Volatile, Microbial, and Sensory Profiles and Consumer Acceptance of Coffee Cascara Kombuchas
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Kombucha Consortium, Black Tea, and Coffee Cascara Kombucha
2.3. pH, Total Titratable Acidity, Total Soluble Solids Determination, and Sugar Analysis
2.4. Analyses of Volatile Organic Compounds
2.5. DNA Extraction, Amplicon Sequencing Data Analysis, and Library Preparation
2.6. Sensory Analysis
2.6.1. Consumer Acceptance and Purchase Intention
2.6.2. Rate All That Apply (RATA)
2.7. Statistics
3. Results and Discussion
3.1. Physicochemical Parameters
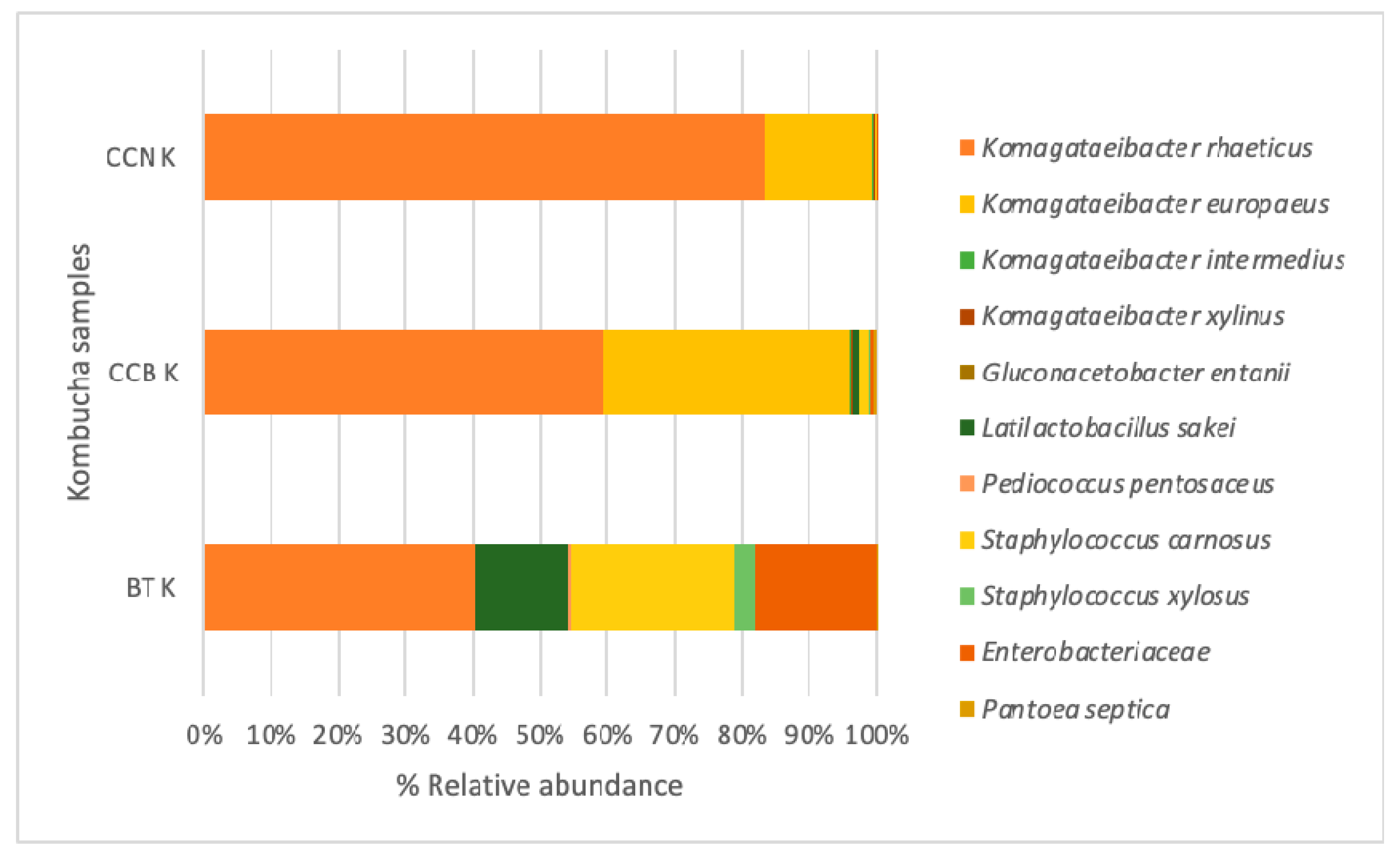
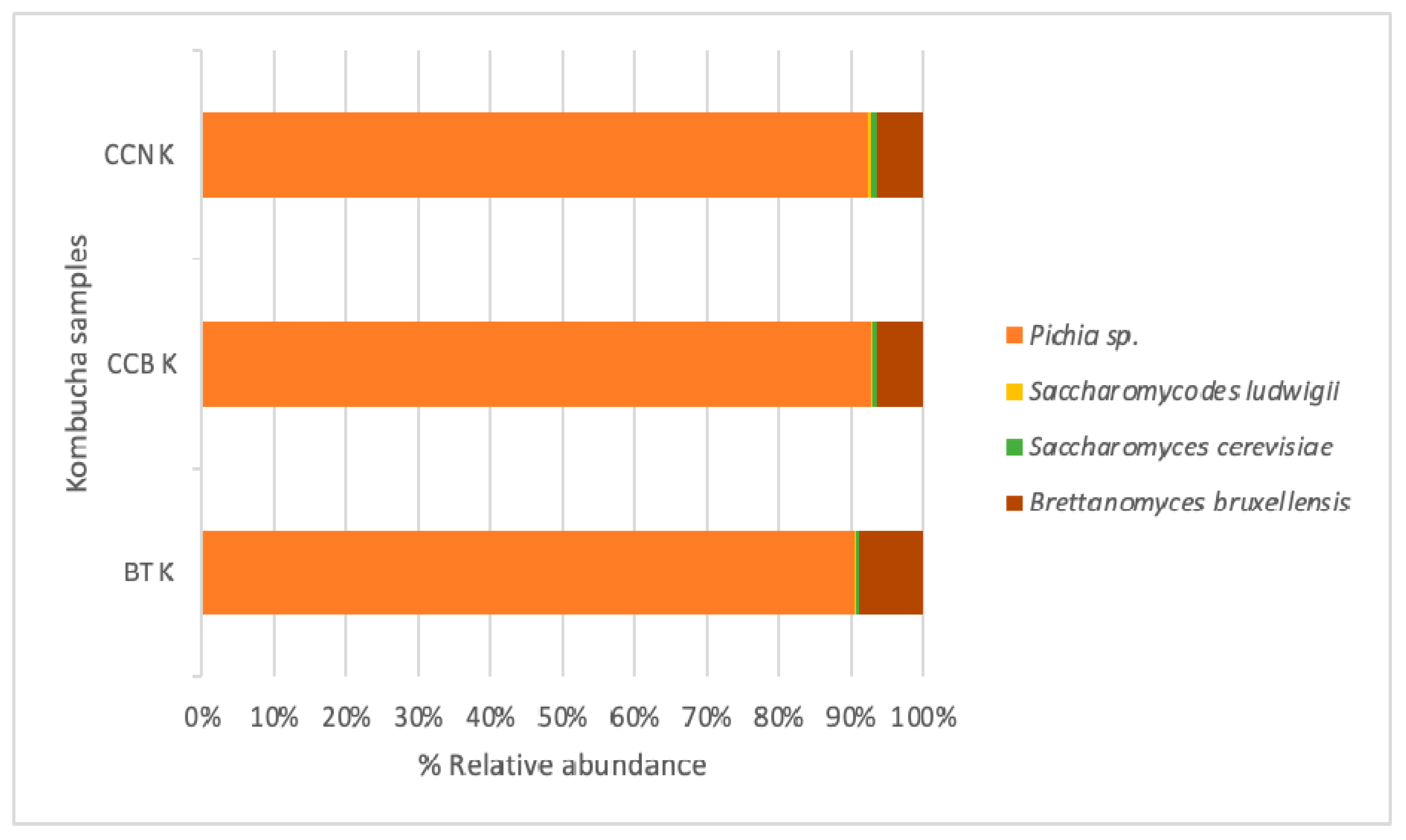
3.2. Microbial Taxonomy
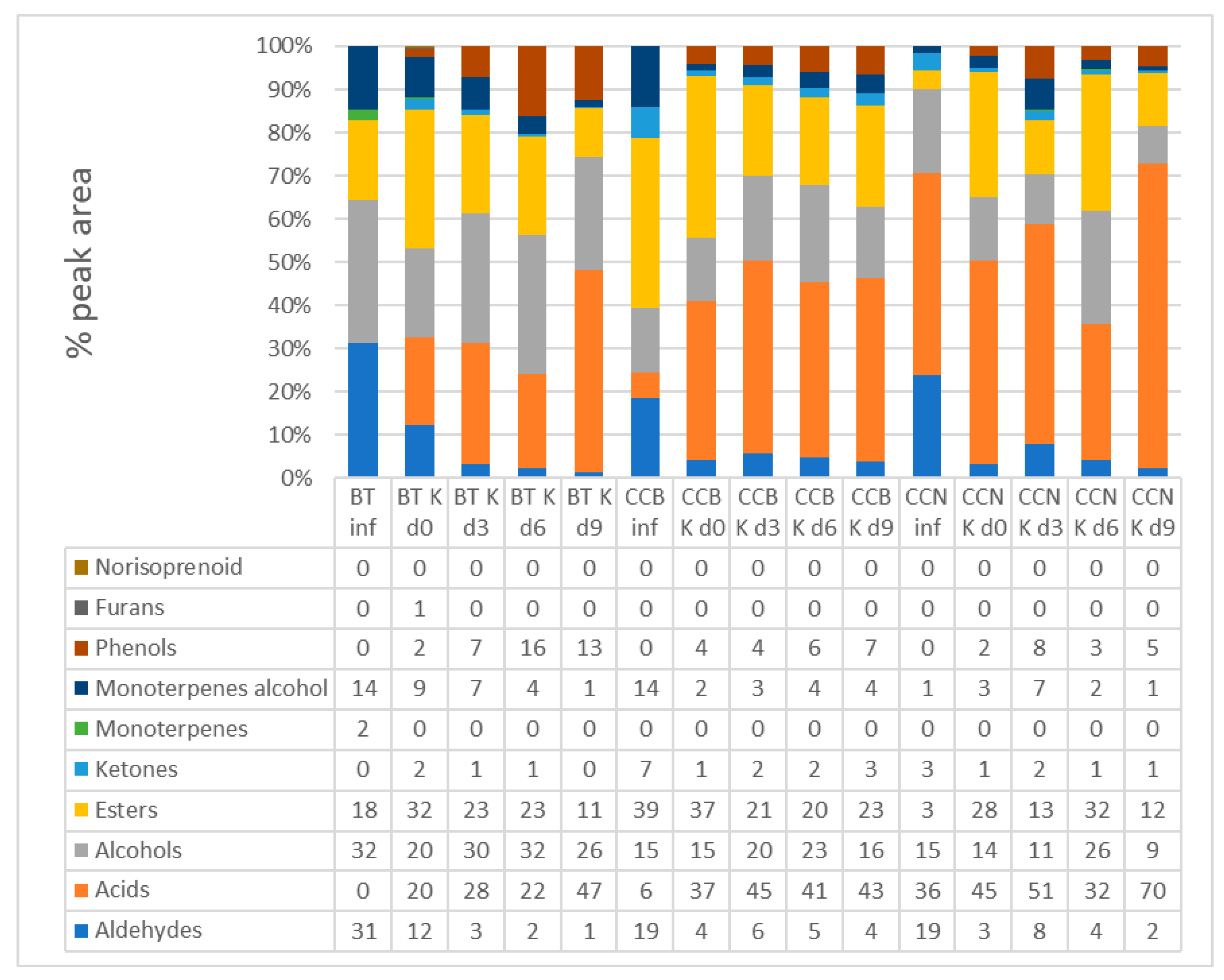
3.3. Volatile Organic Compounds
3.3.1. Black Tea Kombucha
| Volatile Compound | Odor Description | CAS a | ELRI b | LRI c | BT | BT K d0 | BT K d3 | BT K d6 | BT K d9 |
|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||||
| 2-Hexenal | Apple, green, sweet, almond, fruity, leafy, plum, vegetable 1,2 | 505-57-7 | 914 | 939 | □ | ■ | □ | □ | □ |
| 2-Methylbutyraldehyde | Musty, cocoa, phenolic, coffee, nutty, malty, fermented, fatty, alcoholic ² | 96-17-3 | 924 | 927 | ■ | ■ | □ | □ | □ |
| Benzaldehyde * | Almond, burnt sugar, tropical fruit 1,2 | 100-52-7 | 904 | 914 | □ | ■ | □ | □ | □ |
| Dodecanal | Soapy, waxy, aldehydic, citrus, green, floral 2 | 112-54-9 | 927 | 958 | ■ | ■ | ■ | ■ | ■ |
| Ethanal/Acetaldehyde | Pungent, ether, fresh, fruity, musty 1,2 | 75-07-0 | 956 | 983 | □ | ■ | ■ | ■ | ■ |
| Heptanal | Fat, citrus, rancid, fresh, aldehydic, green, herbal, wine-lee, ozone 1,2 | 111-71-7 | 784 | 864 | □ | □ | □ | □ | ■ |
| Hexanal * | Grass, tallow, fat, fresh, green, aldehydic, leafy, fruity, sweaty 1,2 | 66-25-1 | 920 | 943 | ■ | ■ | ■ | □ | □ |
| Isobutyraldehyde | Pungent, malt, green, fresh, aldehydic, floral, green 1,2 | 78-84-2 | 910 | 916 | □ | ■ | □ | □ | □ |
| Isovaleraldehyde | Ethereal, aldehydic, chocolate, peach, fatty 2 | 590-86-3 | 910 | 912 | □ | ■ | ■ | □ | □ |
| Nonanal * | Fat, citrus, fresh, orange, green 1,2 | 124-19-6 | 875 | 897 | ■ | ■ | ■ | ■ | ■ |
| Octanal | Citrus, soap, lemon, herbal, green, honey 1,2 | 124-13-0 | 856 | 927 | ■ | ■ | ■ | ■ | ■ |
| 2-Phenylethanal | Honey, floral, rose, sweet, powdery, fermented, chocolate, earthy, hawthorn, green, hyacinth, clover, cocoa 1, | 122-78-1 | 925 | 942 | ■ | ■ | ■ | ■ | □ |
| Tetradecanal | Fatty, waxy, amber, incense, dry, citrus, peel, musk 2 | 124-25-4 | 705 | 826 | □ | □ | □ | ■ | ■ |
| Acids | |||||||||
| 2-methylbutanoic acid | Pungent, acid, Roquefort cheese 2 | 116-53-0 | 806 | 832 | □ | □ | □ | ■ | ■ |
| Acetic acid | Acidic, sour, pungent, vinegar 1,2 | 64-19-7 | 943 | 956 | □ | ■ | ■ | ■ | ■ |
| Caproic acid | Sweat, sour, fatty, cheese 1,2 | 142-62-1 | 884 | 906 | □ | □ | ■ | ■ | ■ |
| Decanoic acid | Rancid, fat, unpleasant, rancid, sour, fatty, citrus 1,2 | 334-48-5 | 907 | 914 | □ | ■ | ■ | □ | ■ |
| Isovaleric acid | Sweat, acid, rancid, sour, stinky, feet, cheese, tropical 1,2 | 503-74-2 | 876 | 887 | □ | □ | ■ | ■ | ■ |
| Lauric acid | Metal, mild, fatty, coconut, bay, oil 1,2 | 143-07-7 | 755 | 841 | □ | □ | □ | □ | ■ |
| Nonanoic acid | Green, fat, waxy, dirty, cheese, cultured, dairy 1,2 | 112-05-0 | 892 | 898 | □ | ■ | ■ | ■ | ■ |
| Octanoic acid | Acid, sweat, cheese, fruit notes 1,2 | 124-07-2 | 917 | 922 | □ | ■ | ■ | ■ | ■ |
| Alcohols | |||||||||
| 1-penten-3-ol | Ethereal, horseradish, green, radish, chrysanthemum, vegetable, tropical, fruity 2 | 616-25-1 | 737 | 869 | □ | ■ | □ | □ | □ |
| 2-Ethylhexanol | Rose, green, citrus, fresh, floral, oily, sweet 1,2 | 104-76-7 | 954 | 954 | ■ | ■ | ■ | □ | ■ |
| (S)-(–)-2-methyl-1-butanol | Ethereal, fresh 2 | 1565-80-6 | 807 | 858 | □ | □ | □ | □ | ■ |
| 2-methyl-1-butanol | Malt, wine, onion, ethereal, fusel, alcoholic, fatty, greasy, whiskey, leathery, cocoa 1,2 | 137-32-6 | 918 | 923 | □ | ■ | ■ | ■ | □ |
| 3-methyl-1-butanol/isoamyl alcohol | Whiskey, malt, burnt, fusel, oil, alcoholic, fruity, banana 1,2 | 123-51-3 | 903 | 909 | □ | □ | □ | ■ | ■ |
| Phenylethyl alcohol * | Honey, spice, rose, lilac, floral, fresh 1,2 | 60-12-8 | 917 | 939 | □ | ■ | ■ | ■ | ■ |
| (E)-2-Hexenol | Green, leaf, walnut, fresh, fruity, unripe, banana 1,2 | 928-95-0 | 774 | 799 | □ | ■ | □ | □ | □ |
| Amyl alcohol | Fusel, oil, sweet, balsam 2 | 71-41-0 | 811 | 870 | □ | □ | ■ | □ | □ |
| Cedrol | Cedarwood, woody, dry, sweet, soft 2 | 77-53-2 | 611 | 753 | ■ | □ | ■ | □ | □ |
