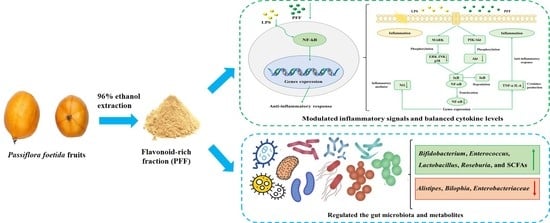
Anti-Inflammatory and Gut Microbiota Modulation Potentials of Flavonoids Extracted from Passiflora foetida Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of PFF
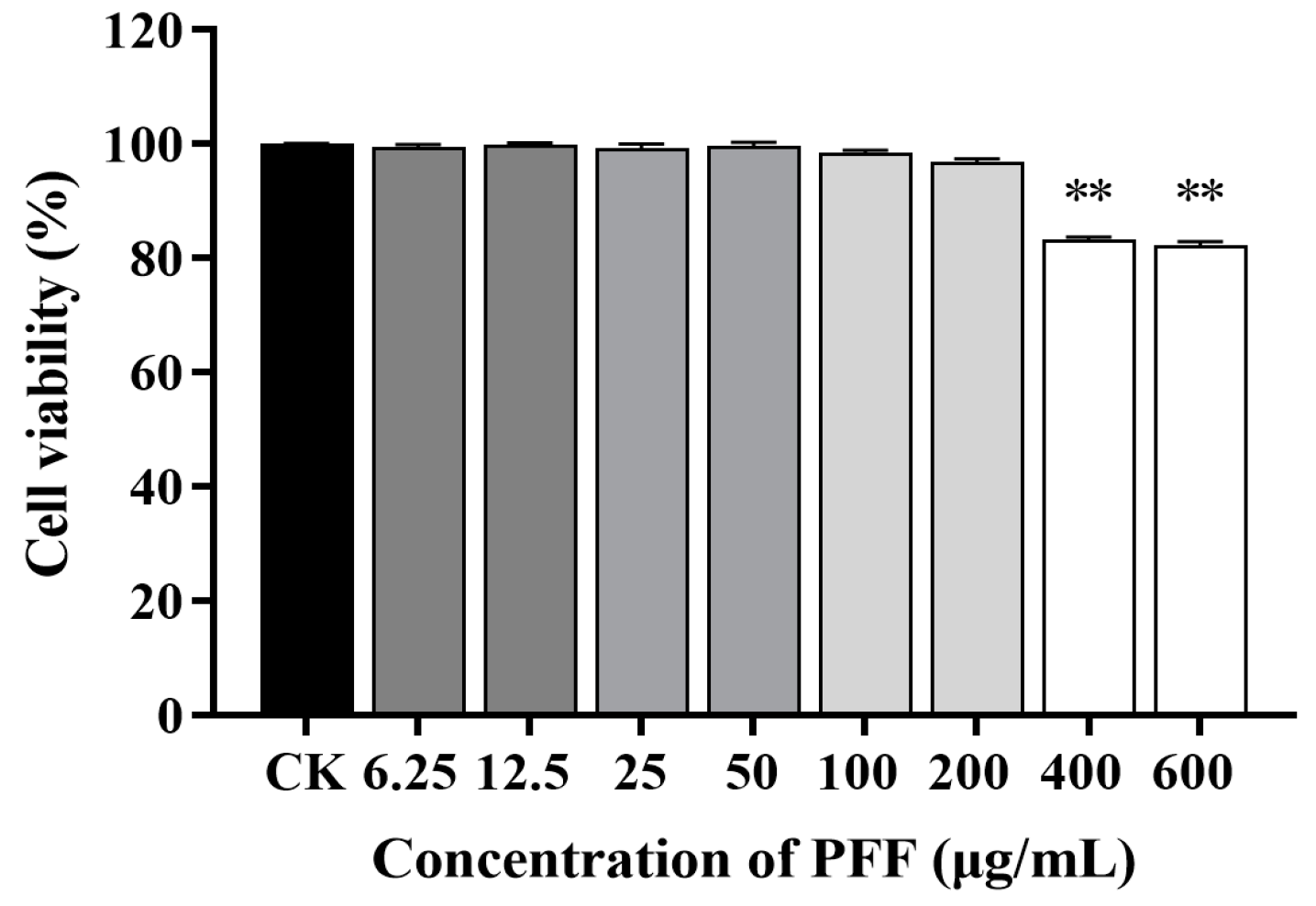
2.3. Cell Culture and Viability Assay
2.4. Determination of NO and Cytokines
2.5. Western Blot Analysis
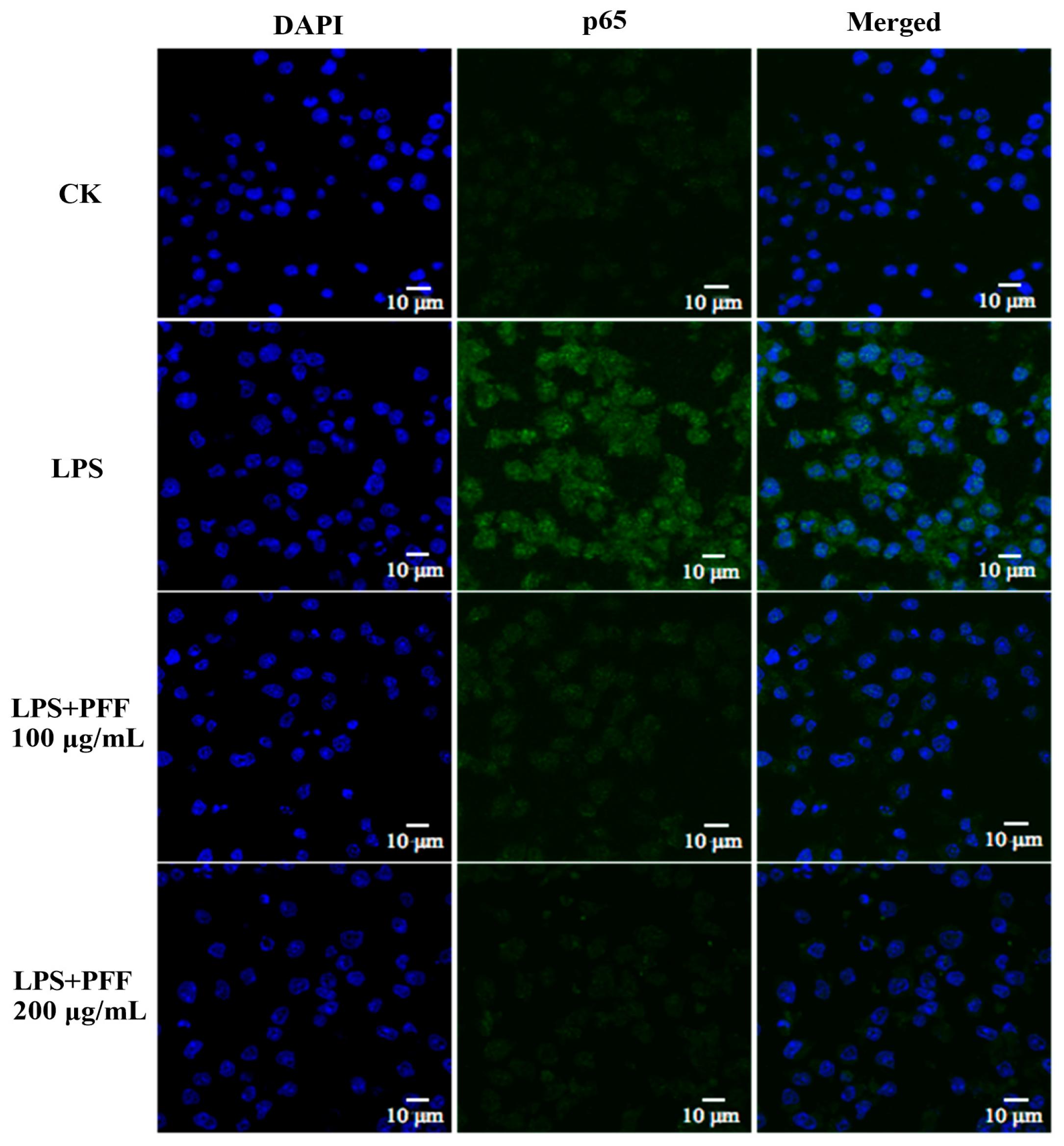
2.6. Immunofluorescence Analysis
2.7. Dynamic Simulator of the Gut Microbiome
2.8. Microbiota Composition Analysis
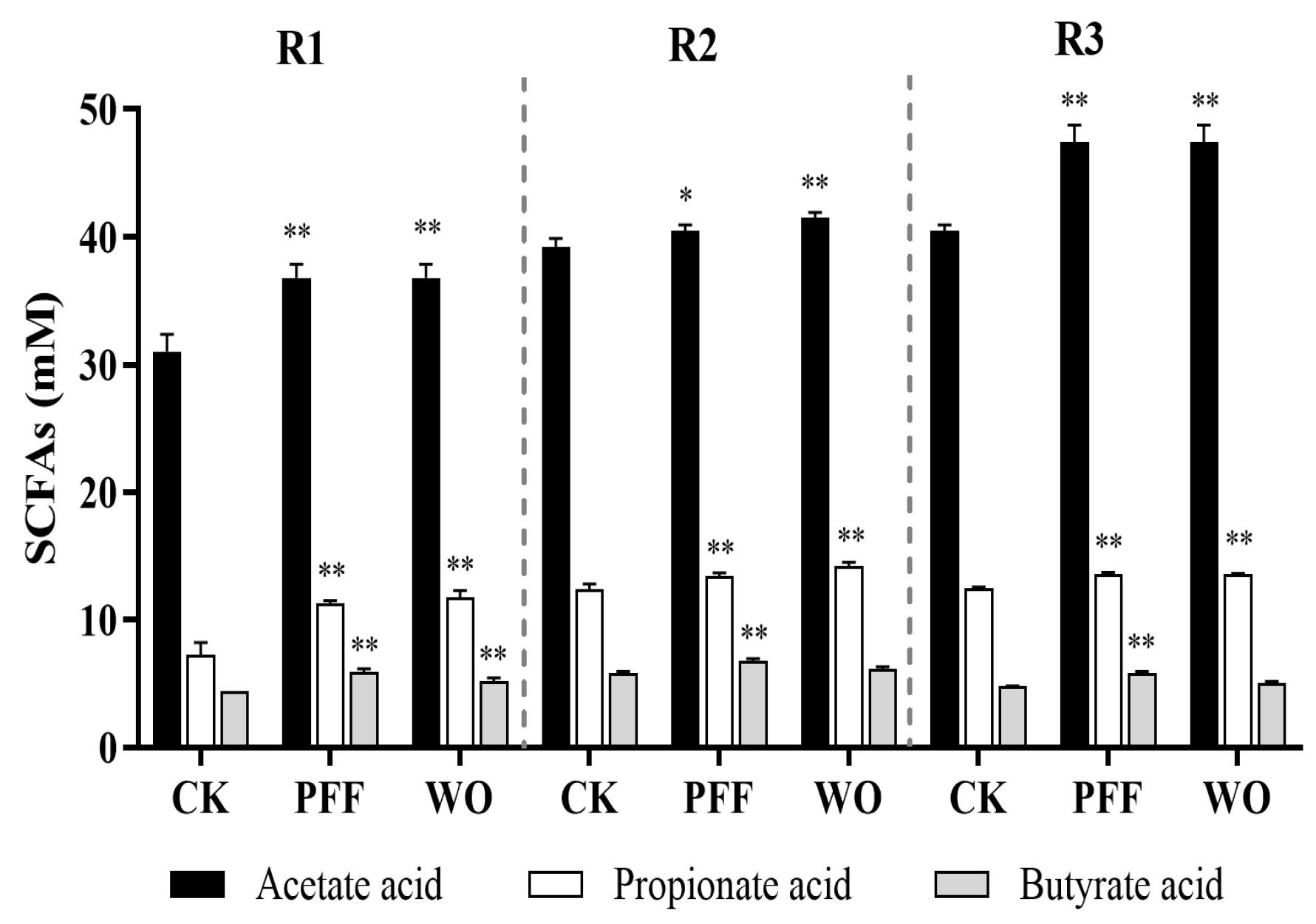
2.9. SCFAs Analysis
2.10. Isolation of the Main Flavonoids in PFF
2.11. Initial Analysis of the Main Flavonoids in PFF
2.12. Statistical Analysis
3. Results and Discussion
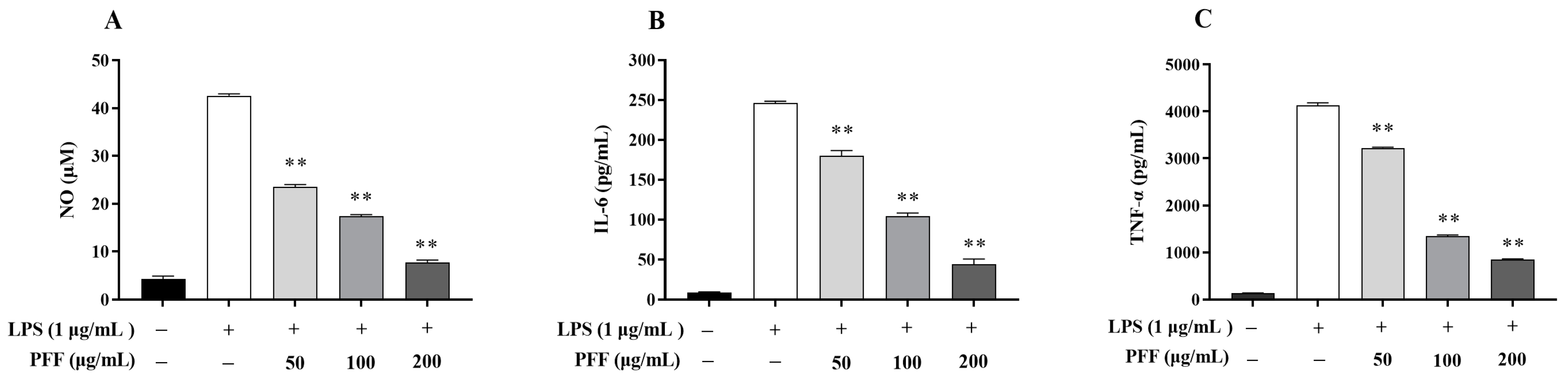
3.1. Effects of PFF on the Level of NO and Cytokines Produced in RAW264.7 Cells
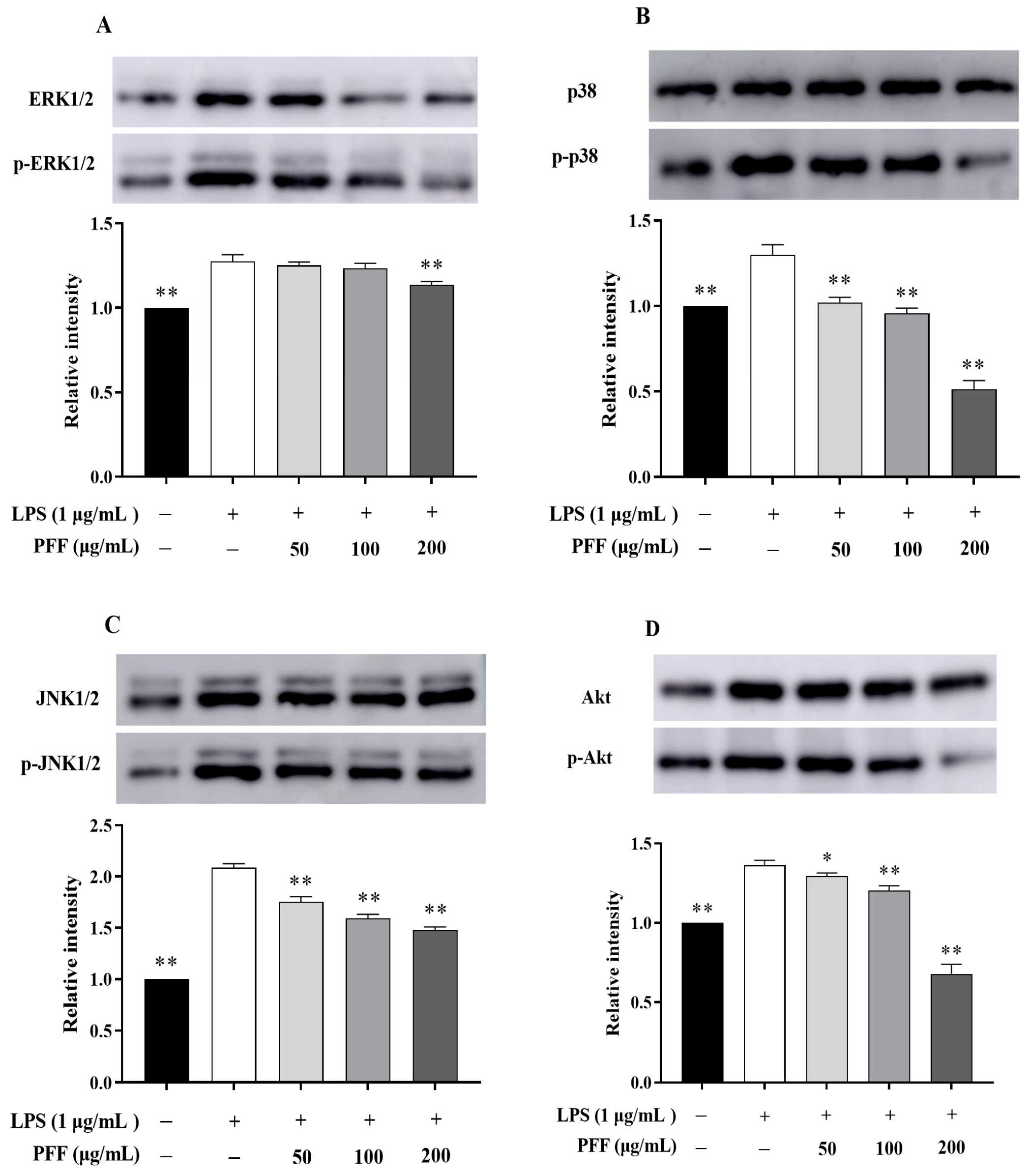
3.2. Effects of PFF on MAPK, PIK/Akt, and NF-κB Signaling Pathways in RAW264.7 Cells
3.3. Intestinal Regulatory Activity of PFF
3.3.1. Effects of PFF on the Gut Microbiota
3.3.2. Effects of PFF on the Metabolites
3.4. Initial Analysis of the Main Flavonoids in PFF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Jung, J.; Kim, H.; Lee, S.; Hong, M.; Hwang, D. Antioxidant and anti-inflammatory activity of Filipendula glaberrima Nakai ethanolic extract and its chemical composition. Molecules 2022, 27, 4628. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, M.; Owlia, S.; Owlia, M.B.; Chi, H.C.; Cho, C.H. Review of anti-inflammatory herbal medicines. Adv. Pharmacol. Sci. 2016, 2016, 9130979. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, J.; Zhang, L.; Qin, N.; Zhu, B.; Xia, X. Jellyfish skin polysaccharides enhance intestinal barrier function and modulate the gut microbiota in mice with DSS-induced colitis. Food Funct. 2021, 12, 10121–10135. [Google Scholar] [CrossRef]
