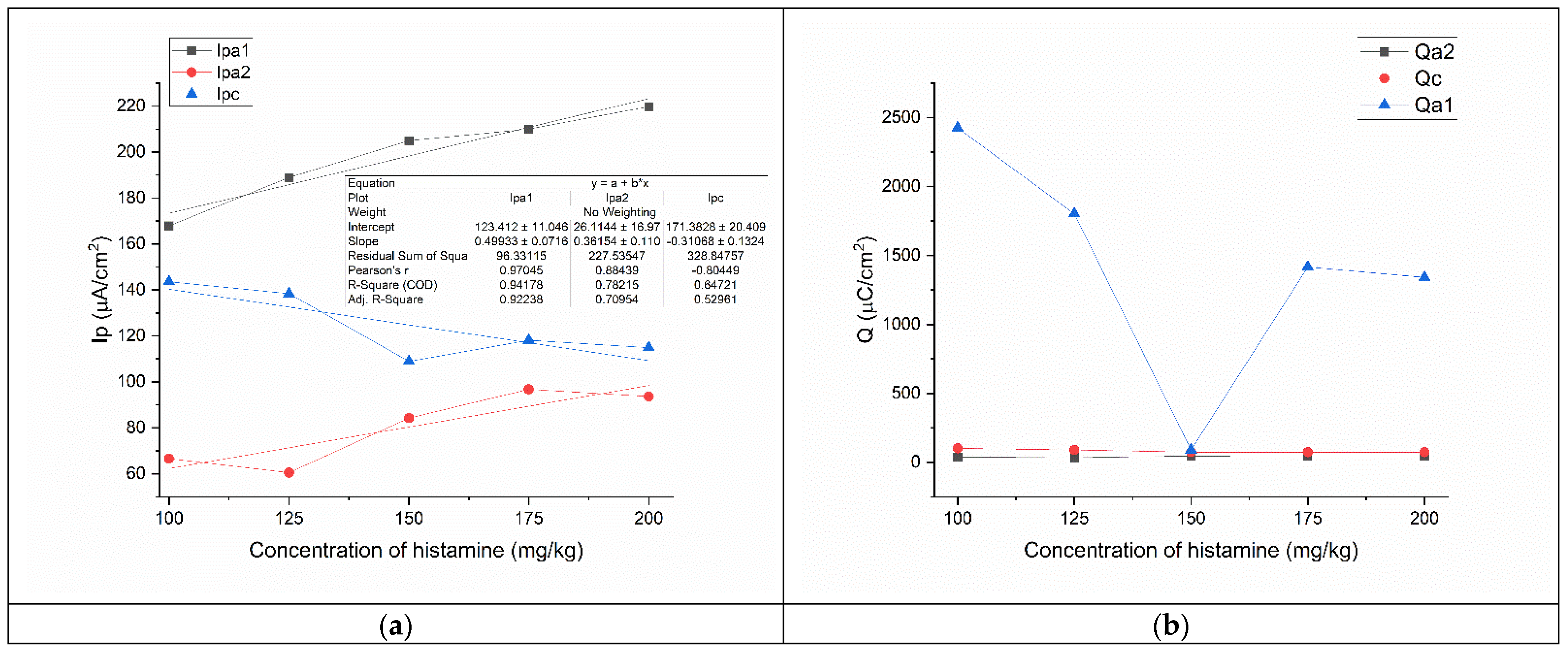
3.5. Evaluation of Histamine in 0.01 M TCA
3.6. Analysis of Interferents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NHS. Food Poisoning. United Kingdom National Health Service. Available online: https://www.nhs.uk/conditions/food-poisoning/ (accessed on 4 September 2022).
- CDC. Food Safety. Center for Disease Control, USA. Available online: https://www.cdc.gov/foodsafety/symptoms.html (accessed on 4 September 2022).