| Ethanol | Sweet 1 | 64-17-5 | 973 | 973 | □ | ■ | ■ | ■ | ■ |
| Octanol | Moss, nut, mushroom, waxy, green, orange, aldehydic, rose 1,2 | 111-87-5 | 810 | 870 | □ | □ | □ | ■ | □ |
| Esters | |||||||||
| 2-methylbutyl acetate | Fruit, over-ripe, sweet, banana, juicy 1,2 | 624-41-9 | 845 | 899 | □ | □ | □ | □ | ■ |
| Ethyl 2–methylbutyrate | Sharp, sweet, green, apple, fruity 2 | 7452-79-1 | 772 | 871 | □ | □ | □ | □ | ■ |
| Ethyl acetate | Pineapple, ethereal, fruity, sweet, weedy, green 1,2 | 141-78-6 | 951 | 961 | □ | ■ | ■ | ■ | ■ |
| Ethyl decanoate | Grape, sweet, waxy, fruity, apple, oily, brandy 1,2 | 110-38-3 | 947 | 983 | □ | □ | ■ | ■ | ■ |
| Ethyl isobutyrate | Sweet, rubber, ethereal, fruity, alcoholic, fusel, rummy 1,2 | 97-62-1 | 754 | 786 | □ | □ | □ | □ | ■ |
| Ethyl laurate | Leaf, sweet, waxy, floral, soapy, clean 1,2 | 106-33-2 | 876 | 901 | ■ | □ | □ | ■ | ■ |
| Ethyl miristate | Sweet, waxy, violet, orris 2 | 124-06-1 | 815 | 848 | ■ | □ | □ | ■ | □ |
| Ethyl octanoate | Fruit, banana, pear 1, | 106-32-1 | 873 | 930 | □ | □ | □ | ■ | ■ |
| Ethyl phenylacetate | Fruit, sweet, floral, honey, rose, balsam, cocoa 1,2 | 101-97-3 | 840 | 870 | □ | □ | □ | ■ | ■ |
| Isopropyl myristate | Faint, oily, fatty 2 | 110-27-0 | 681 | 767 | ■ | ■ | □ | □ | □ |
| Isopropyl palmitate | Fat, bland, oily 1,2 | 142-91-6 | 780 | 735 | □ | □ | ■ | ■ | □ |
| Methyl salicylate | Peppermint 1 | 119-36-8 | 897 | 958 | ■ | ■ | ■ | ■ | □ |
| Ketones | |||||||||
| 3,5-Octadienone | Fruity, fatty, mushroom 2 | 38284-27-4 | 680 | 835 | ■ | ■ | □ | □ | □ |
| Acetylpropionyl | Pungent, sweet, butter, creamy, caramel, nutty, cheese 2 | 600-14-6 | 834 | 912 | □ | □ | ■ | □ | □ |
| Geranyl acetone | Magnolia, green, fresh, fruity, waxy, rose, woody, tropical 1,2 | 3796-70-1 | 648 | 802 | □ | ■ | □ | □ | □ |
| β-damascenone * | Apple, rose, honey, tobacco, sweet 1,2 | 23726-93-4 | 832 | 927 | ■ | ■ | ■ | ■ | ■ |
| Monoterpenes | |||||||||
| (Z)-Sabinene hydrate | Balsam 2 | 15537-55-0 | 818 | 828 | ■ | ■ | □ | □ | □ |
| γ-Terpinene | Terpineol, lilac 2 | 586-81-2 | 871 | 884 | ■ | ■ | □ | □ | □ |
| β-Ionone * | Seaweed, violet, flower, raspberry, woody, sweet, fruity, berry, tropical, beeswax 1,2 | 14901-07-6 | 815 | 825 | ■ | ■ | ■ | ■ | ■ |
| Monoterpenes alcohols | |||||||||
| α-Terpineol | Oil, anise, mint, lemon, citrus 1,2 | 98-55-5 | 801 | 820 | □ | ■ | ■ | ■ | □ |
| 1-Terpinen-4-ol | Turpentine, nutmeg, must, pepper, woody, earth, musty, sweet 1,2 | 562-74-3 | 803 | 868 | □ | ■ | ■ | □ | □ |
| Linalool * | Citrus, flower, lavender, sweet, green 1,2 | 78-70-6 | 921 | 925 | ■ | ■ | ■ | ■ | ■ |
| Linalool oxide | Flower, wood, musty, camphor, fenchyl, alcohol 1,2 | 60047-17-8 | 730 | 778 | ■ | ■ | ■ | ■ | ■ |
| Furans | |||||||||
| Dihydroactinidiolide | Musk, coumarin 2 | 17092-92-1 | 772 | 851 | ■ | ■ | ■ | ■ | ■ |
| Furfuryl alcohol | Burnt, alcoholic, chemical, musty, sweet, caramel, bread, coffee | 98-00-0 | 806 | 849 | □ | □ | □ | □ | ■ |
| Pyrrol | |||||||||
| 1-Ethyl-1H-pyrrole-2-carboxaldehyde | Burnt, roasted, smoky 2 | 2167-14-8 | 792 | 832 | ■ | ■ | □ | □ | □ |
| Phenols | |||||||||
| 4-Ethylguaiacol | Spice, clove, smoky, bacon, phenolic/medicinal 1,2 | 2785-89-9 | 898 | 918 | □ | ■ | ■ | ■ | ■ |
| 4-Ethylphenol | Phenolic/medicinal, castoreum, smoke, guaiacol 2 | 123-07-9 | 917 | 922 | □ | ■ | ■ | ■ | ■ |
| Norisoprenoid | |||||||||
| Theaspirane | Tea, herbal, green, wet, tobacco, leaf, metallic, woody, spicy 2 | 36431-72-8 | 805 | 913 | ■ | ■ | □ | □ | □ |
3.3.2. Coffee Cascara Kombuchas
| Volatile Compound | Odor Description | CAS# a | ELRI b | LRI c | CCB | CCB K d0 | CCB K d3 | CCB K d6 | CCB K d9 | CCN | CCN K d0 | CCN K d3 | CCN K d6 | CCN K d9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | ||||||||||||||
| α-Hexylcinnamaldehyde | Fresh, floral, green, jasmine, herbal, waxy 2 | 101-86-0 | 871 | 908 | □ | □ | □ | □ | ■ | □ | □ | □ | □ | □ |
| 2-methylbutyraldehyde | Musty, cocoa, phenolic, coffee, nutty, malty, fermented, fatty, alcoholic 2 | 96-17-3 | 644 | 754 | □ | ■ | □ | □ | □ | □ | ■ | □ | □ | □ |
| Isovaleraldehyde | Ethereal, aldehydic, chocolate, peach, fatty 2 | 590-86-3 | 777 | 842 | ■ | ■ | □ | □ | □ | □ | ■ | □ | □ | □ |
| Benzaldehyde * | Almond, burnt sugar, tropical fruit 1,2 | 100-52-7 | 736 | 885 | ■ | □ | □ | □ | □ | ■ | □ | □ | □ | □ |
| Decanal | Sweet, citrus, floral, soap, orange peel 1,2 | 112-31-2 | 806 | 846 | ■ | ■ | □ | □ | □ | □ | □ | □ | □ | □ |