- He, X.; Liu, D.; Liu, H.; Wu, D.; Li, H.; Zhang, X.; Gan, R. Prevention of ulcerative colitis in mice by sweet tea (Lithocarpus litseifolius) via the regulation of gut microbiota and butyric-acid-mediated anti-inflammatory signaling. Nutrients 2022, 14, 2208. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.; Machado, A.; Galvez, J.; Cazarin, C.; Marostica, J.M. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020, 258, 118129. [Google Scholar] [CrossRef] [PubMed]
- Marta Wlodarska, A.D.K.A. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe 2015, 5, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Song, Y.; Martínez-Cuesta, M.C.; Peláez, C.; Li, E.; Requena, T.; Wang, H.; Sun, Y. Immunological activity and gut microbiota modulation of pectin from kiwano (Cucumis metuliferus) peels. Foods 2022, 11, 1632. [Google Scholar] [CrossRef]
- Suh, M.G.; Choi, H.; Cho, K.; Park, S.S.; Kim, W.J.; Suh, H.J.; Kim, H. Anti-inflammatory action of herbal medicine comprised of Scutellaria baicalensis and Chrysanthemum morifolium. Biosci. Biotechnol. Biochem. 2020, 84, 1799–1809. [Google Scholar] [CrossRef]
- Chen, G.L.; Fan, M.X.; Wu, J.L.; Li, N.; Guo, M.Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 2019, 277, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xiao, H. Whole food-based approaches to modulating gut microbiota and associated diseases. Annu. Rev. Food Sci. Technol. 2020, 11, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, N.; Wang, Z.; Qiu, T.; Jiang, L.; Zhu, X.; Sun, Y.; Xiong, H. Akebia trifoliata pericarp extract ameliorates inflammation through NF-κB/MAPK signaling pathways and modifies gut microbiota. Food Funct. 