Isolation of Biofilm-Forming Staphylococci from the Bulk-Tank Milk of Small Ruminant Farms in Greece
Abstract
1. Introduction
2. Materials and Methods
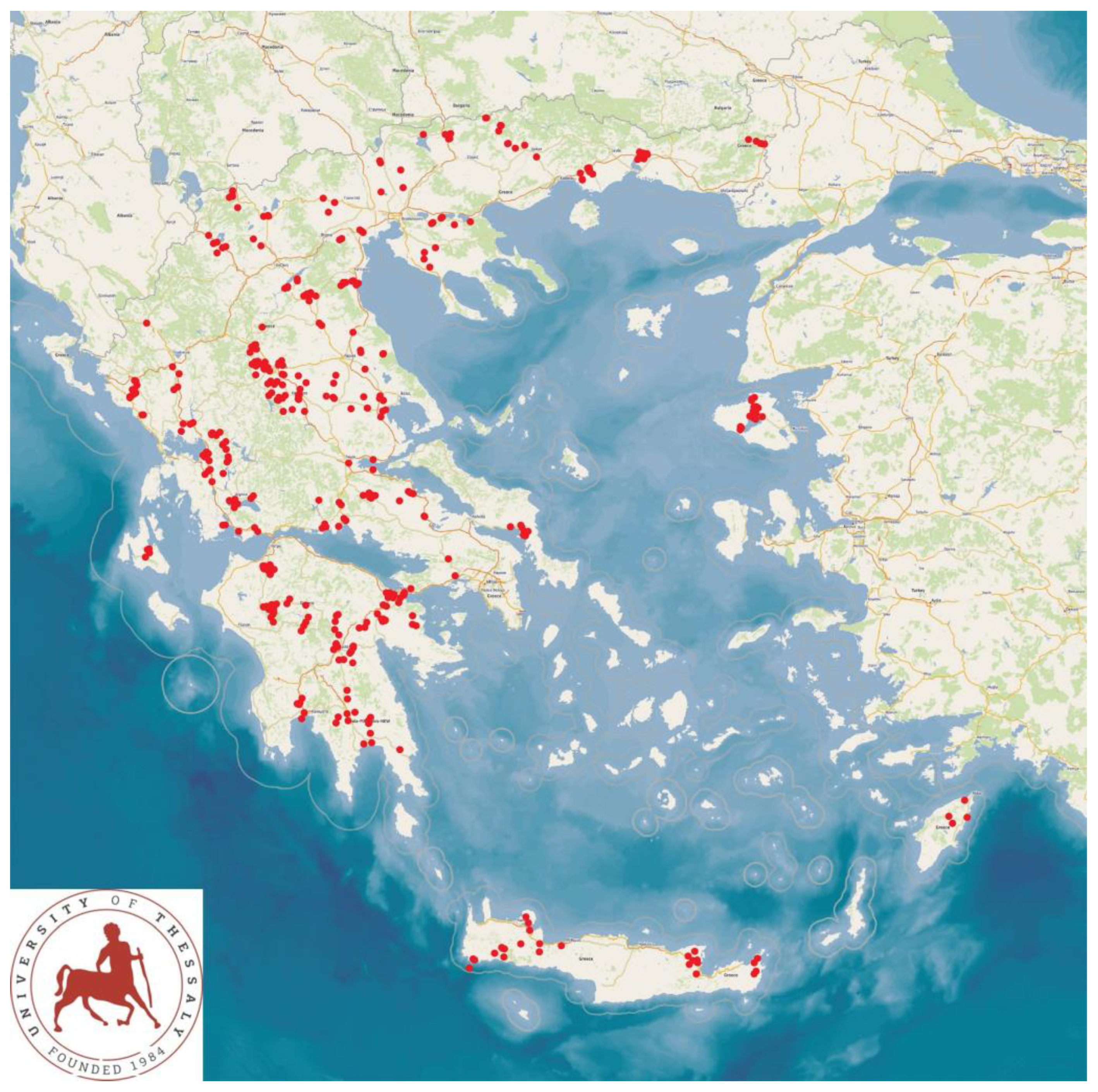
2.1. Visits to Sheep and Goat Farms
2.2. Laboratory Examinations
2.3. Data Management and Analysis
3. Results
3.1. Staphylococcal Recovery and Identity
3.2. Biofilm Formation by Staphylococcal Isolates
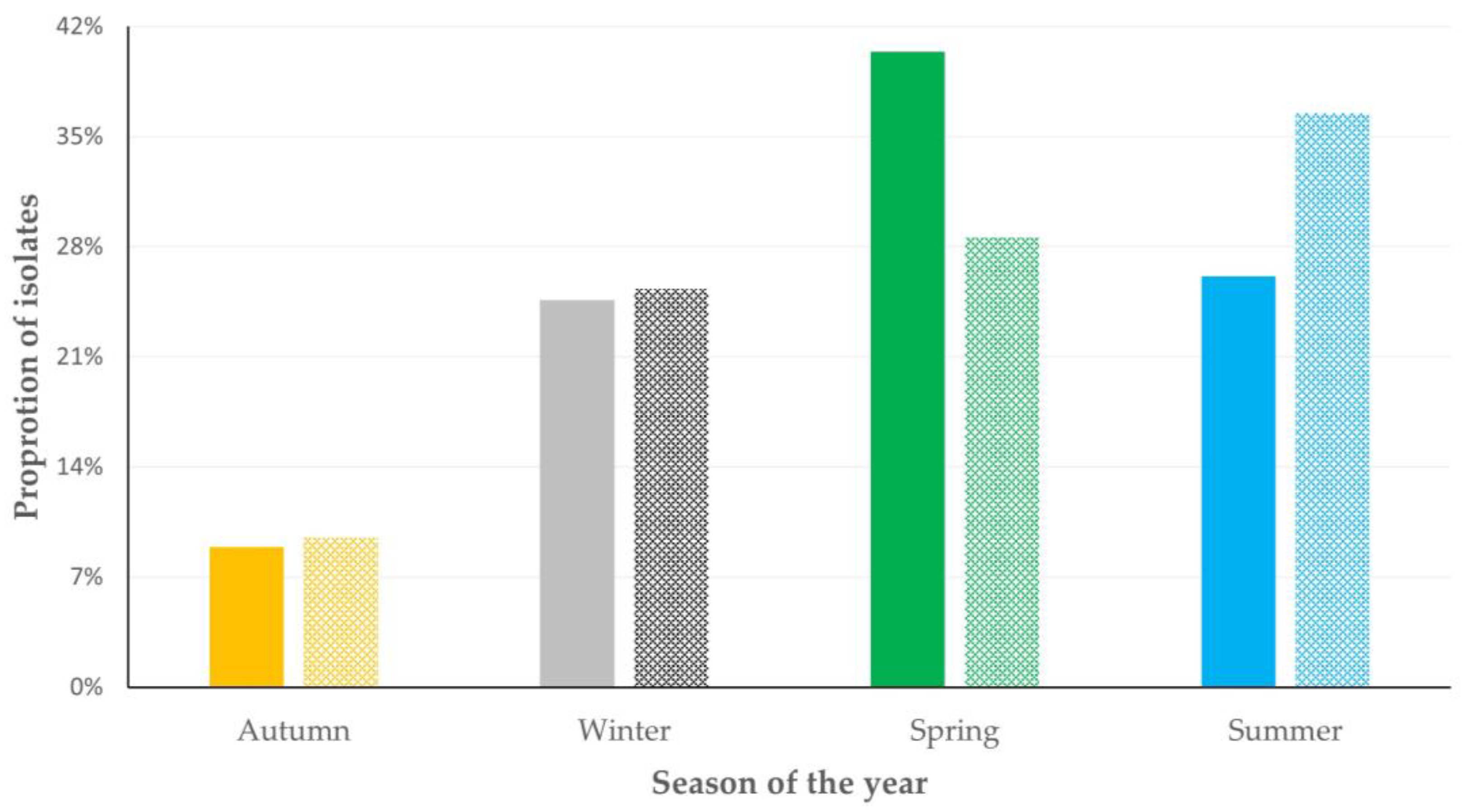
3.3. Variables Associated with Isolation of Biofilm-Forming Staphylococci
3.4. Association of Recovery of Biofilm Formation by Staphylococcal Isolates with Quality of Bulk-Tank Milk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Management system applied in the farm (description (intensive, semi-intensive, semi-extensive, extensive) according to the classification of the European Food Safety Authority [35]) |
| No. of female animals in the farm (no.) |
| Type of milking (hand-milking/machine-milking) |
| Annual frequency of changing teatcups (no. of occasions) |
| Temperature of cleaning water of the milk parlour (°C) |
| Frequency of milk collection from the tank (days) |
| Daily number of milking sessions (no.) |
| Use of teat disinfection after milking (yes/no) |
| Administration of ‘dry-ewe’ treatment at the end of the lactation period (yes/no) |
| Annual frequency of systemic disinfections in the farm |
| Month into the lactation period at sampling |
References
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Inf. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.C.; Fthenakis, G.C. Mastitis in sheep—The last 10 years and the future of research. Vet. Microbiol. 2015, 185, 136–146. [Google Scholar] [CrossRef]
- Perez, M.M.; Prenafeta, A.; Valle, J.; Penades, J.; Rota, C.; Solano, C.; Marco, J.; Grillo, M.J.; Lasa, I.; Irache, J.M.; et al. Protection from Staphylococcus aureus mastitis associated with poly-N-acetyl beta-1,6 glucosamine specific antibody production using biofilm-embedded bacteria. Vaccine 2009, 27, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.K.; Lianou, C.K.; Vasileiou, N.G.C.; Tsilipounidaki, K.; Katsafadou, A.I.; Politis, A.P.; Kordalis, N.G.; Ioannidi, K.S.; Gougoulis, D.A.; Trikalinou, C.; et al. Association of staphylococcal populations on teatcups of milking parlours with vaccination against