| Dodecanal | Soapy, waxy, aldehydic, citrus, green, floral 2 | 112-54-9 | 929 | 963 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Ethanal/Acetaldehyde | Pungent, ether, fresh, fruity, musty 1,2 | 75-07-0 | 980 | 982 | □ | ■ | ■ | ■ | ■ | □ | ■ | ■ | ■ | ■ |
| Heptanal | Fat, citrus, rancid, fresh, aldehydic, green, herbal, wine-lees, ozone 1,2 | 111-71-7 | 789 | 863 | ■ | ■ | ■ | □ | □ | □ | □ | □ | □ | □ |
| Hexanal | Grass, tallow, fat, fresh, green, aldehydic, leafy, fruity, sweaty 1,2 | 66-25-1 | 854 | 892 | ■ | ■ | □ | □ | □ | ■ | ■ | □ | □ | □ |
| Nonanal * | Fat, citrus, fresh, orange, green 1,2 | 124-19-6 | 911 | 918 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Octanal | Citrus, soap, lemon, herbal, green, honey 1,2 | 124-13-0 | 906 | 937 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Palmitaldehyde | Cardboard 1,2 | 629-80-1 | 769 | 843 | ■ | ■ | □ | □ | □ | □ | □ | □ | □ | ■ |
| Phenylethanal/phenylacetaldehyde * | Honey, floral, rose, sweet, powdery, fermented, chocolate, earthy, hawthorn, green, hyacinth, clover, cocoa 1,2 | 122-78-1 | 859 | 930 | □ | □ | ■ | ■ | □ | □ | □ | □ | □ | □ |
| Tetradecanal | Fatty, waxy, amber, incense, dry, citrus, peel, musk 2 | 124-25-4 | 723 | 852 | □ | □ | □ | □ | □ | ■ | ■ | ■ | ■ | □ |
| Undecanal | Waxy, soapy, floral, aldehydic, citrus, green, fatty, fresh, laundry 2 | 112-44-7 | 723 | 865 | □ | □ | □ | □ | □ | ■ | ■ | □ | □ | □ |
| Acids | ||||||||||||||
| Acetic acid | Acidic, sour, pungent, vinegar 1,2 | 64-19-7 | 943 | 956 | □ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Caproic acid | Sweat, sour, fatty, cheese 1,2 | 142-62-1 | 864 | 898 | □ | □ | □ | □ | ■ | □ | □ | □ | ■ | ■ |
| Decanoic acid | Rancid, fat, unpleasant, rancid, sour, fatty, citrus 1,2 | 334-48-5 | 923 | 948 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Isobutyric acid | Rancid, butter, cheese, acidic, sour 1,2 | 79-31-2 | 831 | 861 | □ | □ | □ | □ | □ | ■ | ■ | □ | □ | ■ |
| Isovaleric acid | Sweat, acid, rancid, sour, stinky, feet, cheese, tropical 1,2 | 503-74-2 | 851 | 868 | □ | □ | □ | ■ | ■ | □ | □ | □ | ■ | ■ |
| Lauric acid | Metal, mild, fatty, coconut, bay, oil 1,2 | 143-07-7 | 689 | 826 | □ | □ | □ | □ | ■ | □ | □ | □ | □ | □ |
| Nonanoic acid | Green, fat, waxy, dirty, cheese, cultured, dairy 1,2 | 112-05-0 | 897 | 904 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Octanoic acid | Acid, sweat, cheese, fruit notes 1,2 | 124-07-2 | 922 | 928 | □ | □ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Alcohols | ||||||||||||||
| 1-dodecanol | Earthy, soapy, waxy, fatty, honey, coconut 2 | 112-53-8 | 796 | 924 | ■ | ■ | □ | □ | ■ | □ | □ | □ | □ | □ |
| 1-Heptanol | Musty, leafy, violet, herbal, green, sweet, woody, peony 2 | 111-70-6 | 796 | 834 | □ | □ | □ | □ | □ | □ | □ | ■ | ■ | ■ |
| 2-Ethylhexanol | Rose, green, citrus, fresh, floral, oily, sweet 1,2 | 104-76-7 | 906 | 953 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| 2-Methyl-1-butanol | Malt, wine, onion, ethereal, fusel, alcoholic, fatty, greasy, whiskey, leathery, cocoa 1,2 | 137-32-6 | 858 | 904 | □ | ■ | ■ | ■ | □ | □ | ■ | ■ | □ | □ |
| (S)-(–)-2–methyl-1-butanol | Ethereal, fresh 2 | 1565-80-6 | 796 | 847 | □ | ■ | □ | □ | □ | □ | ■ | □ | □ | □ |
| 3-Methyl-1-butanol/isoamyl alcohol * | Whiskey, malt, burnt, fusel, oil, alcoholic, fruity, banana 1,2 | 123-51-3 | 892 | 899 | □ | ■ | □ | ■ | ■ | □ | ■ | □ | □ | ■ |
| 2-Nonen-1-ol | Sweet, fatty, melon, cucumber, vegetable 2 | 22104-79-6 | 636 | 741 | ■ | ■ | □ | □ | □ | □ | □ | □ | □ | □ |
| (E)-2-Decen-1-ol | Waxy, fresh, air, citrus, rose, rue 2 | 18409-18-2 | 792 | 796 | □ | □ | □ | □ | □ | □ | □ | □ | □ | ■ |
| (E)-2-Octen-1-ol | Green, citrus, vegetable, fatty 2 | 18409-17-1 | 602 | 663 | □ | □ | ■ | ■ | ■ | □ | □ | □ | □ | □ |
| Phenethyl alcohol | Honey, spice, rose, lilac, floral, fresh 1,2 | 60-12-8 | 825 | 885 | ■ | ■ | ■ | ■ | ■ | □ | ■ | ■ | ■ | □ |
| Cedrol | Cedarwood, woody, dry, sweet, soft 2 | 77-53-2 | 653 | 784 | □ | ■ | □ | □ | □ | □ | ■ | ■ | □ | □ |
| Ethanol | Sweet 2 | 64-17-5 | 945 | 945 | □ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Hexadecanol | Flower, wax, clean, greasy, floral, oily 1,2 | 36653-82-4 | 932 | 951 | □ | □ | □ | □ | ■ | ■ | □ | □ | □ | □ |
| Isopulegol | Minty, cooling, medicinal, woody 2 | 89-79-2 | 812 | 816 | □ | □ | □ | □ | □ | ■ | ■ | ■ | ■ | □ |
| Octanol | Moss, nut, mushroom, waxy, green, orange, aldehydic, rose 1,2 | 111-87-5 | 712 | 815 | □ | □ | □ | ■ | ■ | □ | □ | □ | □ | □ |