2020, 5, 4682–4696. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Hubert, J.; Llorach, R.; Scalbert, A. The complex links between dietary phytochemicals and human health deciphered by metabolomics. Mol. Nutr. Food Res. 2009, 53, 1303–1315. [Google Scholar] [CrossRef]
- Zhang, C.H.; Sheng, J.Q.; Sarsaiya, S.; Shu, F.X.; Liu, T.T.; Tu, X.Y.; Ma, G.Q.; Xu, G.L.; Zheng, H.X.; Zhou, L.F. The anti-diabetic activities, gut microbiota composition, the anti-inflammatory effects of Scutellaria-coptis herb couple against insulin resistance-model of diabetes involving the Toll-like receptor 4 signaling pathway. J. Ethnopharmacol. 2019, 237, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Park, J.W.; Kwon, O.K.; Ryu, H.W.; Paik, J.H.; Paryanto, I.; Yuniato, P.; Choi, S.; Oh, D.; Ahn, K.S. Anti-inflammatory effects of Passiflora foetida L. in LPS-stimulated RAW264.7 macrophages. Int. J. Mol. Med. 2018, 41, 3709–3716. [Google Scholar] [CrossRef]
- Song, Y.; Wei, X.; Li, M.; Duan, X.; Sun, Y.; Yang, R.; Su, X.; Huang, R.; Wang, H. Nutritional composition and antioxidant properties of the fruits of a Chinese wild Passiflora foetida. Molecules 2018, 23, 459. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, M.; Hao, H.; Deng, J.; Li, M.; Sun, Y.; Yang, R.; Wang, H.; Huang, R. Structure characterization of a novel polysaccharide from Chinese wild fruits (Passiflora foetida) and its immune-enhancing activity. Int. J. Biol. Macromol. 