staphylococcal mastitis in sheep and goat farms. Pathogens 2021, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Ergun, Y.; Aslantas, O.; Kirecci, E.; Ozturk, F.; Ceylan, A.; Boyar, Y. Antimicrobial susceptibility, presence of resistance genes and biofilm formation in coagulase negative staphlococci isolated from subclinical sheep mastitis. Kafkas Univer. Veter. Fakult. Derg. 2012, 18, 449–456. [Google Scholar] [CrossRef]
- Tel, O.Y.; Aslantas, O.; Keskin, O.; Yilmaz, E.S.; Demir, C. Investigation of the antibiotic resistance and biofilm formation of Staphylococcus aureus strains isolated from gangrenous mastitis of ewes. Acta Vet. Hung. 2012, 60, 189–197. [Google Scholar] [CrossRef]
- Vautor, E.; Carsenti-Dellamonica, H.; Sabah, M.; Mancini, G.; Pepin, M.; Dellamonica, P. Characterization of Staphylococcus aureus isolates recovered from dairy sheep farms (agr group, adherence, slime, resistance to antibiotics). Small Rumin. Res. 2007, 72, 197–199. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Katsafadou, A.I.; Spyrou, V.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Slime-producing staphylococci as causal agents of subclinical mastitis in sheep. Vet. Microbiol. 2018, 224, 93–99. [Google Scholar] [CrossRef]
- Aslantas, O.; Keskin, O.; Yucetepe, A.G. Molecular characterization of Staphylococcus aureus from clinical sheep mastitis cases. Israel J. Vet. Med. 2022, 77, 99–105. [Google Scholar]
- Karahutova, L.; Bujnakova, D. Occurrence and molecular surveillance of pathogenesis risk-associated factors in Staphylococcus aureus recovered from raw sheep’s milk cheese. Small Rumin. Res. 2023, 222, 106967. [Google Scholar] [CrossRef]
- Oknin, H.; Kroupitski, Y.; Shemesh, M.; Blum, S. Upregulation of ica operon governs biofilm formation by a coagulase-negative Staphylococcus caprae. Microorganisms 2023, 11, 1533. [Google Scholar] [CrossRef] [PubMed]
- Lianou, D.T.; Chatziprodromidou, I.P.; Vasileiou, N.G.C.; Michael, C.K.; Mavrogianni, V.S.; Politis, A.P.; Kordalis, N.G.; Billinis, C.; Giannakopoulos, A.; Papadopoulos, E.; et al. A detailed questionnaire for the evaluation of health management in dairy sheep and goats. Animals 2020, 10, 1489. [Google Scholar] [CrossRef]
