Characterization of a Dihydromyricetin/α-Lactoalbumin Covalent Complex and Its Application in Nano-emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of the Covalent Complex
2.3. Determination of the Antioxidant Activity of the Covalent Complex
2.4. Measurement of the α-Glucosidase Inhibitory Activity of the Covalent Complex
2.5. Preparation of the Nanoemulsion Based on the Covalent Complex
2.6. Stability and β-Carotene-Protective Capacity of the Nanoemulsion
2.7. Statistical Analysis
3. Results and Discussion
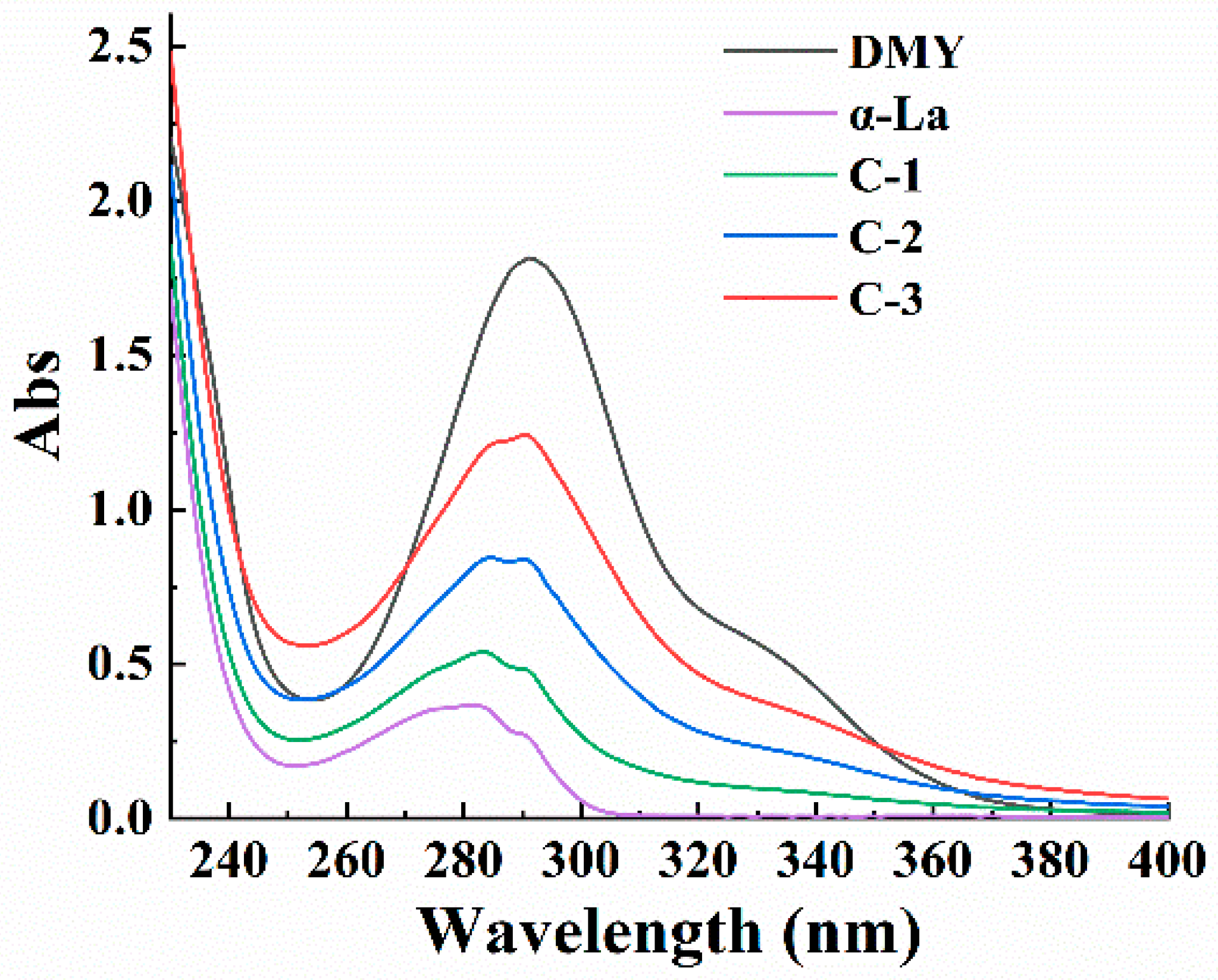
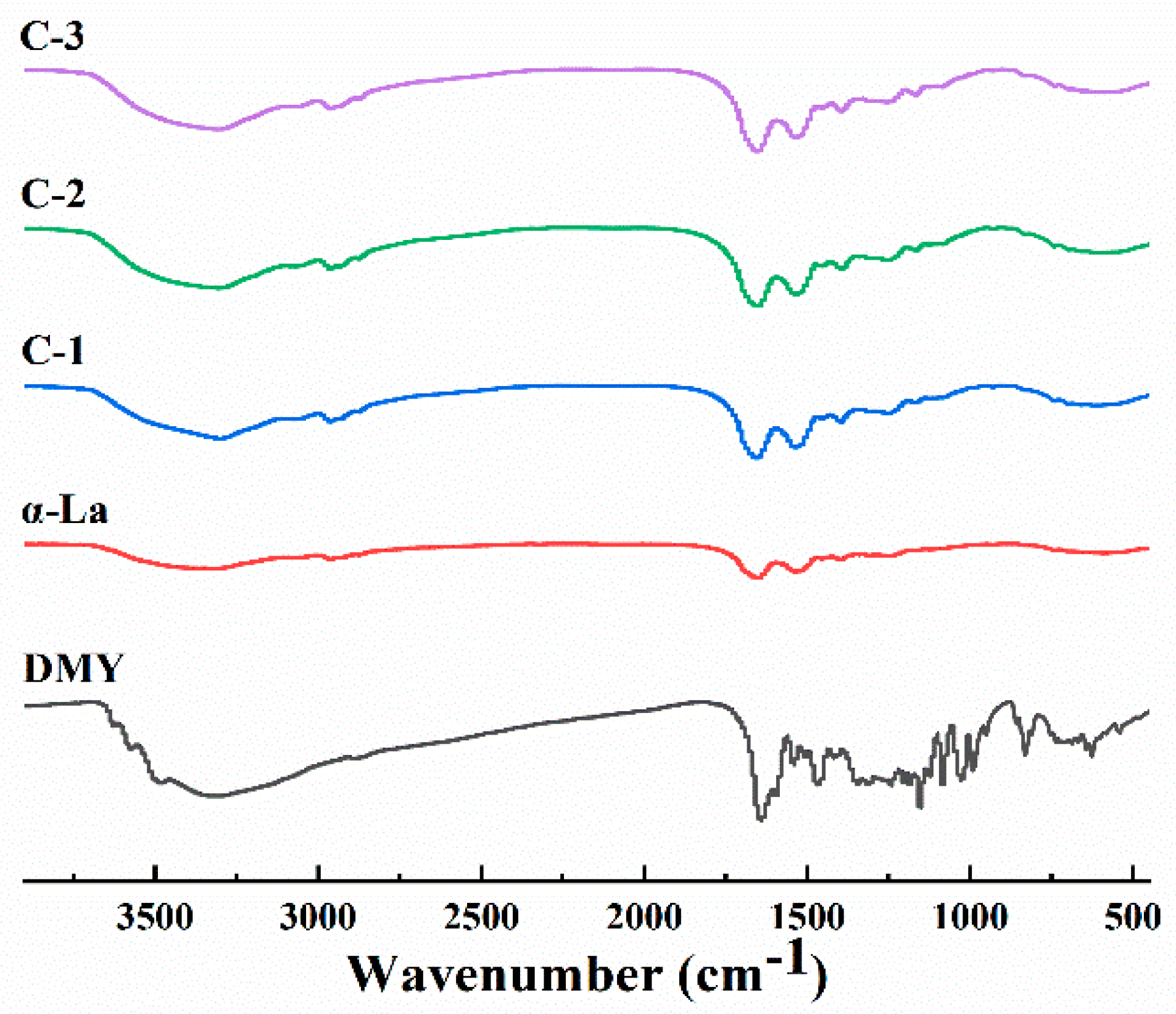
3.1. Characterization of the Covalent Complex
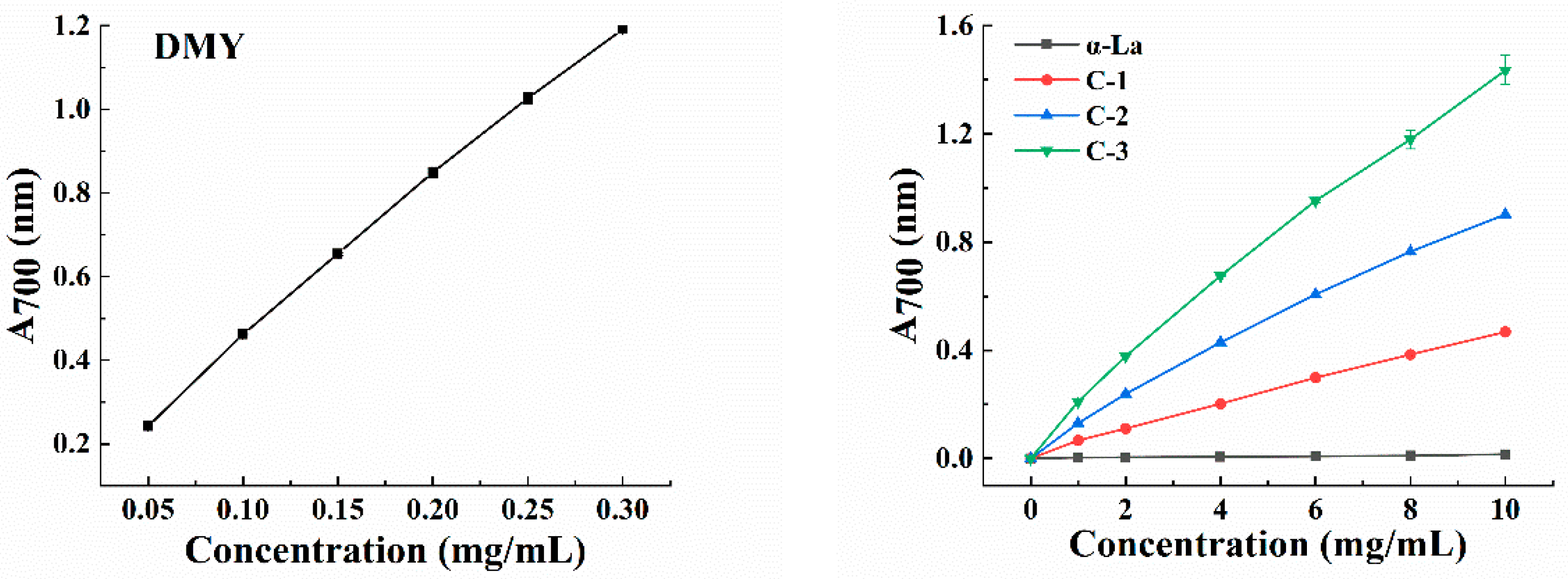
3.2. Antioxidant Activity of the Covalent Complex
3.3. α-Glucosidase Inhibitory Activity of the Covalent Complex
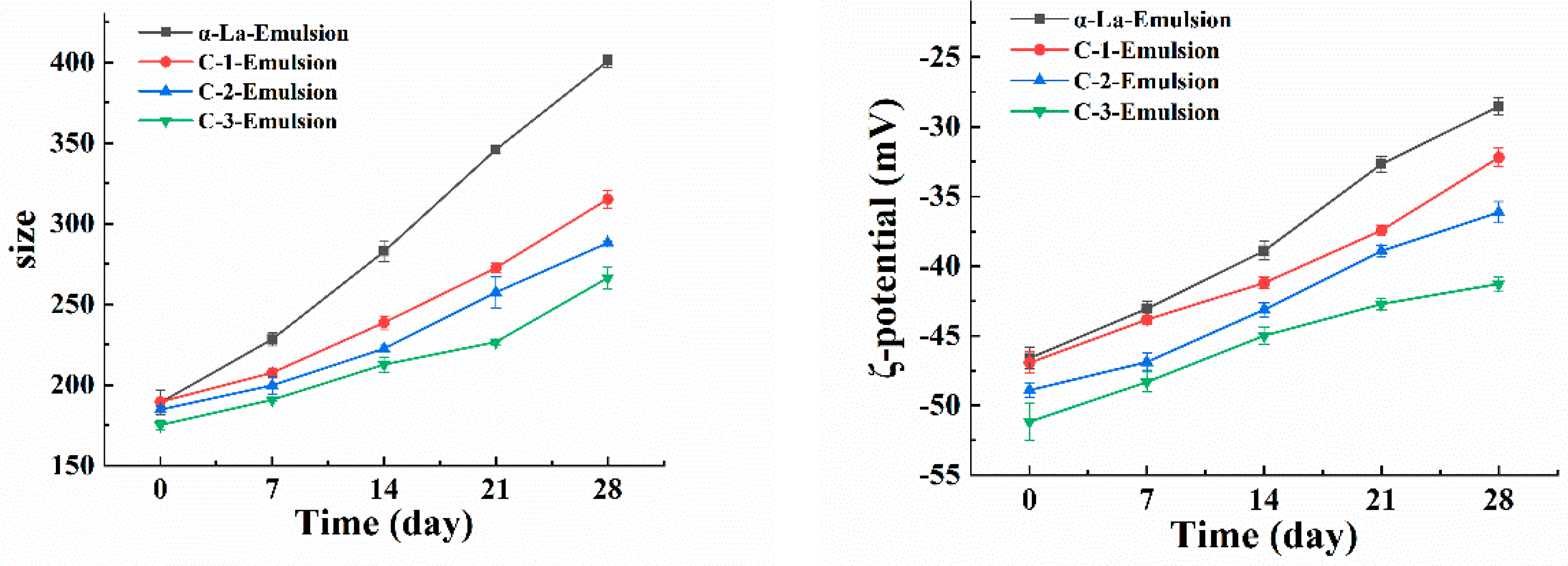
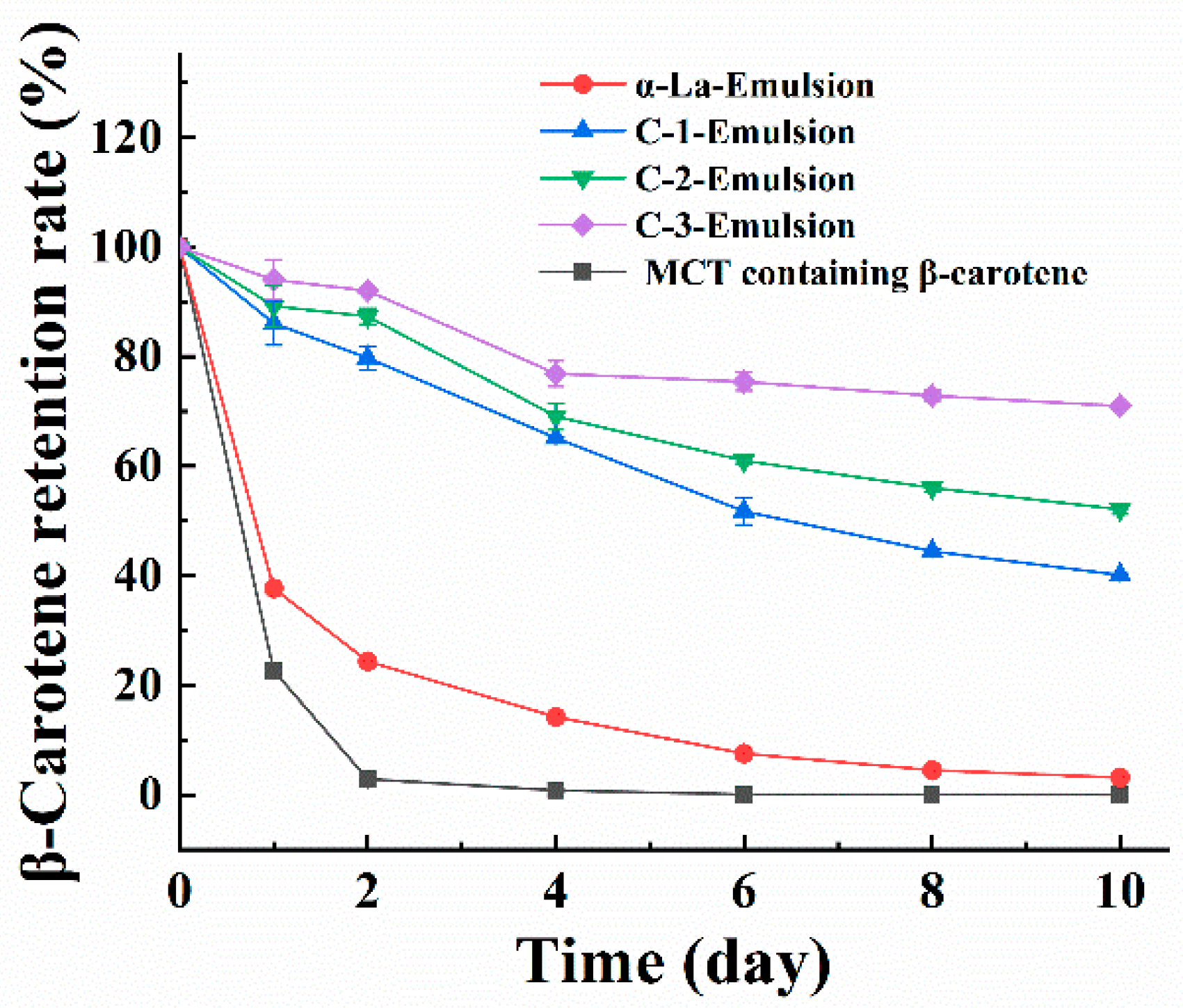
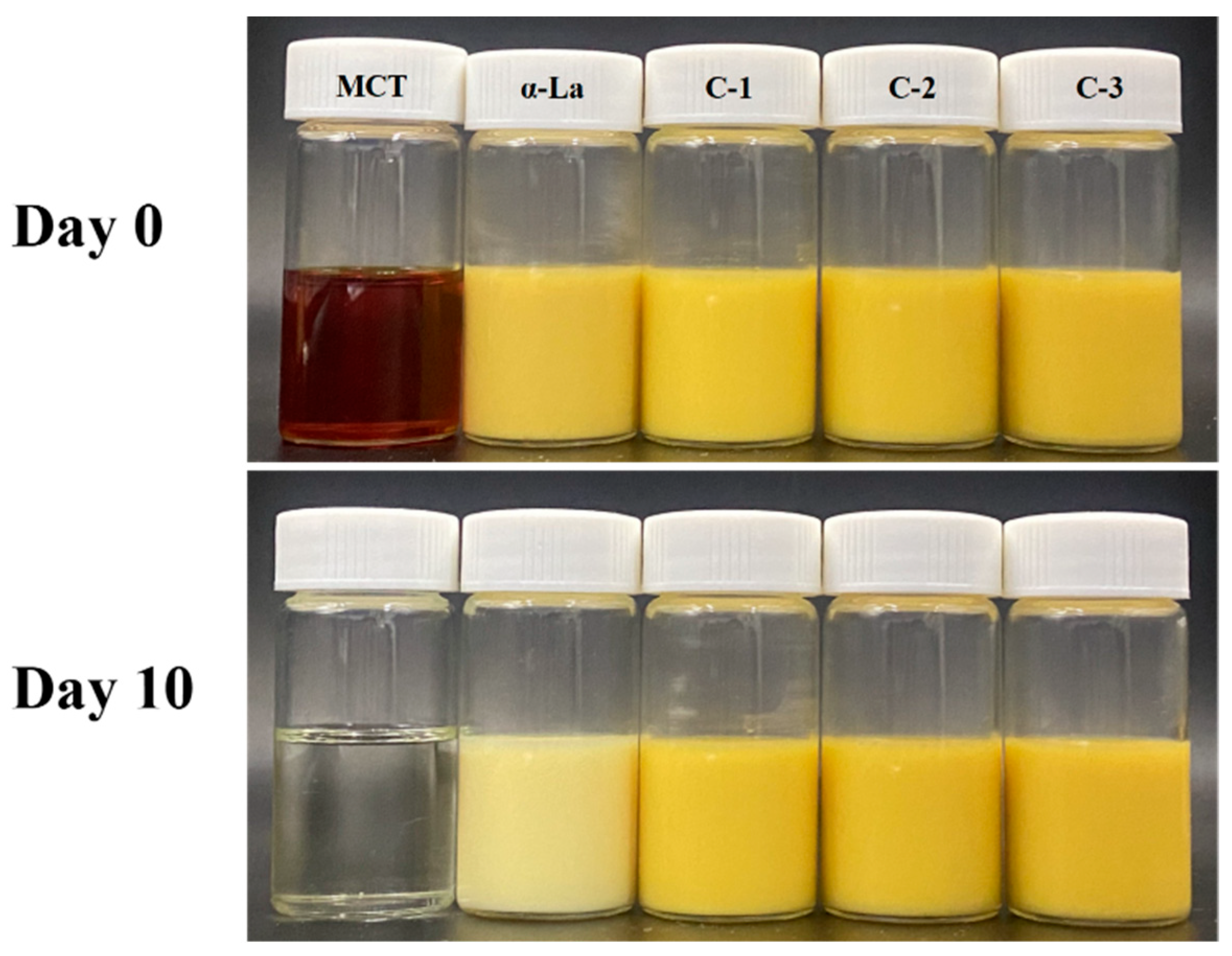
3.4. β-Carotene-Loaded Nanoemulsion Developed with the Covalent Complex