2019, 136, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wen, P.; Hao, H.; Zhu, M.; Sun, Y.; Zou, Y.; Requena, T.; Huang, R.; Wang, H. Structural features of three hetero-galacturonans from Passiflora foetida fruits and their in vitro immunomodulatory effects. Polymers 2020, 12, 615. [Google Scholar] [CrossRef]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.A.; Shen, M. Flavonoids from whole-grain oat alleviated high-fat diet-induced hyperlipidemiavia regulating bile acid metabolism and gut microbiota in mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Che, S.; Zhang, L.; Ruan, Z. Reparative effects of ethanol-induced intestinal barrier injury by flavonoid luteolin via MAPK/NF-κB/MLCK and Nrf2 signaling pathways. J. Agric. Food Chem. 2021, 69, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, J.; Cui, J.; Li, H.; Hasan, B.; Xin, X. Chemical component and in vitro protective effects of Matricaria chamomilla (L.) against lipopolysaccharide insult. J. Ethnopharmacol. 2022, 296, 115471. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Yu, H.; Ruan, Z.; Zhang, L.; Xue, Y.; Yuan, X.; Qi, M.; Yao, Y. Effects of food matrix elements (dietary fibres) on grapefruit peel flavanone profile and on faecal microbiota during in vitro fermentation. Food Chem. 2022, 371, 131065. [Google Scholar] [CrossRef] [PubMed]
- Requena, T.; Song, Y.; Peláez, C.; Martínez-Cuesta, M.C. Modulation and metabolism of obesity-associated microbiota in a dynamic simulator of the human gut microbiota. LWT 2021, 141, 110921. [Google Scholar] [CrossRef]
- Barroso, E.; Montilla, A.; Corzo, N.; Peláez, C.; Martínez-Cuesta, M.C.; Requena, T. Effect of lactulose-derived oligosaccharides on intestinal microbiota during the shift between media with different energy contents. Food Res. Int. 2016, 89, 302–308. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hou, M.; Han, W. Anti-fatigue activity of parsley (Petroselinum crispum) flavonoids via regulation of oxidative stress and gut microbiota in mice. J. Funct. Foods 2022, 89, 104963. [Google Scholar] [CrossRef]