| Esters | ||||||||||||||
| 2-Ethylhexyl salicylate | Mild, orchid, sweet, balsam 2 | 118-60-5 | 863 | 961 | ■ | ■ | □ | □ | □ | ■ | ■ | □ | □ | □ |
| Ethyl 2-methylbutyrate | Sharp, sweet, green, apple, fruity 2 | 7452-79-1 | 877 | 895 | □ | □ | □ | □ | ■ | □ | □ | □ | □ | ■ |
| Ethyl Acetate | Pineapple, ethereal, fruity, sweet, weedy, green 1,2 | 141-78-6 | 940 | 950 | □ | ■ | ■ | ■ | ■ | □ | ■ | ■ | ■ | ■ |
| Ethyl butyrate | Apple, fruity, juicy, fruit, pineapple, cognac 1,2 | 105-54-4 | 765 | 848 | □ | □ | □ | □ | □ | □ | □ | □ | □ | ■ |
| Ethyl decanoate | Grape, sweet, waxy, fruity, apple, oily, brandy 1,2 | 110-38-3 | 915 | 928 | □ | □ | ■ | ■ | ■ | □ | □ | □ | ■ | ■ |
| Ethyl hexanoate * | Apple peel, fruit, sweet, pineapple, waxy, green, banana 1,2 | 123-66-0 | 909 | 912 | □ | ■ | □ | □ | ■ | □ | ■ | □ | □ | ■ |
| Ethyl isobutyrate * | Sweet, rubber, ethereal, fruity, alcoholic, fusel, rummy 1,2 | 97-62-1 | 882 | 895 | □ | □ | □ | □ | ■ | □ | □ | □ | □ | □ |
| Ethyl laurate | Leaf, sweet, waxy, floral, soapy, clean 1,2 | 106-33-2 | 814 | 841 | □ | □ | □ | ■ | ■ | □ | □ | □ | ■ | ■ |
| Ethyl miristate | Sweet, waxy, violet, orris 2 | 124-06-1 | 775 | 780 | □ | □ | □ | □ | □ | □ | □ | □ | □ | ■ |
| Ethyl octanoate * | Fruit, banana, pear 1,2 | 106-32-1 | 913 | 939 | □ | □ | ■ | ■ | ■ | □ | □ | □ | ■ | ■ |
| Ethyl palmitate | Wax, fruity, creamy, milky, balsamic, greasy, oily 1,2 | 628-97-7 | 791 | 833 | ■ | □ | □ | □ | □ | ■ | □ | □ | □ | □ |
| Ethyl phenylacetate | Fruit, sweet, floral, honey, rose, balsam, cocoa 1,2 | 101-97-3 | 874 | 894 | □ | □ | □ | □ | ■ | □ | □ | □ | □ | ■ |
| Homomenthyl salicylate | Mild, menthol 2 | 118-56-9 | 792 | 910 | ■ | ■ | □ | □ | □ | □ | □ | □ | □ | □ |
| Isoamyl acetate * | Banana, sweet, fruity, solvent 1,2 | 123-92-2 | 772 | 878 | ■ | ■ | □ | □ | ■ | □ | □ | □ | □ | □ |
| Isopropyl palmitate | Fat, bland, oily 1,2 | 142-91-6 | 790 | 882 | ■ | ■ | □ | □ | ■ | □ | □ | □ | □ | □ |
| Methyl dihydrojasmonate | Floral, oily, jasmine, green, lactonic, tropical, natural 2 | 24851-98-7 | 785 | 823 | □ | □ | □ | □ | □ | ■ | ■ | □ | □ | □ |
| Methyl palmitate | Oily, waxy, fatty, orris 2 | 112-39-0 | 762 | 848 | □ | □ | □ | ■ | ■ | □ | □ | □ | □ | □ |
| Methyl salicylate | Peppermint 1 | 119-36-8 | 895 | 920 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | □ |
| Ketones | ||||||||||||||
| Geranyl acetone | Magnolia, green, fresh, fruity, waxy, rose, woody, tropical 1,2 | 3796-70-1 | 682 | 777 | □ | □ | □ | □ | □ | ■ | ■ | □ | □ | ■ |
| β-Damascenone * | Apple, rose, honey, tobacco, sweet 1,2 | 23726-93-4 | 878 | 944 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | □ | □ | □ |
| Monoterpenes | ||||||||||||||
| Limonene | Lemon, orange, citrus, herbal, terpene, camphor 1,2 | 138-86-3 | 861 | 893 | □ | □ | □ | □ | ■ | ■ | ■ | ■ | ■ | ■ |
| γ-Nonalactone * | Coconut, peach, creamy, waxy, sweet, buttery, oily 1,2 | 104-61-0 | 859 | 895 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Monoterpenes alcohols | ||||||||||||||
| α-Terpineol | Oil, anise, mint, lemon, citrus 1,2 | 98-55-5 | 871 | 900 | □ | □ | ■ | ■ | ■ | □ | ■ | ■ | □ | □ |
| 1-Terpinen-4-ol | Turpentine, nutmeg, must, pepper, woody, earth, musty, sweet 1,2 | 562-74-3 | 681 | 804 | ■ | ■ | □ | □ | □ | ■ | ■ | ■ | □ | □ |
| Linalool * | Citrus, flower, lavender, sweet, green 1,2 | 78-70-6 | 919 | 925 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Linalool oxide | Flower, wood, musty, camphor, alcohol 1,2 | 60047-17-8 | 891 | 900 | ■ | ■ | ■ | □ | □ | □ | □ | □ | ■ | ■ |
| cis-Linalol oxide | Flower 1 | 5989-33-3 | 838 | 852 | □ | □ | □ | ■ | ■ | □ | □ | □ | □ | □ |
| trans-Linalool oxide | Flower 1,2 | 34995-77-2 | 863 | 878 | □ | □ | □ | □ | □ | ■ | ■ | ■ | □ | □ |
| Furans | ||||||||||||||
| Furfural * | Bread, almond, sweet, woody 1,2 | 98-01-1 | 924 | 924 | ■ | ■ | □ | □ | □ | ■ | ■ | □ | □ | □ |
| Phenols | ||||||||||||||
| 4-Ethylguaiacol | Spice, clove, smoky, bacon, phenolic/medicinal 1,2 | 2785-89-9 | 862 | 881 | □ | ■ | ■ | ■ | ■ | □ | ■ | ■ | ■ | ■ |
| 4-Ethylphenol | Phenolic/medicinal, castoreum, smoke, guaiacol 2 | 123-07-9 | 858 | 889 | □ | ■ | ■ | ■ | ■ | □ | ■ | ■ | ■ | ■ |
3.4. Sensory Tests
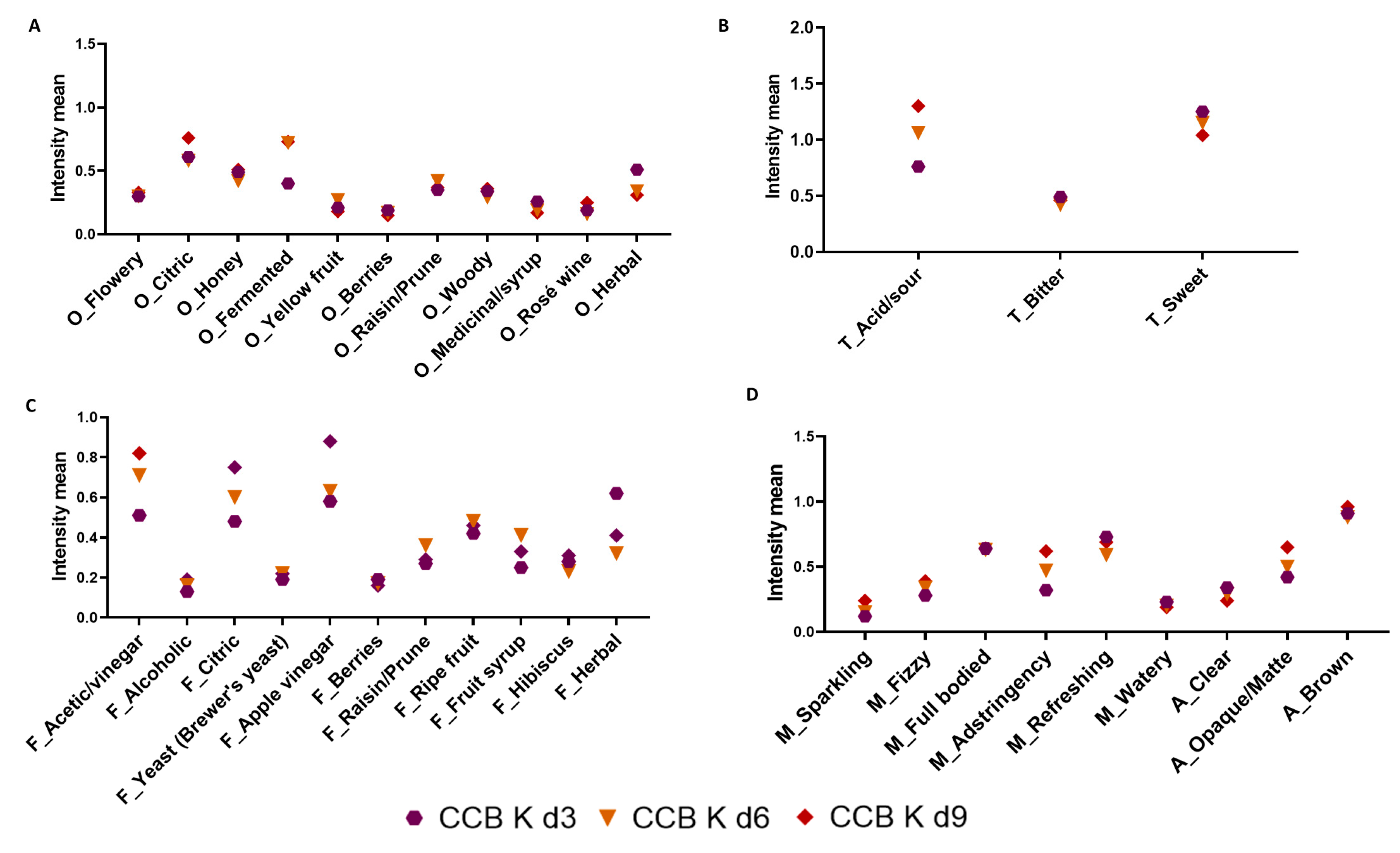
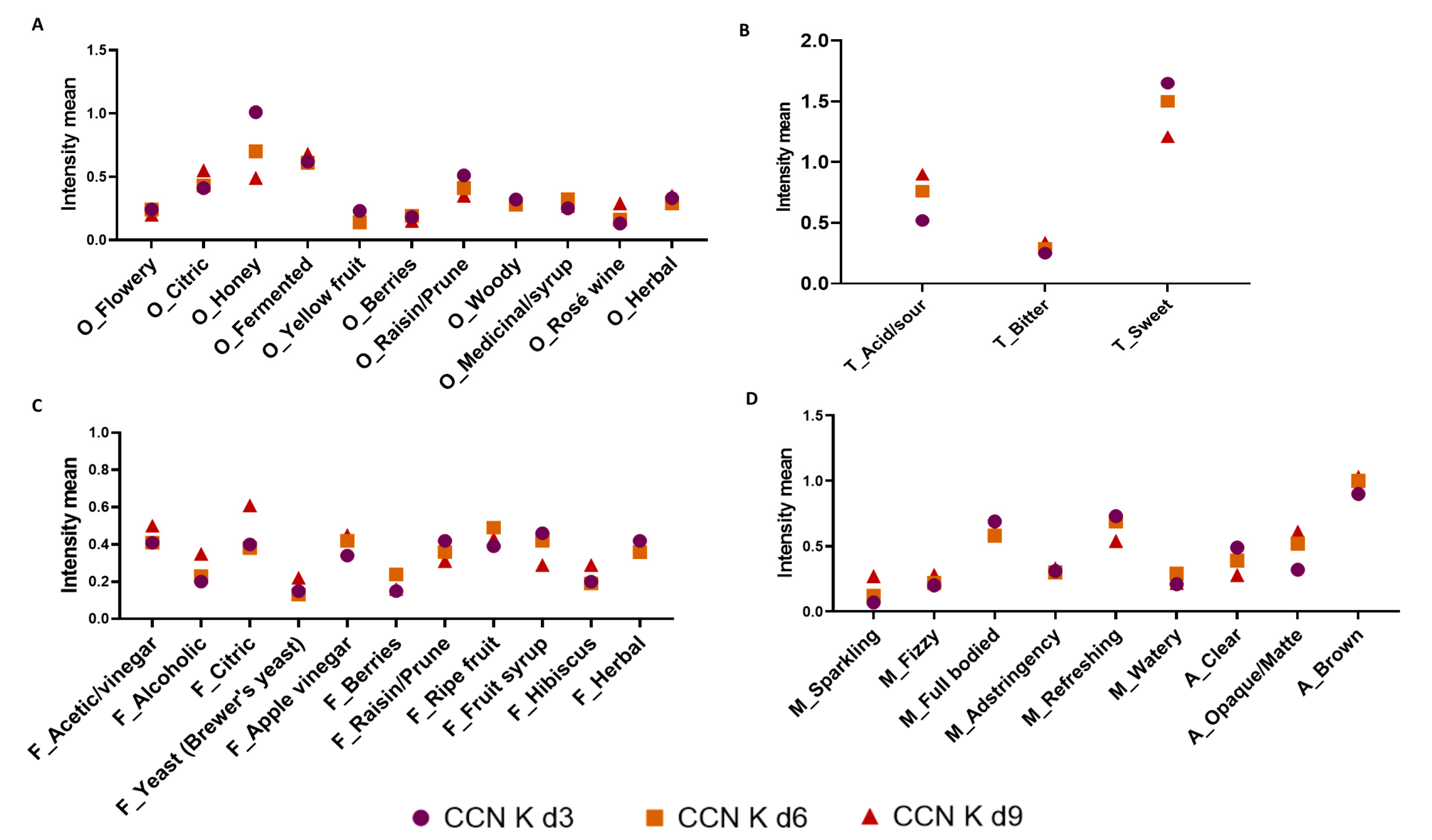
3.4.1. Rate All That Apply (RATA)
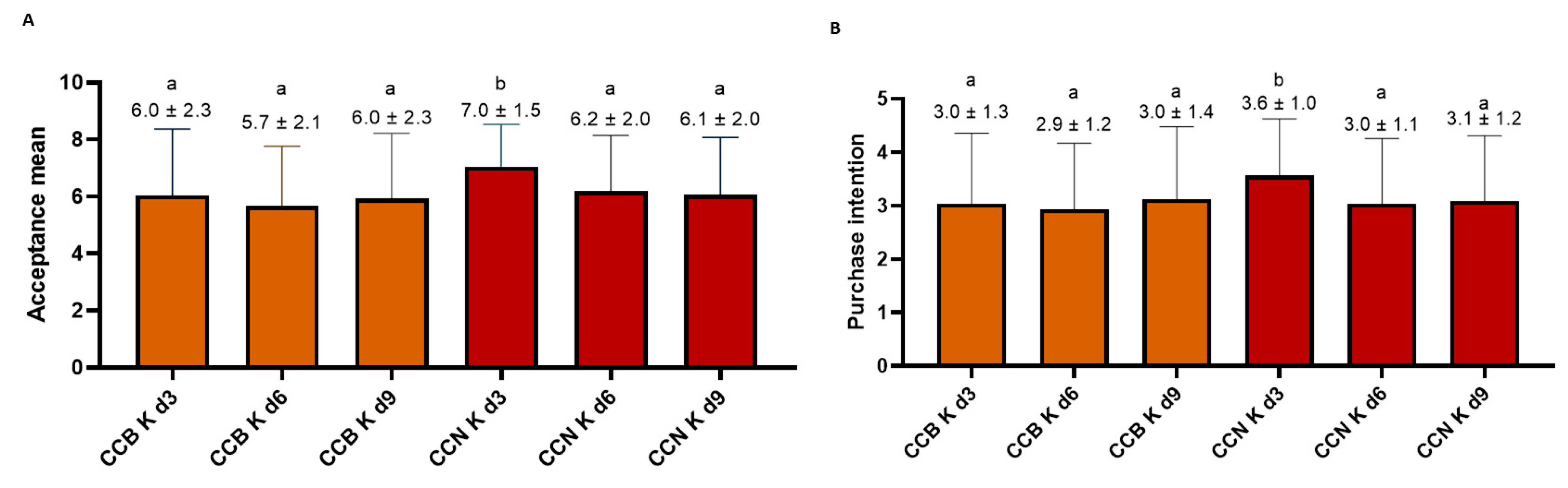
3.4.2. Consumer Acceptance and Purchase Intent Test Scores