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shrestha, S.; Hag, L.V.; Haritos, V.S.; Dhital, S. Lentil and Mungbean protein isolates: Processing, functional properties, and potential food applications. Food Hydrocoll. 2023, 135, 108142. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Xing, X.; Wang, S. Health benefits of dietary polyphenols: Insight into interindividual variability in absorption and metabolism. Curr. Opin. Food Sci. 2022, 48, 100941. [Google Scholar] [CrossRef]
- Zhang, Y.; Balasooriya, H.; Sirisena, S.; Ng, K. The effectiveness of dietary polyphenols in obesity management: A systematic review and meta-analysis of human clinical trials. Food Chem. 2023, 404, 134668. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sarteshnizi, R.A.; Udenigwe, C.C. Recent advances in protein–polyphenol interactions focusing on structural properties related to antioxidant activities. Curr. Opin. Food Sci. 2022, 45, 100840. [Google Scholar] [CrossRef]
- Baba, W.N.; McClements, D.J.; Maqsood, S. Whey protein–polyphenol conjugates and complexes: Production, characterization, and applications. Food Chem. 2021, 365, 130455. [Google Scholar] [CrossRef] [PubMed]
- Parolia, S.; Maley, J.; Sammynaiken, R.; Green, R.; Nickerson, M.; Ghosh, S. Structure–Functionality of lentil protein-polyphenol conjugates. Food Chem. 2022, 367, 130603. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A.; Plundrich, N.; Schneider, M.; Campbell, C.; Lila, M.A. Protein-polyphenol particles for delivering structural and health functionality. Food Hydrocoll. 2017, 72, 163–173. [Google Scholar] [CrossRef]
- Liu, X.; Xue, F.; Adhikari, B. Production of hemp protein isolate-polyphenol conjugates through ultrasound and alkali treatment methods and their characterization. Future Foods. 2023, 7, 100210. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, L.; Luo, D.; Xie, Y.; Zhang, Y.; Zhang, Y.; Jiao, W.; Su, G.; Zhao, M. Fabrication and characterization of anchovy protein hydrolysates-polyphenol conjugates with stabilizing effects on fish oil emulsion. Food Chem. 2021, 351, 129324. [Google Scholar] [CrossRef]
- Lajnaf, R.; Attia, H.; Ayadi, M.A. Technological properties and biological activities of camel α-lactalbumin—A review. Int. Dairy. J. 2023, 139, 105563. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Liu, Y.; Gantumur, M.A.; Sukhbaatar, N.; Zhao, P.; Oh, K.C.; Jiang, Z.; Hou, J. Improving gas-water interface properties and bioactivities of α-lactalbumin induced by three structurally different saponins. Food Hydrocoll. 