- Xu, X.; Xie, H.; Hao, J.; Jiang, Y.; Wei, X. Flavonoid glycosides from the seeds of Litchi chinensis. J. Agric. Food Chem. 2011, 59, 1205–1209. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, H.W.; Lee, M.K.; Kim, H.J.; Kim, J.B.; Choe, J.S.; Lee, Y.M.; Jang, H.H. Antioxidant and Anti-inflammatory activities in relation to the flavonoids composition of pepper (Capsicum annuum L.). Antioxidants 2020, 9, 986. [Google Scholar] [CrossRef]
- Chao, L.; Lin, J.; Zhou, J.; Du, H.; Chen, X.; Liu, M.; Qu, Q.; Lv, W.; Guo, S. Polyphenol rich Forsythia suspensa extract alleviates DSS-induced ulcerative colitis in mice through the Nrf2-NLRP3 pathway. Antioxidants 2022, 11, 475. [Google Scholar] [CrossRef]
- Yang, A.; Fan, H.; Zhao, Y.; Chen, X.; Zhu, Z.; Zha, X.; Zhao, Y.; Chai, X.; Li, J.; Tu, F.; et al. An immune-stimulating proteoglycan from the medicinal mushroom Huaier up-regulates NF-κB and MAPK signaling via Toll-like receptor 4. J. Biol. Chem. 2019, 294, 2628–5268. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Yuan, R.; Huang, L.; Liu, Z.; Huang, D.; Huang, L.; Gao, H.; Liu, Y.; Xu, Q.; Yang, S. Atypical nitrogen-containing flavonoid in the fruits of cumin (Cuminum cyminum L.) with anti-inflammatory activity. J. Agric. Food Chem. 2019, 67, 8339–8347. [Google Scholar] [CrossRef]