- Li, S.; Zhong, T.; Long, Q.; Huang, C.; Chen, L.; Lu, D.; Li, X.; Zhang, Z.; Shen, G.; Hou, X. A gold nanoparticles-based molecularly imprinted electrochemical sensor for histamine specific-recognition and determination. Microchem. J. 2021, 171, 106844. [Google Scholar] [CrossRef]
- Morrow, J.D.; Margolies, G.R.; Rowland, J.; Roberts, L.J. Evidence That Histamine Is the Causative Toxin of Scombroid-Fish Poisoning. N. Engl. J. Med. 1991, 324, 716–720. [Google Scholar] [CrossRef]
- Tu, A. Food Poisoning—Handbook of Natural Toxins; Marcel Dekker, Inc.: New York, NY, USA, 1992. [Google Scholar]
- Lee, Y.-C.; Kung, H.-F.; Wu, C.-H.; Hsu, H.-M.; Chen, H.-C.; Huang, T.-C.; Tsai, Y.-H. Determination of histamine in milkfish stick implicated in food-borne poisoning. J. Food Drug Anal. 2016, 24, 63–71. [Google Scholar] [CrossRef]
- Taylor, S.L.; Stratton, J.E.; Nordlee, J.A. Histamine Poisoning (Scombroid Fish Poisoning): An Allergy-Like Intoxication. J. Toxicol. Clin. Toxicol. 1989, 27, 225–240. [Google Scholar] [CrossRef]
- Taylor, S.L.; Eitenmiller, R.R. Histamine Food Poisoning: Toxicology and Clinical Aspects. CRC Crit. Rev. Toxicol. 1986, 17, 91–128. [Google Scholar] [CrossRef] [PubMed]
- Koo, P.-L.; Lim, G.-K. A review on analytical techniques for quantitative detection of histamine in fish products. Microchem. J. 2023, 189, 108499. [Google Scholar] [CrossRef]
- Sagratini, G.; Fernández-Franzón, M.; De Berardinis, F.; Font, G.; Vittori, S.; Mañes, J. Simultaneous determination of eight underivatised biogenic amines in fish by solid phase extraction and liquid chromatography–tandem mass spectrometry. Food Chem. 2012, 132, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Carneiro, L.S.; Vianna, M.S.; Romani, E.C.; Aucelio, R.Q. Determination of histamine in tuna fish by photoluminescence sensing using thioglycolic acid modified CdTe quantum dots and cationic solid phase extraction. J. Lumin. 2017, 182, 71–78. [Google Scholar] [CrossRef]
- Vidal-Carou, M.C.; Veciana-Nogués, M.T.; Mariné-Font, A. Spectrofluorometric determination of histamine in fish and meat products. J. AOAC Int. 1990, 73, 565–567. [Google Scholar] [CrossRef]
- Alizadeh, N.; Kamalabadi, M.; Mohammadi, A. Determination of Histamine and Tyramine in Canned Fish Samples by Headspace Solid-Phase Microextraction Based on a Nanostructured Polypyrrole Fiber Followed by Ion Mobility Spectrometry. Food Anal. Methods 2017, 10, 3001–3008. [Google Scholar] [CrossRef]
- Cao, D.; Xu, X.; Xue, S.; Feng, X.; Zhang, L. An in situ derivatization combined with magnetic ionic liquid-based fast dispersive liquid-liquid microextraction for determination of biogenic amines in food samples. Talanta 2019, 199, 212–219. [Google Scholar] [CrossRef]
- Peng, J.-F.; Fang, K.-T.; Xie, D.-H.; Ding, B.; Yin, J.-Y.; Cui, X.-M.; Zhang, Y.; Liu, J.-F. Development of an automated on-line pre-column derivatization procedure for sensitive determination of histamine in food with high-performance liquid chromatography–fluorescence detection. J. Chromatogr. A 2008, 1209, 70–75. [Google Scholar] [CrossRef]