2023, 138, 108463. [Google Scholar] [CrossRef]
- Zhen, S.; Chen, S.; Geng, S.; Zhang, H.; Chen, Y.; Liu, B. Ultrasound-assisted natural deep eutectic solvent extraction and bioactivities of flavonoids in Ampelopsis grossedentata Leaves. Foods 2022, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Yuan, Y.; Jiang, X.; Zhang, R.; Ma, H.; Liang, G.; Liu, B. An investigation on pickering nano-emulsions stabilized by dihydromyricetin/high-amylose corn starch composite particles: Preparation conditions and carrier properties. Curr. Res. Food Sci. 2023, 6, 100458. [Google Scholar] [CrossRef]
- Zhen, S.; Geng, S.; Ma, H.; Liu, B. Interaction of α-lactalbumin with dihydromyricetin and its application in nano-emulsion. Int. J. Food Sci. Technol. 2023, 58, 1120–1129. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Lupina, K.; Kowalczyk, D.; Zieba, E.; Kazimierczak, W.; Mezynska, M.; Basiura-Cembala, M.; Wiacek, A.E. Edible films made from blends of gelatin and polysaccharide-based emulsifiers—A comparative study. Food Hydrocoll. 2019, 96, 555–567. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J.; Ibrahim, M.S.; Osman, H. Antioxidant activities of mangrove Rhizophora apiculate bark extracts. Food Chem. 2008, 107, 200–207. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- He, C.; Liu, X.; Jiang, Z.; Geng, S.; Ma, H.; Liu, B. Interaction mechanism of flavonoids and α-glucosidase: Experimental and molecular modelling studies. Foods 2019, 8, 355. [Google Scholar] [CrossRef]
- Li, J.; Geng, S.; Zhen, S.; Lv, X.; Liu, B. Fabrication and characterization of oil-in-water emulsions stabilized by whey protein isolate/phloridzin/sodium alginate ternary complex. Food Hydrocoll. 2022, 129, 107625. [Google Scholar] [CrossRef]
- Liu, X.; Geng, S.; He, C.; Sun, J.; Ma, H.; Liu, B. Preparation and characterization of a dihydromyricetin–sugar beet pectin covalent polymer. Food Chem. 2022, 376, 131952. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jeong, W.S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Hum. Well. 2022, 11, 502–511. [Google Scholar] [CrossRef]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Geng, S.; Chen, C.; Liang, G.; Liu, B. Preparation