- Yuan, L.; Li, X.; He, S.; Gao, C.; Wang, C.; Shao, Y. Effects of natural flavonoid isoorientin on growth performance and gut microbiota of mice. J. Agric. Food Chem. 2018, 66, 9777–9784. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, Y.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Luo, J.; Han, Y.; Du, H.; Liu, J.; He, W.; Zhu, J.; Xiao, J.; Wang, J.; Cao, Y.; et al. Dietary tangeretin alleviated dextran sulfate sodium-induced colitis in mice via inhibiting inflammatory response, restoring intestinal barrier function, and modulating gut microbiota. J. Agric. Food Chem. 2021, 69, 7663–7674. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Xia, Y.; Guo, H.; He, X.; Li, H.; Wu, D.; Geng, F.; Lin, F.; Li, H.; et al. Screening and process optimization of ultrasound-assisted extraction of main antioxidants from sweet tea (Lithocarpus litseifolius [Hance] Chun). Food Biosci. 2021, 43, 101277. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Zhang, F.; He, F.; Li, L.; Guo, L.; Zhang, B.; Yu, S.; Zhao, W. Bioavailability based on the gut microbiota: A new perspective. Microbiol. Mol. Biol. Rev. 2020, 84, e00072. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kim, E.Y.; Kim, W.J.; Kim, W.K.; Kim, C.M. Antioxidant constituents from Setaria viridis. Arch. Pharmacal Res. 2002, 25, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yang, J.; Dong, Y.; Zhou, L.; Lin, G. Flavone C-glycosides from flowers of Trollius ledebouri. Phytochemistry 2005, 66, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Velozo, L.S.M.; Ferreira, M.J.P.; Santos, M.I.S.; Moreira, D.L.; Guimarães, E.F.; Emerenciano, V.P.; Kaplan, M.A.C. C-glycosyl flavones from Peperomia blanda. Fitoterapia 2009, 80, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Guvenalp, Z.; Ozbek, H.; Karadayi, M.; Gulluce, M.; Kuruuzum-Uz, A.; Salih, B.; Demirezer, O. Two antigenotoxic chalcone glycosides from Mentha longifolia subsp. longifolia. Pharm. Biol. 2015, 53, 888–896. [Google Scholar] [CrossRef]