- Lianou, D.T.; Michael, C.K.; Fthenakis, G.C. Data on mapping 444 dairy small ruminant farms during a countrywide investigation performed in Greece. Animals 2023, 13, 2044. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.T.; Gambrel-Lenarz, S.A.; Scher, F.M.; Graham, T.E.; Reddy, R. Microbiological Count Methods. In Standard Methods for the Examination of Dairy Products, 17th ed.; Wehr, H.M., Frank, J.F., Eds.; APHA Press: Washington, DC, USA, 2004; pp. 153–186. [Google Scholar]
- Barrow, G.I.; Feltham, R.K.A. Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Euzeby, J.P. List of bacterial names with standing in nomenclature: A folder available on the Internet. Int. J. Syst. Bacteriol. 1997, 47, 590–592. [Google Scholar] [CrossRef]
- Freeman, D.J.; Falkiner, F.R.; Keane, C.T. New method of detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef]
- Fabres-Klein, M.H.; Santos, M.J.C.; Klein, R.C.; de Souza, G.N.; Ribon, A.D.B. An association between milk and slime increases biofilm production by bovine Staphylococcus aureus. BMC Vet. Res. 2015, 11, 3. [Google Scholar] [CrossRef]
- Vasudevan, P.; Nair, M.K.M.; Annamalai, T.; Venkitanarayanan, K.S. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol. 2003, 92, 179–185. [Google Scholar] [CrossRef]
- Wiggans, G.R.; Shook, G.E. A lactation measure of somatic cell count. J. Dairy Sci. 1987, 70 (Suppl. 13), 2666–2672. [Google Scholar] [CrossRef]
- Franzoi, M.; Manuelian, C.L.; Penasa, M.; De Marchi, M. Effects of somatic cell score on milk yield and mid-infrared predicted composition and technological traits of Brown Swiss, Holstein Friesian, and Simmental cattle breeds. J. Dairy Sci. 2020, 103, 791–804. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Sarrou, S.; Fragkou, I.; Katsafadou, A.I.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Role of staphylococci in mastitis in sheep. J. Dairy Res. 2019, 86, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.H.; Fischer, U. Description of a new species of the genus Staphylococcus: Staphylococcus carnosus. Int. J. Syst. Bacteriol. 1982, 32, 153–156. [Google Scholar] [CrossRef]
- Lianou, D.T.; Petinaki, E.; Cripps, P.J.; Gougoulis, D.A.; Michael, C.K.; Tsilipounidaki, K.; Skoulakis, A.; Katsafadou, A.I.; Vasileiou, N.G.C.; Giannoulis, T.; et al. Antibiotic resistance of staphylococci from bulk-tank milk of sheep flocks: Prevalence, patterns, association with biofilm formation, effects on milk quality, and risk factors. Biology 2021, 10, 1016. [Google Scholar] [CrossRef]