4. Conclusions and Final Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coffee Production by Exporting Countries. Available online: https://www.ico.org/prices/po-production.pdf (accessed on 16 December 2022).
- DePaula, J.; Cunha, S.; Cruz, A.; Sales, A.L.; Revi, I.; Fernandes, J.; Ferreira, I.M.P.L.V.O.; Miguel, M.A.L.; Farah, A. Volatile fingerprinting and sensory profiles of coffee cascara teas produced in Latin American countries. Foods 2022, 11, 3144. [Google Scholar]
- Heuzé, V.; Tran, G. Coffee Hulls, Fruit Pulp and by-Products. Feedipedia, a Programme by INRAE, CIRAD. AFZ and FAO. 2015. Available online: https://www.feedipedia.org/node/549 (accessed on 25 May 2023).
- Esquivel, P.; Viñas, M.; Steingass, C.B.; Gruschwitz, M.; Guevara, E.; Carle, R.; Schweiggert, R.M.; Jiménez, V.M. Coffee (Coffea arabica L.) by-products as a source of carotenoids and phenolic compounds—Evaluation of varieties with different peel color. Front. Sustain. Food Syst. 2020, 4, 590597. [Google Scholar]
- DePaula, J.; Farah, A. Phenolic compounds in coffee and by-products. In Coffee Science—Biotechnological Advances, 1st ed.; Economics and Health Benefits, Ramakrishna, A., Parvatam, G., Jeszka-Skowron, M., Eds.; Taylor and Francis (CRC Press): Oxfordshire, UK, 2022; Volume 1, pp. 129–147. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens). Safety of Dried Coffee Husk (Cascara) from Coffea arabica L. as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, 7085. [Google Scholar]
- Rios, M.B.; Iriondo-DeHond, A.; Iriondo-DeHond, M.; Herrera, T.; Velasco, D.; Gómez-Alonso, S.; Callejo, M.J.; del Castillo, M.D. Effect of coffee cascara dietary fiber on the physicochemical, nutritional and sensory properties of a gluten-free bread formulation. Molecules 2020, 25, 1358. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, M.; Iriondo-DeHond, A.; Herrera, T.; Fernández-Fernández, A.M.; Sorzano, C.O.S.; Miguel, E.; del Castillo, M.D. Sensory acceptance, appetite control and gastrointestinal tolerance of yogurts containing coffee-cascara extract and inulin. Nutrients 2020, 12, 627. [Google Scholar] [CrossRef]
- Heeger, A.; Kosinska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, M.; Lebber, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea—Microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538. [Google Scholar] [CrossRef]
- Leal, J.M.; Suárez, L.V.; Jayabalan, R.; Oros, J.H.; Ecalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CYTA J. Food 2018, 16, 390. [Google Scholar]
- Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of kombucha obtained from green, oolong, and black teas on tnhibition of pathogenic bacteria, antioxidation and toxicity on colorectal cancer cell line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; D’Almeida, C.T.S.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; de Barros, F.A.R. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in kombucha tea extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Srihari, T.; Arunkumar, R.; Arunakaran, J.; Satyanarayana, U. Downregulation of signalling molecules involved in angiogenesis of prostate cancer cell line (PC-3) by kombucha (lyophilized). Biomed. Prev. Nutr. 2013, 3, 53. [Google Scholar] [CrossRef]
- Srihari, T.; Kathikesan, K.; Ashokkumar, N.; Satyanarayana, U. Antihyperglycaemic efficacy of kombucha in streptozotocin-induced rats. J. Funct. Foods 2013, 5, 1794–1802. [Google Scholar] [CrossRef]
- Jayabalan, R.; Subathradevi, P.; Marimuthu, S.; Sathishkumar, M.; Swaminathan, K. Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chem. 2008, 109, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Hepatoprotective properties of kombucha tea against TBHP-induced oxidative stress via suppression of mitochondria dependent apoptosis. Pathophysiology 2011, 18, 221. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.L.; Iriondo-DeHond, A.; DePaula, J.; Ribeiro, M.; Ferreira, I.M.P.L.V.O.; Miguel, M.A.L.; del Castillo, M.D.; Farah, A. Intracellular antioxidant and anti-inflammatory effects and bioactive profiles of coffee cascara and black tea kombucha beverages. Foods 2023, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Adhikari, K. Current trends in kombucha: Marketing perspectives and the need for improved sensory research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef]
- Grand View Research. Kombucha Market Size, Share & Trends Analysis Report by Product (Conventional, Hard), by Distribution Channel (On-Trade, Off-Trade), by Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/kombucha-market (accessed on 13 September 2022).