- Harmoko, H.; Kartasasmita, R.E.; Munawar, H.; Rakhmawati, A.; Budiawan, B. Determination of histamine in different compositions of commercially canned fish in Indonesia by modified QuEChERS and LC-MS/MS. J. Food Compos. Anal. 2021, 105, 104256. [Google Scholar] [CrossRef]
- Fukushima, Y.; Aikawa, S. Colorimetric sensing of histamine in aqueous solution by a system composed of alizarin complexone and Ni2+ complex via indicator displacement approach. Tetrahedron Lett. 2021, 72, 153088. [Google Scholar] [CrossRef]
- Gagic, M.; Nejdl, L.; Xhaxhiu, K.; Cernei, N.; Zitka, O.; Jamróz, E.; Švec, P.; Richtera, L.; Kopel, P.; Milosavljevic, V.; et al. Fully automated process for histamine detection based on magnetic separation and fluorescence detection. Talanta 2020, 212, 120789. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Feng, S.; Park, C.Y.; Park, K.Y.; Song, J.; Park, J.P.; Chun, H.S.; Park, T.J. Fluorescence detection of histamine based on specific binding bioreceptors and carbon quantum dots. Biosens. Bioelectron. 2020, 167, 112519. [Google Scholar] [CrossRef]
- Zhou, T.; Fan, M.; You, R.; Lu, Y.; Huang, L.; Xu, Y.; Feng, S.; Wu, Y.; Shen, H.; Zhu, L. Fabrication of Fe3O4/Au@ATP@Ag Nanorod sandwich structure for sensitive SERS quantitative detection of histamine. Anal. Chim. Acta 2020, 1104, 199–206. [Google Scholar] [CrossRef]
- Li, P.; Zhou, B.; Ge, M.; Jing, X.; Yang, L. Metal coordination induced SERS nanoprobe for sensitive and selective detection of histamine in serum. Talanta 2021, 237, 122913. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, G.; Bao, J.; Liu, C.; Li, W.; Kong, Z.; Sun, X.; Li, J.; Lu, R. Fabrication of an Ag-based SERS nanotag for histamine quantitative detection. Talanta 2023, 256, 124256. [Google Scholar] [CrossRef]
- Yang, F.; Xu, L.; Dias, A.C.; Zhang, X. A sensitive sandwich ELISA using a modified biotin-streptavidin amplified system for histamine detection in fish, prawn and crab. Food Chem. 2021, 350, 129196. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Lin, Z.-Z.; Hong, C.-Y.; Huang, Z.-Y. Histamine detection in fish samples based on indirect competitive ELISA method using iron-cobalt co-doped carbon dots labeled histamine antibody. Food Chem. 2020, 345, 128812. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, H.; Tang, R.; Tan, Y.; Xia, X.; Zhang, X. Construction of histamine aptamer sensor based on Au NPs nanozyme for ultrasensitive SERS detection of histamine. J. Food Compos. Anal. 2023, 120. [Google Scholar] [CrossRef]
- DeBeeR, J.; Bell, J.W.; Nolte, F.; Arcieri, J.; Correa, G. Histamine Limits by Country: A Survey and Review. J. Food Prot. 2021, 84, 1610–1628. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hong, C.; Lin, Z.; Huang, Z. Rapid detection of histamine in fish based on the fluorescence characteristics of carbon nitride. J. Food Compos. Anal. 2022, 112, 104659. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Sahudin, M.A.; Tan, L.L.; Su’Ait, M.S.; Karim, N.H.A.; Mackeen, M.M. Regenerable and selective histamine impedimetric sensor based on hydroxyl functionalised Schiff base complex electrode. Electrochim. Acta 2021, 379, 138186. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, Y.; Jia, Y.; Ye, B.-C. Synthesis of MOF-derived Ni@C materials for the electrochemical detection of histamine. Talanta 2020, 219, 121360. [Google Scholar] [CrossRef]