- Lianou, D.T.; Petinaki, E.; Cripps, P.J.; Gougoulis, D.A.; Michael, C.K.; Tsilipounidaki, K.; Skoulakis, A.; Katsafadou, A.I.; Vasileiou, N.G.C.; Giannoulis, T.; et al. Prevalence, patterns, association with biofilm formation, effects on milk quality and risk factors for antibiotic resistance of staphylococci from bulk-tank milk of goat herds. Antibiotics 2021, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Bremer, P.; Brooks, J. Biofilms in dairy manufacturing plant—Description, current concerns and methods of control. Biofouling 1997, 11, 81–97. [Google Scholar] [CrossRef]
- Tattersall, B. Effect of Long Pasteurization Run Times on Bacterial Numbers in Milk. Master of Science Dissertation, Utah State University, Logan, UT, USA, 2020; 79p. [Google Scholar]
- Jindal, S.; Anand, S.; Metzger, L.; Amamcharl, J. A comparison of biofilm development on stainless steel and modified-surface plate heat exchangers during a 17-h milk pasteurization run. J. Dairy Sci. 2018, 101, 2921–2926. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm formation and control of foodborne pathogenic bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Vithanage, N.R.; Dissanayake, M.; Bolge, G.; Palombo, E.A.; Yeager, T.R.; Datta, N. Biodiversity of culturable psychrotrophic microbiota in raw milk attributable to refrigeration conditions, seasonality and their spoilage potential. Int. Dairy J. 2016, 57, 80–90. [Google Scholar] [CrossRef]
- Else, T.A.; Pantle, C.R.; Amy, P.S. Boundaries for biofilm formation: Humidity and temperature. Appl. Environ. Microbiol. 2003, 69, 5006–5010. [Google Scholar] [CrossRef]
- Katsarou, E.I.; Lianou, D.T.; Papadopoulos, E.; Fthenakis, G.C. Long-term climatic changes in small ruminant farms in Greece and potential associations with animal health. Sustainability 2022, 14, 1673. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on the welfare risks related to the farming of sheep for wool, meat and milk production. EFSA J. 2014, 12, 3933–4060. [Google Scholar]



| Staphylococcal Isolates | Frequency of Isolates (n) (Proportion of Isolation 1) | |||
|---|---|---|---|---|
| Animals in Farms | Milking Mode in Farms | |||