- Leonarski, E.; Guimarães, A.C.; Cesca, K.; Poletto, P. Production process and characteristics of kombucha fermented from alternative raw materials. Food Biosci. 2022, 49, 101841. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.-P.; Taillandier, P.; Beaufort, S. Metabolome-microbiome signatures in the fermented beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef] [PubMed]
- Nummer, B.A. Kombucha brewing under the Food and Drug Administration model food code: Risk analysis and processing guidance. J. Environ. Health 2013, 76, 8–11. [Google Scholar]
- Instituto Adolfo Lutz. Métodos Físico-Químicos para Análise de Alimentos, 4th ed.; Instituto Adolfo Lutz: São Paulo, Brazil, 2008. Available online: http://www.ial.sp.gov.br/ial/publicacoes/livros/metodos-fisico-quimicos-para-analise-de-alimentos (accessed on 5 May 2023).
- Wischral, D.; Arias, J.M.; Modesto, L.F.; Passos, D.F.; Pereira, N., Jr. Lactic acid production from sugarcane bagasse hydrolysates by Lactobacillus pentosus: Integrating xylose and glucose fermentation. Biotechnol. Prog. 2019, 35, e2718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, B.; Jing, W.; Yi, Z.; Zhang, Y.; Ren, D.; Yi, L. Effects of Different Steeping Temperatures on the Leaching of Aroma Components in Black Tea by SPME–GC–MS Coupled with Chemometric Method. J. AOAC Int. 2019, 102, 1834. [Google Scholar] [CrossRef]
- NIST V2.2 (National Institute of Standards and Technology, USA) Library Database. Available online: https://webbook.nist.gov/chemistry/name-ser/ (accessed on 25 May 2023).
- Galvan-Lima, A.; Cunha, S.C.; Martins, Z.E.; Soares, A.G.; Ferreira, I.M.P.L.V.O.; Farah, A. Headspace volatolome of peel flours from citrus fruits grown in Brazil. Food Res. Int. 2021, 150, 110801. [Google Scholar] [CrossRef]
- Yamanaka, L.E.; Abdala, P.; Christoff, A.P.; Extração de DNA: Avaliação da Metodologia Utilizada pela Neoprospecta. Neoprospecta Microbiome Technologies. Nota Técnica: Extração de DNA Neo. 2018. Available online: http://neoprospecta.com (accessed on 26 August 2022).
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Techniques. In Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 1, pp. 173–186. [Google Scholar]
- Damasio, M.H.; Costell, E. Análisis sensorial descriptivo: Generación de descriptores y selección de catadores. Rev. Agroquim. Tecnol. Aliment. 1991, 31, 165–177. [Google Scholar]
- Ares, G.; Giménez, A.; Barreiro, C.; Gámbaro, A. Use of an open-ended question to identify drivers of liking of milk desserts. Comparison with preference mapping techniques. Food Qual. Prefer. 2010, 21, 4286–4294. [Google Scholar] [CrossRef]
- Ares, G.; Bruzzone, F.; Vidal, L.; Cadena, R.S.; Giménez, A.; Pineau, B.; Hunter, D.C.; Paisley, A.G.; Jaeger, S.R. Evaluation of a rating-based variant of check-all-that-apply questions: Rate-all-that-apply (RATA). Food Qual. Prefer. 2014, 36, 87–95. [Google Scholar] [CrossRef]
- Meyners, M.; Jaeger, S.R.; Ares, G. On the analysis of Rate-All-That-Apply (RATA) data. Food Qual. Prefer. 2016, 49, 1–10. [Google Scholar] [CrossRef]
- De Noronha, M.C.; Cardoso, R.R.; D’Almeida, C.T.S.; do Carmo, M.A.V.; Azevedo, L.; Maltarollo, V.G.; Ribeiro Júnior, J.I.; Eller, M.R.; Cameron, L.C.; Ferreira, M.S.L.; et al. Black tea kombucha: Physicochemical, microbiological and comprehensive phenolic profile changes during fermentation, and antimalarial activity. Food Chem. 2022, 384, 132515. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- May, A.; Narayanan, S.; Alcock, J.; Varsani, A.; Maley, C.; Aktips, A. Kombucha: A novel model system for cooperation and conflict in a complex multi-species microbial ecosystem. PeerJ 2019, 7, e7565. [Google Scholar] [CrossRef]
- Ulusoy, A.; Tamer, C.E. Determination of suitability of black carrot (Daucus carota L. spp. sativus var. atrorubens Alef.) juice concentrate, cherry laurel (Prunus laurocerasus), blackthorn (Prunus spinosa) and red raspberry (Rubus ideaus) for kombucha beverage production. J. Food Meas. Charact. 2019, 13, 1524–1536. [Google Scholar] [CrossRef]
- Da Silva Júnior, J.C.; Magnani, M.; da Costa, W.K.A.; Madruga, M.S.; Olegário, L.S.; Borges, G.S.C.; Santas, A.M.; Lima, M.S.; de Lima, L.C.; Brito, I.L.; et al. Traditional and flavored kombuchas with pitanga and umbu-cajá pulps: Chemical properties, antioxidants, and bioactive compounds. Food Biosci. 2021, 44, 101380. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and microbiological impacts on the chemical and flavor profile. Food Chem. A 2022, 1, 100025. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Arikan, M.; Mitchell, A.L.; Finn, R.D.; Gürel, F. Microbial composition of Kombucha determined using amplicon sequencing and shotgun metagenomics. J. Food Sci. 2020, 82, 455–464. [Google Scholar] [CrossRef]
- Marsh, A.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Cannazza, P.; Rissanen, A.J.; Guizelini, D.; Losoi, P.; Sarlin, E.; Romano, D.; Santala, V.; Mangayil, R. Characterization of Komagataeibacter Isolate Reveals New Prospects in Waste Stream Valorization for Bacterial Cellulose Production. Microorganisms 2021, 9, 2230. [Google Scholar] [CrossRef] [PubMed]
- Landis, E.A.; Fogarty, E.; Edwards, J.C.; Popa, O.; Eren, A.M.; Wolfe, B.E. Microbial diversity and interaction specificity in kombucha tea fermentations. mSystems 2022, 7, e0015722. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Sci. Rep. 2018, 8, 8612. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Yang, H.; Sun, L.; Xia, H.; Sun, W.; Wang, Z.; Zhang, J. Bacterial Communities Related to Aroma Formation during Spontaneous Fermentation of ‘Cabernet Sauvignon’ Wine in Ningxia, China. Foods 2022, 11, 2775. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.D.; Uetanabaro, A.P.T.; Santos, W.C.R.; Caetano, R.G.; Albano, H.; Kato, R.; Consenza, G.P.; Azeredo, A.; Góes-Neto, A.; Rosa, C.A.; et al. Microbial–physicochemical integrated analysis of kombucha fermentation. LWT—Food Sci. Technol. 2021, 148, 111788. [Google Scholar] [CrossRef]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Couloumme-Labarthe, L.; Fall, A.; Daube, G.; Conto, E. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Karabiyikli, S. Importance of acetic acid bacteria in food industry. Food Control 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Gaggia, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha beverage from green, black and rooibos teas: A comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Nguyen, P.B.; Nguyen, H.Y.; Le, P.H. Screening the optimal ratio of symbiosis between isolated yeast and acetic acid bacteria strain from traditional kombucha for high-level production of glucuronic acid. LWT Food Sci. Technol. 2015, 64, 1149–1155. [Google Scholar] [CrossRef]
- Singh, N.; Anand, S. Enterobacteriaceae. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: London, UK, 2011; pp. 67–71. [Google Scholar]
- Walterson, A.M.; Stavrinides, J. Pantoea: Insights into a highly versatile and diverse genus within the Enterobacteriaceae. FEMS Microbiol. Rev. 2015, 39, 968–984. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.-R.; Park, S.-H.; Lee, M.-A. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. LWT Food Sci. Technol. 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Wang, Y.; Li, L.; Huang, J.; Yang, X.; Chen, S.; Zhao, Y. Contribution of microbial community to flavor formation in tilapia sausage during fermentation with Pediococcus pentosaceus. LWT Food Sci. Technol. 2022, 154, 112628. [Google Scholar] [CrossRef]
- Khusro, A.; Aarti, C. Metabolic heterogeneity and techno-functional attributes of fermentedfoods-associated coagulase-negative staphylococci. Food Microbiol. 2022, 105, 104028. [Google Scholar] [CrossRef]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 23, 9861–9874. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High Potential of Pichia kluyveri and Other Pichia Species in Wine Technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.L.; Heard, G.; Cox, J. Yeast ecology of Kombucha fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—Fromspoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef]
- Ferremi Leali, N.; Binati, R.L.; Martelli, F.; Gatto, V.; Luzzini, G.; Salini, A.; Slaghenaufi, D.; Fusco, S.; Ugliano, M.; Torriani, S.; et al. Reconstruction of Simplified Microbial Consortia to Modulate Sensory Quality of Kombucha Tea. Foods 2022, 11, 3045. [Google Scholar] [CrossRef]
- Coppeti, M.V. Yeasts and molds in fermented food production: An ancient bioprocess. Curr. Opin. Food Sci. 2019, 25, 57–61. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Vejarano, R. Saccharomycodes ludwigii, control and potential uses in winemaking process. Fermentation 2018, 4, 71. [Google Scholar] [CrossRef]
- Ellis, D.J.; Kerr, E.D.; Schenk, G.; Schulz, B.L. Metabolomics of Non-Saccharomyces Yeasts in Fermented Beverages. Beverages 2022, 8, 41. [Google Scholar] [CrossRef]
- Vaughan, M.J.; Mitchell, T.; McSpadden Gardener, B.B. What’s inside that seed we brew? A new approach to mining the coffee microbiome. Appl. Environ. Microbiol. 2015, 19, 6518–6527. [Google Scholar] [CrossRef]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Torres, J.; Falconi, C.; Moccand, C.; Weckx, S.; De Vuyst, L. Following coffee production from cherries to cup: Microbiological and metabolomic analysis of wet processing of Coffea arabica. Appl. Environ. Microbiol. 2019, 85, e02635-18. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Kania-Zelada, I.; del Barrio-Galán, R.; Medel-Marabolí, M.; Gil, M.; Peña-Neira, A. Study of the changes in volatile compounds, aroma and sensory attributes during the production process of sparkling wine by traditional method. Food Res. Int. 2019, 119, 554–563. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and technological parameters impacting the chemical composition and sensory quality of kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.-P.; Zhang, Y.; Sai, W.-D.; Guo, L.; Tan, J.-F.; Peng, Q.-H.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Flavornet and Human Odor Space. Available online: http://www.flavornet.org (accessed on 7 June 2023).