- Gazola, A.C.; Costa, G.M.; Zucolotto, S.M.; Castellanos, L.; Ramos, F.A.; de Lima, T.C.M.; Schenkel, E.P. The sedative activity of flavonoids from Passiflora quadrangularis is mediated through the GABAergic pathway. Biomed. Pharmacother. 2018, 100, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Xiao, Q.; Qu, Z.; Zhao, Y.; Yang, L.; Gao, P. Orientin ameliorates LPS-induced inflammatory responses through the inhibitory of the NF-κB pathway and NLRP3 inflammasome. Evid. Based Complement. Altern. Med. 2017, 2017, 2495496. [Google Scholar] [CrossRef]
- Yu, Y.; Pei, F.; Li, Z. Orientin and vitexin attenuate lipopolysaccharide-induced inflammatory responses in RAW264.7 cells: A molecular docking study, biochemical characterization, and mechanism analysis. Food Sci. Hum. Wellness. 2022, 11, 1273–1281. [Google Scholar] [CrossRef]
- Rosa, S.I.G.; Rios-Santos, F.; Balogun, S.O.; Martins, D.T.D.O. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef]
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Group | Compartment | CK | PFF | WO |
|---|---|---|---|---|
| Alistipes | R1 | 7.07 ± 0.01 | 5.31 ± 0.21 ** | 5.38 ± 0.06 ** |
| R2 | 8.69 ± 0.07 | 8.58 ± 0.14 | 8.36 ± 0.06 * | |
| R3 | 8.75 ± 0.03 | 8.45 ± 0.09 * | 8.46 ± 0.14 * | |
| Bifidobacterium | R1 | 8.84 ± 0.31 | 8.65 ± 0.12 | 8.36 ± 0.09 |
| R2 | 8.30 ± 0.38 | 8.65 ± 0.20 | 8.46 ± 0.08 | |
| R3 | 8.30 ± 0.38 | 8.65 ± 0.20 | 8.12 ± 0.18 | |
| Bilophia | R1 | 8.37 ± 0.01 | 8.27 ± 0.10 | 8.22 ± 0.27 |
| R2 | 8.39 ± 0.01 | 8.16 ± 0.09 | 8.23 ± 0.24 | |
| R3 | 8.38 ± 0.20 | 7.99 ± 0.20 | 8.30 ± 0.18 | |
| Enterbacteriaceae | R1 | 8.60 ± 0.00 | 8.26 ± 0.02 ** | 7.83 ± 0.05 ** |