| Sheep 1 | Goats | Machine-Milking | Hand-Milking | |
| S. aureus | 54 (23.3%) | 21 (26.3%) | 51 (22.5%) | 24 (28.2%) |
| S. auricularis | 3 (1.3%) | 1 (1.3%) | 2 (0.9%) | 2 (2.4%) |
| S. capitis | 6 (2.6%) | 6 (7.5%) | 7 (3.1%) | 5 (5.9%) |
| S. carnosus | 2 (0.9%) | 0 (0.0%) | 2 (0.9%) | 0 (0.0%) |
| S. chromogenes | 13 (5.6%) | 1 (1.3%) | 9 (4.0%) | 5 (0.8%) |
| S. cohnii subsp. cohnii | 4 (1.7%) | 1 (1.3%) | 5 (2.2%) | 0 (0.0%) |
| S. cohnii subsp. urealyticum | 3 (1.3%) | 2 (2.5%) | 4 (1.8%) | 1 (1.2%) |
| S. epidermidis | 4 (1.7%) | 1 (1.3%) | 5 (2.2%) | 0 (0.0%) |
| S. equorum | 23 (9.9%) | 11 (13.8%) | 23 (10.1%) | 11 (12.9%) |
| S. haemolyticus | 22 (9.5%) | 4 (5.0%) | 22 (9.7%) | 4 (4.7%) |
| S. hominis | 2 (0.9%) | 1 (1.3%) | 1 (0.4%) | 2 (2.4%) |
| S. intermedius | 6 (2.6%) | 1 (1.3%) | 3 (1.3%) | 4 (4.7%) |
| S. kloosii | 7 (3.0%) | 3 (3.8%) | 8 (3.5%) | 2 (2.4%) |
| S. lentus | 12 (5.2%) | 5 (6.3%) | 14 (6.2%) | 3 (3.5%) |
| S. lugdunensis | 11 (4.7%) | 2 (2.5%) | 11 (4.8%) | 2 (2.4%) |
| S. pasteuri | 2 (0.9%) | 0 (0.0%) | 2 (0.9%) | 0 (0.0%) |
| S. pettenkoferi | 0 (0.0%) 2 | 3 (3.8%) 2 | 2 (0.9%) | 1 (1.2%) |
| S. saprophyticus | 4 (1.7%) | 0 (0.0%) | 3 (1.3%) | 1 (1.2%) |
| S. sciuri | 3 (1.3%) | 0 (0.0%) | 3 (1.3%) | 0 (0.0%) |
| S. simulans | 35 (15.1%) | 9 (11.3%) | 33 (14.5%) | 11 (12.9%) |
| S. vitulinus | 3 (1.3%) | 4 (5.0%) | 4 (1.8%) | 3 (3.5%) |
| S. warneri | 9 (3.9%) | 2 (2.5%) | 9 (4.0%) | 2 (2.4%) |
| S. xylosus | 4 (1.7%) | 2 (2.5%) | 4 (1.8%) | 2 (2.4%) |
| Total | 232 | 80 | 227 | 85 |
| Staphylococcal Isolates | No. of Isolates | No. of Biofilm-Forming Isolates | Proportion of Biofilm-Forming Isolates among Species | p-Value |
|---|---|---|---|---|
| S. aureus | 75 | 58 | 77.3% | 0.22 |
| S. auricularis | 4 | 4 | 100.0% | 0.21 |
| S. capitis | 12 | 10 | 83.3% | 0.37 |
| S. carnosus | 2 | 2 | 100.0% | 0.37 |
| S. chromogenes | 14 | 9 | 64.3% | 0.52 |
| S. cohnii subsp. cohnii | 5 | 2 | 40.0% | 0.11 |
| S. cohnii subsp. urealyticum | 5 | 4 | 80.0% | 0.68 |
| S. epidermidis | 5 | 4 | 80.0% | 0.68 |
| S. equorum | 34 | 24 | 70.6% | 0.87 |
| S. haemolyticus | 26 | 12 | 46.2% | 0.002 |
| S. hominis | 3 | 2 | 66.7% | 0.84 |
| S. intermedius | 7 | 4 | 57.1% | 0.38 |
| S. kloosii | 10 | 9 | 90.0% | 0.19 |
| S. lentus | 17 | 10 | 58.8% | 0.22 |