- The Good Scents Company Information System. Available online: https://www.thegoodscentscompany.com/ (accessed on 7 June 2023).
- Kliks, J.; Kawa-Rygielska, J.; Gasinski, A.; Rebas, J.; Szumny, A. Changes in the volatile composition of apple and apple/pear ciders affected by the different dilution rates in the continuous fermentation system. LWT 2021, 147, 111630. [Google Scholar] [CrossRef]
- Farah, A. Flavor Development during Roasting. In Drying and Roasting of Cocoa and Coffee; Hii, C.L., Borém, F.M., Eds.; Taylor and Francis (CRC Press): Oxfordshire, UK, 2019; Volume 1, p. 43. [Google Scholar]
- Nieto-Rojo, R.; Ancín-Azpilicueta, C.; Garrido, J.J. Sorption of 4-ethylguaiacol and 4-ethylphenol on yeast cell walls, using a synthetic wine. Food Chem. 2014, 152, 399–406. [Google Scholar] [CrossRef]
- Savary, O.; Mounier, J.; Thierry, A.; Poirier, E.; Jourdren, J.; Maillard, M.-B.; Penland, M.; Decamps, C.; Coton, E.; Coton, M. Tailor-made microbial consortium for Kombucha fermentation: Microbiota-induced biochemical changes and biofilm formation. Food Res. Int. 2021, 147, 110549. [Google Scholar] [CrossRef]
- Tran, T.; Billet, K.; Torres-Cobos, B.; Vichi, S.; Verdier, F.; Martin, A.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Use of a minimal microbial consortium to determine the origin of kombucha flavor. Front. Microbiol. 2022, 13, 836617. [Google Scholar] [CrossRef]
- Zhang, J.; Mullen, J.V.; Dias, D.R.; Schwan, R.F. The chemistry and sensory characteristics of new herbal tea-based kombuchas. J. Food Sci. 2021, 83, 740–748. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, J.; Lu, J.; Chen, D.; Song, S.; Wang, H.; Sun, M.; Feng, T. Revealing the influence of microbiota on the flavor of kombucha during natural fermentation process by metagenomic and GC-MS analysis. Food Res. Int. 2023, 169, 112909. [Google Scholar] [CrossRef]
- Pua, A.; Choo, W.X.D.; Goh, R.M.; Liu, S.Q.; Cornuz, M.; Ee, K.-H.; Sun, J.; Lassabliere, B.; Yu, B. A systematic study of key odourants, non-volatile compounds, and antioxidant capacity of cascara (dried Coffea arabica pulp). LWT 2021, 138, 110630. [Google Scholar] [CrossRef]
- Nunes, C.; Coimbra, M.A.; Saraiva, J.; Rocha, S.M. Study of the volatile components of a candied plum and estimation of their contribution to the aroma. Food Chem. 2008, 111, 897–905. [Google Scholar] [CrossRef]
- Javed, H.U.; Wang, D.; Wu, G.-F.; Kallem, Q.M.; Duan, C.-Q.; Shi, Y. Post-storage changes of volatile compounds in air- and sun-dried raisins with different packaging materials using HS-SPME with GC/MS. Food Res. Int. 2019, 119, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Wang, H.; Jiao, S.; Wu, J.; Hou, Y.; Sun, J.; Yuan, J. Chemical profile and antioxidant capacity of kombucha tea by the pure cultured kombucha. LWT Food Sci. Technol. 2022, 168, 113931. [Google Scholar] [CrossRef]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research progress of wine aroma components: A critical review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, P.; Steger, M.C.; Bellucci, A.Q.; Segatz, V.; Rieke-Zapp, J.; Sommerfeld, K.; Schwarz, S.; Einfalt, D.; Lachenmeier, D.W. Production of coffee cherry spirits from Coffea arabica varieties. Foods 2022, 11, 1672. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Zini, C.A. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, B.-Q.; Duan, C.Q.; Yan, G.-Y. Effects of different pre-fermentation cold maceration time on aroma compounds of Saccharomyces cerevisiae co-fermentation with Hansenia sporaopuntiae or Pichia kudriavzevii. LWT Food Sci. Technol. 2018, 92, 177–186. [Google Scholar] [CrossRef]
- Buck, N.; Wohlt, D.; Winter, A.R.; Ortner, E. Aroma-Active Compounds in Robusta Coffee Pulp Puree—Evaluation of Physicochemical and Sensory Properties. Molecules 2021, 26, 3925. [Google Scholar] [CrossRef] [PubMed]
- Einfalt, D.; Meissner, K.; Kurz, L.; Intani, K.; Müller, J. Fruit Spirit Production from Coffee Cherries—Process Analysis and Sensory Evaluation. Beverages 2020, 6, 57. [Google Scholar] [CrossRef]
- Andresen, M.; Kazantseva, J.; Kuldjärv, R.; Malv, E.; Vaikma, H.; Kaleda, A.; Kütt, M.-L.; Vilu, R. Characterization of chemical, microbial and sensory profiles of commercial kombuchas. Int. J. Food Microbiol. 2022, 373, 109715. [Google Scholar]
- Mao, Y.; Tian, S.; Qin, Y.; Chen, S. Sensory sweetness and sourness interactive response of sucrose-citric acid mixture based on synergy and antagonism. NPJ Sci. 2022, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Cardeal, Z.L.; Marriott, P.J. Comprehensive two-dimensional gas chromatography–mass spectrometry analysis and comparison of volatile organic compounds in Brazilian cachaça and selected spirits. Food Chem. 2009, 112, 747–755. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Lotti, C.; Mesuero, D.; Carlin, S.; Weingart, G.; Mattivi, F. Quantitative metabolic profiling of grape, apple and raspberryvolatile compounds (VOCs) using a GC/MS/MS method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 966, 132–139. [Google Scholar] [CrossRef]
- Rey-Serra, P.; Mnejja, M.; Monfort, A. Inheritance of esters and other volatile compounds responsiblefor the fruity aroma in strawberry. Front. Plant Sci. 2022, 13, 959155. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Grisenti, M.; Ajelli, M.; Betta, E.; Algarra, A.A.; Cappellin, L.; Aprea, E.; Gasperi, F.; Biasoli, F.; et al. Exploring Blueberry Aroma Complexity by Chromatographic and Direct-Injection Spectrometric Techniques. Front. Plant Sci. 2017, 8, 617. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Yue, Y.; Shi, T.; Liu, J.; Tian, Q.; Yang, X.; Wang, L. Genomic, metabonomic and transcriptomic analyses of sweet osmanthus varieties provide insights into floral aroma formation. Sci. Hortic. 2022, 306, 111442. [Google Scholar] [CrossRef]
- Yin, P.; Kong, Y.-S.; Liu, P.-P.; Wang, J.-J.; Zhu, Y.; Wang, G.-M.; Sun, M.-F.; Chen, Y.; Guo, G.-Y.; Liu, Z.-H. A critical review of key odorants in green tea: Identification and biochemical formation pathway. Trends Food Sci. Technol. 2022, 129, 221–232. [Google Scholar] [CrossRef]
- Zannou, O.; Kelebek, H.; Selli, S. Elucidation of key odorants in Beninese Roselle (Hibiscus sabdariffa L.) infusions prepared by hot and cold brewing. Food Res. Int. 2020, 133, 109133. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Moreira, R.F.A.; Trugo, L.C.; Pietroluongo, M.; De Maria, C.A.B. Flavor composition of cashew (Anacardium occidentale) and marmeleiro (Croton Species) honeys. J. Agric. Food Chem. 2002, 50, 7616–7621. [Google Scholar] [CrossRef]
- Milheiro, J.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. A simple, cheap and reliable method for control of 4-ethylphenol and 4-ethylguaiacol in red wines. Screening of fining agents for reducing volatile phenols levels in red wines. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1041–1042, 183–190. [Google Scholar]
- Ménager, I.; Jost, M.; Aubert, C. Changes in physicochemical characteristics and volatile constituents of strawberry (Cv. Cigaline) during maturation. J. Agric. Food Chem. 2004, 52, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, J.; Fillmore, S.; Pang, X.; Zhang, Z. Effect of high temperature on color, chlorophyll fluorescence and volatile biosynthesis in green-ripe banana fruit. Postharvest Biol. Technol. 2011, 62, 246–257. [Google Scholar] [CrossRef]
- Ma, T.; Sam, F.E.; Didi, S.A.; Atuna, R.A.; Amagloh, F.K.; Zhang, B. Contribution of edible flowers on the aroma profile of dealcoholized pinot noir rose wine. LWT 2022, 170, 114034. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, C.; Li, Z.; Ji, W.; Zhu, Y.; Zhang, S. Enhanced removal of VOCs from wood by coupling extraction with surfactant and, oxidation using ultrasound-activated persulfate. Ind. Crops Prod. 2022, 189, 115818. [Google Scholar] [CrossRef]
- Ghadiriasli, R.; Mahmoud, M.A.A.; Wagenstaller, M.; van de Kuilen, J.-W.; Buettner, A. Chemo-sensory characterization of aroma active compounds of native oak wood in relation to their geographical origins. Food Res. Int. 2021, 150, 110776. [Google Scholar] [CrossRef]
- Pinho, O.; Ferreira, I.M.; Santos, L.H. Method optimization by solid-phase microextraction in combination with gas chromatography with mass spectrometry for analysis of beer volatile fraction. J. Chromatogr. A 2006, 1121, 145–153. [Google Scholar] [CrossRef]
- Bicas, J.L.; Molina, C.; Dionísio, A.P.; Barros, F.F.C.; Wagner, R.; Maróstica, M.R., Jr.; Pastore, G.M. Volatile constituents of exotic fruits from Brazil. Food Res. Int. 2011, 44, 1843–1855. [Google Scholar] [CrossRef]
- Janzantti, N.S.; Monteiro, M. Changes in the aroma of organic passion fruit (Passiflora edulis Sims f. flavicarpa Deg.) during ripeness. LWT Food Sci. Technol. 2014, 59, 612–620. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Q.; Wu, C.; Li, T.; Tu, K. Characterization of soluble sugars, glycosidically bound and free volatiles in fresh-cut pineapple stored at different temperature. Food Biosci. 2021, 43, 101329. [Google Scholar] [CrossRef]
- European Food Safety Authority. Added and Free Sugars Should Be as Low as Possible. 2022. Available online: https://www.efsa.europa.eu/en/news/added-and-free-sugars-should-be-low-possible (accessed on 7 June 2023).