| R2 | 8.49 ± 0.01 | 8.12 ± 0.05 ** | 7.90 ± 0.02 ** | |
| R3 | 8.48 ± 0.05 | 7.91 ± 0.13 ** | 8.03 ± 0.04 ** | |
| Enterococcus | R1 | 6.48 ± 0.08 | 8.56 ± 0.21 ** | 8.87 ± 0.12 ** |
| R2 | 6.42 ± 0.13 | 8.84 ± 0.13 ** | 8.82 ± 0.01 ** | |
| R3 | 7.09 ± 0.04 | 8.44 ± 0.21 ** | 8.40 ± 0.02 ** | |
| Lactobacillus | R1 | 3.68 ± 0.00 | 3.99 ± 0.02 ** | 5.54 ± 0.04 ** |
| R2 | 3.60 ± 0.03 | 4.44 ± 0.15 ** | 5.41 ± 0.00 ** | |
| R3 | 3.98 ± 0.00 | 4.42 ± 0.07 ** | 5.28 ± 0.05 ** | |
| Roseburia | R1 | 5.64 ± 0.09 | 6.47 ± 0.19 ** | 4.93 ± 0.02 ** |
| R2 | 6.23 ± 0.07 | 6.58 ± 0.06 ** | 5.56 ± 0.03 ** | |
| R3 | 6.01 ± 0.03 | 6.47 ± 0.10 ** | 5.29 ± 0.06 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Song, Y.; Huang, R.; Zhu, M.; Li, M.; Requena, T.; Wang, H. Anti-Inflammatory and Gut Microbiota Modulation Potentials of Flavonoids Extracted from Passiflora foetida Fruits. Foods 2023, 12, 2889. https://doi.org/10.3390/foods12152889
Han X, Song Y, Huang R, Zhu M, Li M, Requena T, Wang H. Anti-Inflammatory and Gut Microbiota Modulation Potentials of Flavonoids Extracted from Passiflora foetida Fruits. Foods. 2023; 12(15):2889. https://doi.org/10.3390/foods12152889
Chicago/Turabian StyleHan, Xiangpeng, Ya Song, Riming Huang, Minqian Zhu, Meiying Li, Teresa Requena, and Hong Wang. 2023. "Anti-Inflammatory and Gut Microbiota Modulation Potentials of Flavonoids Extracted from Passiflora foetida Fruits" Foods 12, no. 15: 2889. https://doi.org/10.3390/foods12152889
APA StyleHan, X., Song, Y., Huang, R., Zhu, M., Li, M., Requena, T., & Wang, H. (2023). Anti-Inflammatory and Gut Microbiota Modulation Potentials of Flavonoids Extracted from Passiflora foetida Fruits. Foods, 12(15), 2889. https://doi.org/10.3390/foods12152889