and characterization of β-carotene nanoemulsions stabilized by complexes of tartary buckwheat bran protein and rutin. J. Food Process. Preserv. 2021, 45, e15961. [Google Scholar] [CrossRef]
- Abd El-Maksoud, A.A.; Abd El-Ghany, I.H.; El-Beltagi, H.S.; Anankanbil, S.; Banerjee, C.; Petersen, S.V.; Perez, B.; Guo, Z. Adding functionality to milk-based protein: Preparation, and physicochemical characterization of β-lactoglobulin-phenolic conjugates. Food Chem. 2018, 241, 281–289. [Google Scholar] [CrossRef]
- Singh, A.; Singh, K.; Sharma, A.; Kaur, K.; Kaur, K.; Chadha, R.; Bedi, P.M.S. Recent developments in synthetic α-glucosidase inhibitors: A comprehensive review with structural and molecular insight. J. Mol. Struct. 2023, 1281, 135115. [Google Scholar] [CrossRef]
- Geng, S.; Chen, Y.; Abbasi, A.M.; Ma, H.; Mo, H.; Liu, B. Tannin fraction from Ampelopsis grossedentata leaves tea (Tengcha) as an antioxidant and alpha-glucosidase inhibitory nutraceutical. Int. J. Food Sci. Technol. 2016, 51, 2692–2700. [Google Scholar] [CrossRef]