- Wang, R.; Mao, Y.; Wang, L.; Qu, H.; Chen, Y.; Zheng, L. Solution-gated graphene transistor based sensor for histamine detection with gold nanoparticles decorated graphene and multi-walled carbon nanotube functionalized gate electrodes. Food Chem. 2021, 347, 128980. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Waterhouse, G.I.; Qiao, X.; Xu, Z. A surface-imprinted surface-enhanced Raman scattering sensor for histamine detection based on dual semiconductors and Ag nanoparticles. Food Chem. 2021, 369, 130971. [Google Scholar] [CrossRef]
- Gumpu, M.; Nesakumar, N.; Sethuraman, S.K.U.; Rayappan, J. Development of electrochemical biosensor with ceria–PANIcore–shell nano-interface for the detection of histamine. Sens. Actuators B 2014, 199, 330–338. [Google Scholar] [CrossRef]
- Xu, S.; Wu, F.; Mu, F.; Dai, B. The preparation of Fe-based peroxidase mimetic nanozymes and for the electrochemical detection of histamine. J. Electroanal. Chem. 2022, 908, 116088. [Google Scholar] [CrossRef]
- Tian, X.; Liu, H.; Liu, H.; Wang, X. Immobilizing diamine oxidase on electroactive phase-change microcapsules to construct thermoregulatory smart biosensor for enhancing detection of histamine in foods. Food Chem. 2022, 397, 133759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, Q.; Guo, J.; Jiao, X.; Kong, X.; Yu, Q. Surface-enhanced Raman spectroscopy tandem with derivatized thin-layer chromatography for ultra-sensitive on-site detection of histamine from fish. Food Control. 2022, 138, 108987. [Google Scholar] [CrossRef]
- Saghatforoush, L.; Hasanzadeh, M.; Shadjou, N. Polystyrene–graphene oxide modified glassy carbon electrode as a new class of polymeric nanosensors for electrochemical determination of histamine. Chin. Chem. Lett. 2014, 25, 655–658. [Google Scholar] [CrossRef]
- Kumar, N.; Goyal, R.N. Silver nanoparticles decorated graphene nanoribbon modified pyrolytic graphite sensor for determination of histamine. Sens. Actuators B Chem. 2018, 268, 383–391. [Google Scholar] [CrossRef]
- Peeters, M.; Kobben, S.; Jiménez-Monroy, K.; Modesto, L.; Kraus, M.; Vandenryt, T.; Gaulke, A.; van Grinsven, B.; Ingebrandt, S.; Junkers, T.; et al. Thermal detection of histamine with a graphene oxide based molecularly imprinted polymer platform prepared by reversible addition–fragmentation chain transfer polymerization. Sens. Actuators B Chem. 2014, 203, 527–535. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Chen, X. Competitive electrochemical immunosensor for the detection of histamine based on horseradish peroxidase initiated deposition of insulating film. J. Electroanal. Chem. 2015, 736, 88–92. [Google Scholar] [CrossRef]
- Gonçalves, L.M. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2020, 25, 100640. [Google Scholar] [CrossRef]
- Oberhaus, F.V.; Frense, D. Catalysing electropolymerization: High-quality polythiophene films for electrochemical sensors by the utilization of fluorine based Lewis acid catalysts. Electrochim. Acta 2021, 402, 139536. [Google Scholar] [CrossRef]