- United States Department of Health and Human Services. 2015–2020 Dietary Guidelines for Americans, 8th ed, December 2015, United States. Available online: https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 11 May 2023).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa de Orçamentos Familiares 2017–2018; Análise do Consumo Alimentar Pessoal no Brasil: Rio de Janeiro, Brazil, 2020. [Google Scholar]
- Ayed, L.; Abid, S.B.; Hamdi, M. Development of a beverage from red grape juice fermented with the Kombucha consortium. Ann. Microbiol. 2017, 67, 111–121. [Google Scholar] [CrossRef]
- Lima, J.P.; Farah, A. Methylxanthines in stimulant foods and beverages commonly consumed in Brazil. J. Food Compos. Anal. 2019, 78, 75–78. [Google Scholar] [CrossRef]

| Days of Fermentation | Titrable Acidity (mEq/L) | pH | Soluble Solids (°Brix) | Sucrose (g/100 mL) | |
|---|---|---|---|---|---|
| Black tea | 0 | 0.1 ± 0.00 a | 3.8 ± 0.07 a | 10.4 ± 0.07 a | 10.5 ± 0.75 a |
| 3 | 0.2 ± 0.05 a | 3.5 ± 0.00 b | 10.8 ± 0.14 a | 9.1 ± 0.75 b | |
| 6 | 0.3 ± 0.06 b | 3.5 ± 0.00 b | 9.7 ± 0.00 b | 8.2 ± 0.75 c | |
| 9 | 0.3 ± 0.06 b | 3.4 ± 0.00 b | 9.3 ± 0.28 b | 7.9 ± 0.75 d | |
| CCB K | 0 | 0.1 ± 0.00 a | 3.7 ± 0.07 a | 11.5 ± 0.63 a | 10.6 ± 0.26 a |
| 3 | 0.2 ± 0.00 a | 3.6 ± 0.07 a | 11.3 ± 0.00 a | 10.5 ± 0.25 a | |
| 6 | 0.2 ± 0.00 a | 3.6 ± 0.07 a | 10.4 ± 0.14 b | 9.4 ± 0.75 b | |
| 9 | 0.2 ± 0.00 a | 3.5 ± 0.00 a | 10.0 ± 0.42 b | 8.2 ± 0.75 c | |
| CCN K | 0 | 0.04 ± 0.00 a | 3.8 ± 0.21 a | 11.6 ± 0.14 a | 10.5 ± 0.75 a |
| 3 | 0.2 ± 0.00 b | 3.6 ± 0.07 a | 10.9 ± 0.14 a | 9.0 ± 0.75 b | |
| 6 | 0.2 ± 0.05 b | 3.5 ± 0.00 a | 10.6 ± 0.49 a | 8.5 ± 0.75 c | |
| 9 | 0.4 ± 0.08 b | 3.5 ± 0.00 a | 9.9 ± 0.56 b | 8.1 ± 0.75 d |
| Gender | Age | ||||
|---|---|---|---|---|---|
| Male | Female | 18–24 | 25–34 | 34–44 | 45–59 |
| 39% | 61% | 47% | 38% | 6% | 8% |
| Level of Education | |||||
| Basic education | Undergraduate | Incompletegraduation | Completegraduation | Master’s ordoctoral degree | |
| 0% | 12% | 40% | 10% | 38% | |
| Family Income (MW: Minimum Wages) | |||||
| 1 MW | 2–3 MW | 4–5 MW | >5 MW | ||
| 18% | 39% | 9% | 7% | ||
| Know Kombucha | Drink Kombucha | ||||
| Yes | No | Yes | No | ||
| 68% | 32% | 9% | 81% | ||
| Sparkling Beverage/Soft Drink Consumption | |||||
| Sparkling water | Apple juice | Soda | Tonic water | Sparkling wine | Cider |
| 50% | 10% | 73% | 32% | 42% | 15% |
| Aroma and Flavor Attributesfrom RATA Test | Respective Volatile Compounds Identified in the Literature and in the Present Study | References |
|---|---|---|
 Alcoholic Alcoholic | Isoamyl alcohol, ethyl acetate, ethyl hexanoate, ethyl decanoate, linalool oxide | [97] |
 Apple Apple | Dodecanal, heptanal benzaldehyde, decanal, decanoic acid, ethyl 2-methylbutyrate, ethyl butyrate, methyl salicylate | [98] |
 Berries Berries | Hexanal, decanal, benzaldehyde, octanoic acid, ethyl acetate, ethyl hexanoate, ethyl octanoate, linalool | [98,99,100] |
 Citric Citric | Nonanal, octanal, dodecanal, limonene α-terpineol, linalool | [30] |
 Fermented Fermented | Hexanal, benzaldehyde, nonanal, phenylacetaldehyde, ethanol, octanoic acid | [101] |
 Floral/flowery Floral/flowery | Phenylethyl alcohol, β-damascenone, linalool, linalool oxide, cis/trans-linalool oxide | [102] |
 Herbal Herbal | Hexanal, heptanal, nonanal, octanal, phenylacetaldehyde, phenylethyl alcohol, linalool | [103] |
 Hibiscus Hibiscus | Dodecanal, nonanal, 2-ethylhexanol, acetic acid, linalool | [104] |
 Honey Honey | Octanal, benzaldehyde, phenylacetaldehyde, phenylethyl alcohol, isovaleric acid, β-damascenone, cis/trans-linalool oxide, linalool, α-terpineol | [105,106] |
 Medicinal syrup Medicinal syrup | 4-ethylguiacol, 4-ethylphenol | [107] |
 Raisin/prune Raisin/prune | Nonanal, benzaldehyde, ethyl octanoate β-damascenone, γ-nonalactone, linalool | [85,86] |
 Ripe fruit Ripe fruit | Hexanal, hexanoic acid, octanoic acid, ethanol, ethyl acetate, isoamyl acetate, cis/trans linalool-oxide, linalool | [108,109] |
 Rosé wine Rosé wine | Nonanal, 1-dodecanol, 1-heptanol, octanoic acid, decanoic acid, ethyl hexanoate, ethyl octanoate, ethyl decanoate, β-damascenone, linalool | [110] |
 Sparkling wine Sparkling wine | Hexanoic acid, octanoic acid, ethyl isobutyrate, ethyl hexanoate, isoamyl acetate | [71] |
 Vinegar Vinegar | Acetic acid | [72] |
 Woody Woody | Hexanal, nonanal, cedrol, acetic acid, decanoic acid | [111,112] |
 Brewer’s yeast Brewer’s yeast | Nonanal, ethanol, octanoic acid, isoamyl alcohol, ethyl decanoate, ethyl hexanoate | [113] |
 Yellow fruit Yellow fruit | Benzaldehyde, heptanal, nonanal, octanal, ethyl butyrate, ethyl octanoate, ethyl acetate, β-damascenone, limonene, α-terpineol | [114,115,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, A.L.; Cunha, S.C.; Morgado, J.; Cruz, A.; Santos, T.F.; Ferreira, I.M.P.L.V.O.; Fernandes, J.O.; Miguel, M.A.L.; Farah, A. Volatile, Microbial, and Sensory Profiles and Consumer Acceptance of Coffee Cascara Kombuchas. Foods 2023, 12, 2710. https://doi.org/10.3390/foods12142710
Sales AL, Cunha SC, Morgado J, Cruz A, Santos TF, Ferreira IMPLVO, Fernandes JO, Miguel MAL, Farah A. Volatile, Microbial, and Sensory Profiles and Consumer Acceptance of Coffee Cascara Kombuchas. Foods. 2023; 12(14):2710. https://doi.org/10.3390/foods12142710
Chicago/Turabian StyleSales, Amanda Luísa, Sara C. Cunha, Jéssika Morgado, Adriano Cruz, Thiago F. Santos, Isabel M.P.L.V.O. Ferreira, José O. Fernandes, Marco Antonio L. Miguel, and Adriana Farah. 2023. "Volatile, Microbial, and Sensory Profiles and Consumer Acceptance of Coffee Cascara Kombuchas" Foods 12, no. 14: 2710. https://doi.org/10.3390/foods12142710
APA StyleSales, A. L., Cunha, S. C., Morgado, J., Cruz, A., Santos, T. F., Ferreira, I. M. P. L. V. O., Fernandes, J. O., Miguel, M. A. L., & Farah, A. (2023). Volatile, Microbial, and Sensory Profiles and Consumer Acceptance of Coffee Cascara Kombuchas. Foods, 12(14), 2710. https://doi.org/10.3390/foods12142710