- Buffoni, J.N.; Bonizzi, I.; Pauciullo, A.; Ramunno, L.; Feligini, M. Characterization of the major whey proteins from milk of Mediterranean water buffalo (Bubalus bubalis). Food Chem. 2011, 127, 1515–1520. [Google Scholar] [CrossRef]
- Geng, S.; Li, Y.; Lv, J.; Ma, H.; Liang, G.; Liu, B. Fabrication of food-grade Pickering high internal phase emulsions (HIPEs) stabilized by a dihydromyricetin and lysozyme mixture. Food Chem. 2022, 373, 131576. [Google Scholar] [CrossRef]
- Singh, R.K.; Sambyal, K. An overview of β-carotene production: Current status and future prospects. Food Biosci. 2022, 47, 101717. [Google Scholar] [CrossRef]
- Dalcin, A.J.F.; Roggia, I.; Felin, S.; Vizzotto, B.S.; Mitjans, M.; Vinardell, M.P.; Schuch, A.P.; Ourique, A.F.; Gomes, P. UVB photoprotective capacity of hydrogels containing dihydromyricetin nanocapsules to UV-induced DNA damage. Colloid. Surf. B. 2021, 197, 111431. [Google Scholar] [CrossRef] [PubMed]
| Sample | Total Phenol Content (mg/g) |
|---|---|
| α-La | 35.78 ± 0.27 a |
| C-1 | 42.75 ± 0.31 b |
| C-2 | 62.34 ± 0.47 c |
| C-3 | 82.66 ± 1.01 d |
| Sample | IC50 (mg/mL) |
|---|---|
| DMY | 0.03 ± 0.00 a |
| α-La | 58.37 ± 0.52 e |