- Tanaka, K.; Shichiri, T.; Wang, S.; Yamabe, T. A study of the electropolymerization of thiophene. Synth. Met. 1988, 24, 203–215. [Google Scholar] [CrossRef]
- Surucu, O.; Bolat, G.; Abaci, S. Electropolymerization of thiophene on gold nanoparticle modified electrode in aqueous media. J. Electroanal. Chem. 2013, 701, 20–24. [Google Scholar] [CrossRef]
- Najafisayar, P.; Bahrololoom, M. The effect of pulse electropolymerization on the electrochemicalproperties of polythiophene films. Electrochim. Acta 2013, 114, 462–473. [Google Scholar] [CrossRef]
- Lungu, C.; Grigorescu, C.; Rusu, M.; Jepu, I.; Porosnicu, C.; Lungu, A.; Feraru, I.; Savastru, D. Nanodiamond crystallites embedded in carbon films prepared by thermionic vacuum arc method. Diam. Relat. Mater. 2011, 20, 1061–1064. [Google Scholar] [CrossRef]
- Bloino, J.; Baiardi, A.; Biczysko, M. Aiming at an accurate prediction of vibrational and electronic spectra for medium-to-large molecules: An overview. Int. J. Quantum Chem. 2016, 116, 1543–1574. [Google Scholar] [CrossRef]
- Fazzi, D.; Canesi, E.V.; Bertarelli, C.; Castiglioni, C.; Negri, F.; Zerbi, G. Raman spectroscopic characterization of a thiophene-based active material for resistive organic nonvolatile memories. J. Raman Spectrosc. 2009, 41, 406–413. [Google Scholar] [CrossRef]
- Barclay, M.S.; Quincy, T.J.; Williams-Young, D.B.; Caricato, M.; Elles, C.G. Accurate Assignments of Excited-State Resonance Raman Spectra: A Benchmark Study Combining Experiment and Theory. J. Phys. Chem. A 2017, 121, 7937–7946. [Google Scholar] [CrossRef]
- Iordache, A.-M.; Cristescu, R.; Fagadar-Cosma, E.; Popescu, A.C.; Ciucu, A.A.; Iordache, S.M.; Balan, A.; Nichita, C.; Stamatin, I.; Chrisey, D.B. Histamine detection using functionalized porphyrin as electrochemical mediator. Comptes Rendus Chim. 2018, 21, 270–276. [Google Scholar] [CrossRef]
- Iordache, A.; Iordache, S.; Bohiltea, R.; Barna, V.; Rizea, C.; Mazlum, A.; Capatina, V.; Grigorescu, C. Spectroscopic identification of histamine using metalloporphyrins and their electrochemical characterization. J. Optoelectron. Adv. Mater. 2021, 23, 630–633. [Google Scholar]
- Food and Drug Administration. Chapter 7—Scombrotoxin (histamine) formation. In Fish and Fishery Products Hazards and controls Guide; Department of Health and Human Services—Office of Seafood: Washington, DC, USA, 2001; pp. 73–93. [Google Scholar]
- Centers for Disease Control and Prevention. Scombroid Fish Poisoning—Pennsylvania. 1998. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4918a2.htm (accessed on 20 July 2023).
- European Union. Commission Regulation (EU) No 1019/2013; Official Journal of the European Union: Brussels, Belgium, 2013. [Google Scholar]
- Bartholomev, B.; Berry, P.; Rodhouse, J.; Gilhouse, R. Scombrotoxic fish poisoning in Britain: Features of over 250 suspected incidents from 1976 to 1986. Epidemiol Infect 1987, 99, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Flick, G.J.; Oria, M.P.; Douglas, L. Potential Hazards in Cold-Smoked Fish: Biogenic Amines. J. Food Sci. 2001, 66, S1088–S1099. [Google Scholar] [CrossRef]