| S. lugdunensis | 13 | 5 | 38.5% | 0.006 |
| S. pasteuri | 2 | 2 | 100.0% | 0.37 |
| S. pettenkoferi | 3 | 3 | 100.0% | 0.28 |
| S. saprophyticus | 4 | 4 | 100.0% | 0.21 |
| S. sciuri | 3 | 3 | 100.0% | 0.28 |
| S. simulans | 44 | 32 | 72.7% | 0.88 |
| S. vitulinus | 7 | 6 | 85.7% | 0.41 |
| S. warneri | 11 | 10 | 90.9% | 0.15 |
| S. xylosus | 6 | 5 | 83.3% | 0.53 |
| Total | 312 | 224 | 71.8% |
| Variable | Odds Ratios 1 (95% Confidence Intervals) | p Value |
|---|---|---|
| Farms applying machine-milking | ||
| Temperature of the water used to clean the milking parlour | 0.024 | |
| <50 °C (88.9% 2) | 12.507 (2.791–56.046) | 0.001 |
| 50–69 °C (47.9%) | 1.438 (0.873–2.369) | 0.15 |
| 70–89 °C (39.0%) | reference | - |
| ≥90 °C (56.0%) | 1.990 (0.856–4.628) | 0.11 |
| Farms applying hand-milking | ||
| Frequency of milk collection from the tank | 0.08 | |
| Daily (38.1%) | reference | - |
| At least every two days (48.5%) | 1.526 (0.992–2.346) 2 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lianou, D.T.; Michael, C.K.; Solomakos, N.; Vasileiou, N.G.C.; Petinaki, E.; Mavrogianni, V.S.; Tzora, A.; Voidarou, C.; Fthenakis, G.C. Isolation of Biofilm-Forming Staphylococci from the Bulk-Tank Milk of Small Ruminant Farms in Greece. Foods 2023, 12, 2836. https://doi.org/10.3390/foods12152836
Lianou DT, Michael CK, Solomakos N, Vasileiou NGC, Petinaki E, Mavrogianni VS, Tzora A, Voidarou C, Fthenakis GC. Isolation of Biofilm-Forming Staphylococci from the Bulk-Tank Milk of Small Ruminant Farms in Greece. Foods. 2023; 12(15):2836. https://doi.org/10.3390/foods12152836
Chicago/Turabian StyleLianou, Daphne T., Charalambia K. Michael, Nikolaos Solomakos, Natalia G. C. Vasileiou, Efthymia Petinaki, Vasia S. Mavrogianni, Athina Tzora, Chrysoula Voidarou, and George C. Fthenakis. 2023. "Isolation of Biofilm-Forming Staphylococci from the Bulk-Tank Milk of Small Ruminant Farms in Greece" Foods 12, no. 15: 2836. https://doi.org/10.3390/foods12152836
APA StyleLianou, D. T., Michael, C. K., Solomakos, N., Vasileiou, N. G. C., Petinaki, E., Mavrogianni, V. S., Tzora, A., Voidarou, C., & Fthenakis, G. C. (2023). Isolation of Biofilm-Forming Staphylococci from the Bulk-Tank Milk of Small Ruminant Farms in Greece. Foods, 12(15), 2836. https://doi.org/10.3390/foods12152836