| C-1 | 4.35 ± 0.10 d |
| C-2 | 1.72 ± 0.02 c |
| C-3 | 1.05 ± 0.01 b |
| Sample | IC50 (mg/mL) |
|---|---|
| DMY | 0.06 ± 0.00 a |
| C-DMY/α-La-1 | 37.08 ± 1.29 c |
| C-DMY/α-La-2 | 4.81 ± 0.16 b |
| C-DMY/α-La-3 | 1.78 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Wu, L.; Zhen, S.; Liu, B. Characterization of a Dihydromyricetin/α-Lactoalbumin Covalent Complex and Its Application in Nano-emulsions. Foods 2023, 12, 2783. https://doi.org/10.3390/foods12142783
Lu N, Wu L, Zhen S, Liu B. Characterization of a Dihydromyricetin/α-Lactoalbumin Covalent Complex and Its Application in Nano-emulsions. Foods. 2023; 12(14):2783. https://doi.org/10.3390/foods12142783
Chicago/Turabian StyleLu, Ninghai, Limin Wu, Shiyu Zhen, and Benguo Liu. 2023. "Characterization of a Dihydromyricetin/α-Lactoalbumin Covalent Complex and Its Application in Nano-emulsions" Foods 12, no. 14: 2783. https://doi.org/10.3390/foods12142783
APA StyleLu, N., Wu, L., Zhen, S., & Liu, B. (2023). Characterization of a Dihydromyricetin/α-Lactoalbumin Covalent Complex and Its Application in Nano-emulsions. Foods, 12(14), 2783. https://doi.org/10.3390/foods12142783