| Material | Supporting Electrolyte | Concentration Range (mg∙kg−1) | LOD (mg∙kg−1) | LOQ (mg∙kg−1) | Maximum Limit of Detection (mg∙kg−1) | Response Time (s) | Sensitivity (µA/cm2 × mg∙kg−1) | Reproducibility | Repeatability | Signal Recovery |
|---|---|---|---|---|---|---|---|---|---|---|
| Graphene-thiophene/Au | 0.01 M TCA | 100–200 | 13.8 | 46.06 ppm | 551 | 17.82 | 0.49933 | 1.13–2.3% (n = 5) | 1.5 (n = 3) | 73.99–109% |
| Sensors | Method | Concentration Range (mg/kg) | LOD (mg/kg) | [Ref] |
|---|---|---|---|---|
| AuNP/p-aminobenzene sulfonic acid/GCE | DPV | 1420–3799 | 21.3 | [3] |
| Fe3O4/Au@ATP@Ag | SERS | 71–710 | 35.5 | [20] |
| Au NPs-nitrilotriacetic acid-Ni2+ (NTANi2+) | SERS | 35.5–3550 | 35.5 | [21] |
| Fe3O4@SiO2–COOH -Ag NPs with glycine (Gly) and (3-Aminopheyonyl) boronic acid (APBA) | SERS | 0.355–3550 | 0.257 | [22] |
| Hydroxyl functionalized Schiff base zinc(II) complex/TiO2 NP/FTO | EIS | 3.5–355 | 3.5 | [29] |
| Ni@C metallic-organic framework/GCE | CV | 3550–35,500 | 11,360 | [30] |
| Graphene/MWCNT/AuNP | Transconductance | 106.5–3550 | 3.55 | [31] |
| Apt/AuNFs/ITO | DPV | 0.03–177.5 | 0.02 | [34] |
| Diamine oxidase phase-change microcapsules | CV | 3550–284 × 103 | 16.8 | [35] |
| Graphene/antibodies with HRP-tagged histamine | Square Wave Voltammetry | 0.00032–0.3195 | 0.00016 | [40] |
| Fe2O3-TiO2-CdSe | Fluorescence | 2485–159,750 | 56.8 | [18] |
| Alizarin complexone (ALC) and Ni2+ | UV-VIS | 177.5–5325 | 272.64 | [17] |
| Polystyrene–graphene oxide nanocomposite | DPV | 3.55–106.5 | 1.065 | [37] |
| Graphene nanoribbons-AgNPs | Square Wave Voltammetry | 2130–17,750 | 0.6745 | [38] |
| Our sensor (graphene-thiophene) in 0.01M histamine-TCA solution | CV | 100–200 | 13.8 | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iordache, S.-M.; Iordache, A.-M.; Zubarev, A.; Caramizoiu, S.; Grigorescu, C.E.A.; Marinescu, S.; Giuglea, C. Spectro-Electrochemical Properties of A New Non-Enzymatic Modified Working Electrode Used for Histamine Assessment in the Diagnosis of Food Poisoning. Foods 2023, 12, 2908. https://doi.org/10.3390/foods12152908
Iordache S-M, Iordache A-M, Zubarev A, Caramizoiu S, Grigorescu CEA, Marinescu S, Giuglea C. Spectro-Electrochemical Properties of A New Non-Enzymatic Modified Working Electrode Used for Histamine Assessment in the Diagnosis of Food Poisoning. Foods. 2023; 12(15):2908. https://doi.org/10.3390/foods12152908
Chicago/Turabian StyleIordache, Stefan-Marian, Ana-Maria Iordache, Alexei Zubarev, Stefan Caramizoiu, Cristiana Eugenia Ana Grigorescu, Silviu Marinescu, and Carmen Giuglea. 2023. "Spectro-Electrochemical Properties of A New Non-Enzymatic Modified Working Electrode Used for Histamine Assessment in the Diagnosis of Food Poisoning" Foods 12, no. 15: 2908. https://doi.org/10.3390/foods12152908
APA StyleIordache, S.-M., Iordache, A.-M., Zubarev, A., Caramizoiu, S., Grigorescu, C. E. A., Marinescu, S., & Giuglea, C. (2023). Spectro-Electrochemical Properties of A New Non-Enzymatic Modified Working Electrode Used for Histamine Assessment in the Diagnosis of Food Poisoning. Foods, 12(15), 2908. https://doi.org/10.3390/foods12152908






