Comparison of Drying Techniques for Extraction of Bioactive Compounds from Olive-Tree Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents and Standards
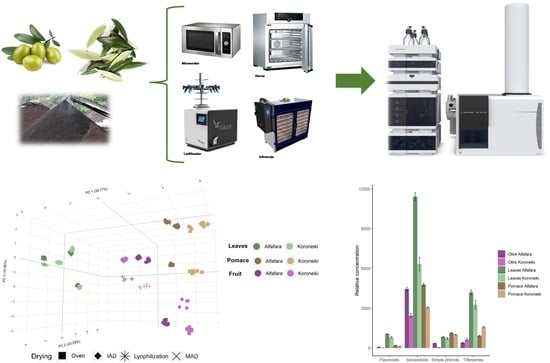
2.3. Drying of Samples
2.4. Metabolites Extraction
2.5. LC–QTOF MS/MS Analysis
2.6. Data Processing and Statistical Analysis
3. Results and Discussion
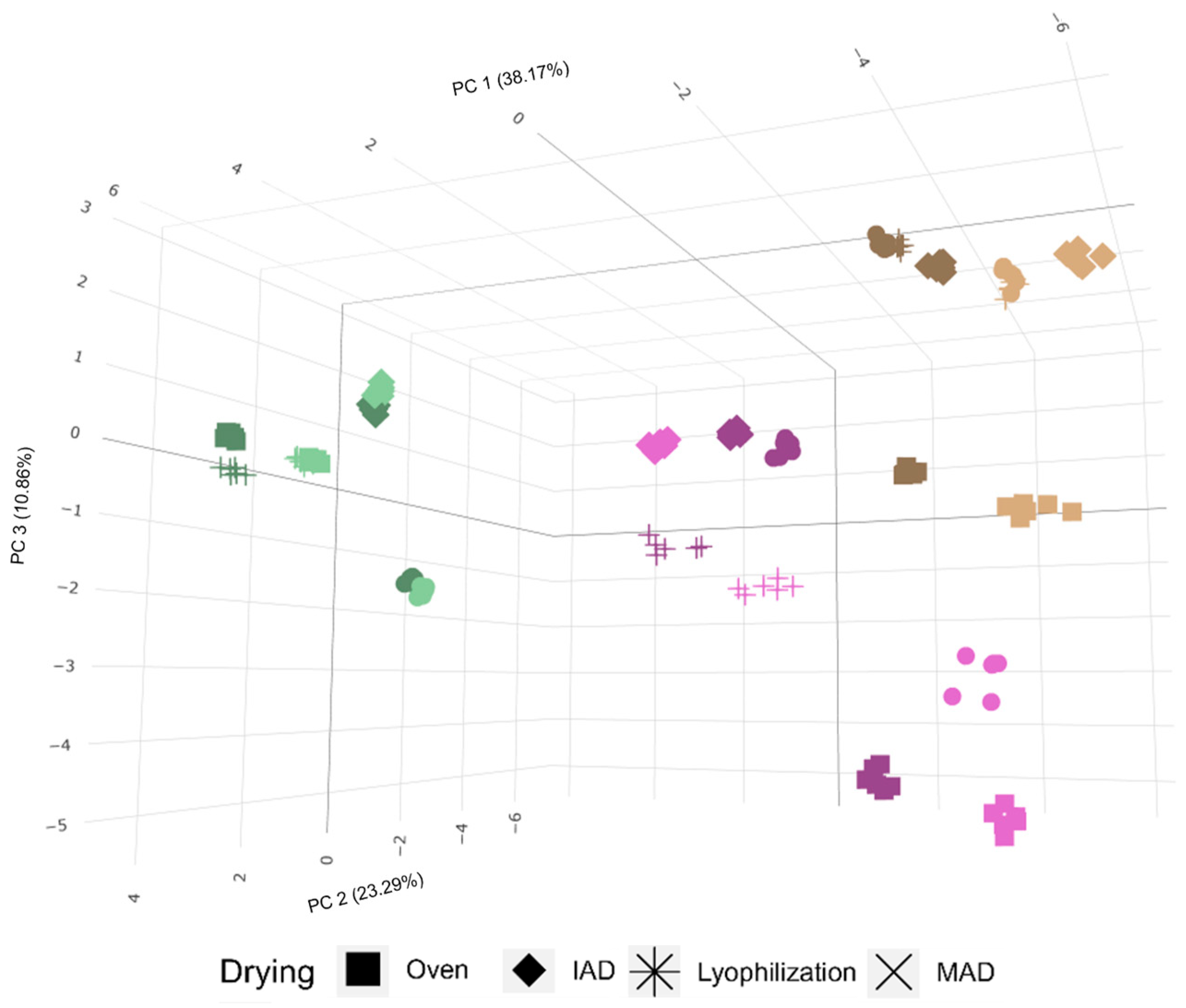
3.1. Characterization of Bioactive Extracts from Selected Raw Materials
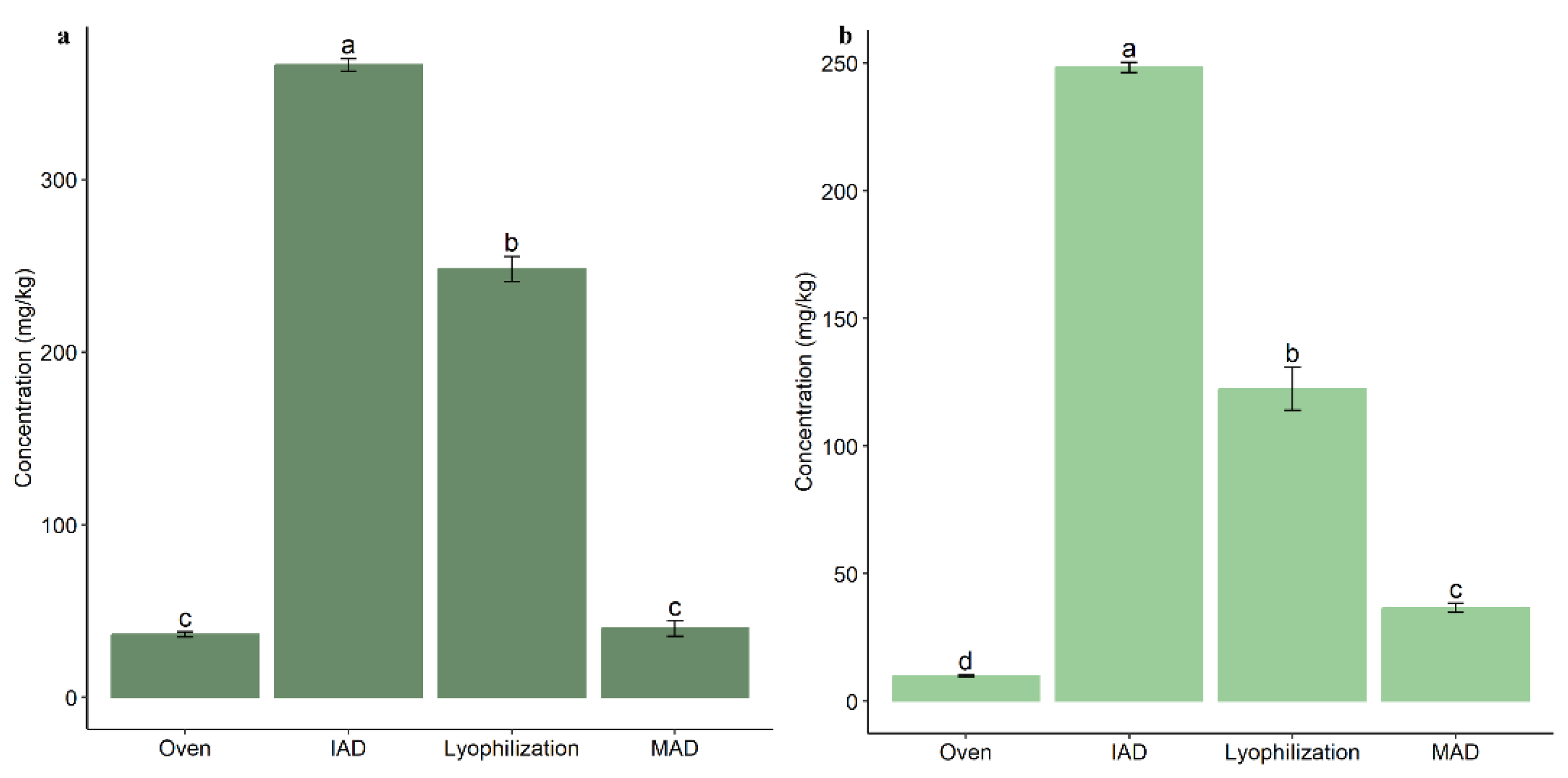
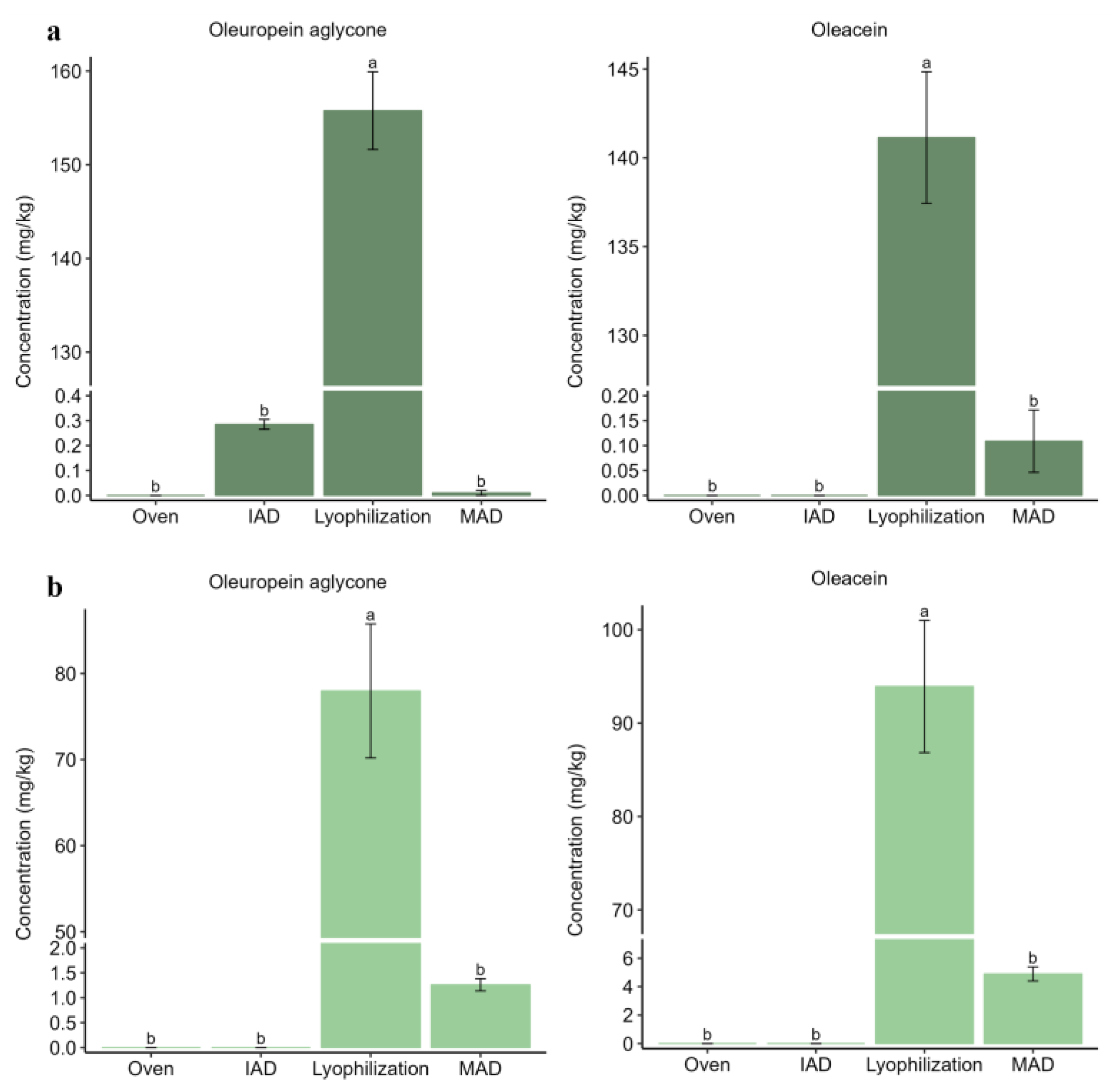
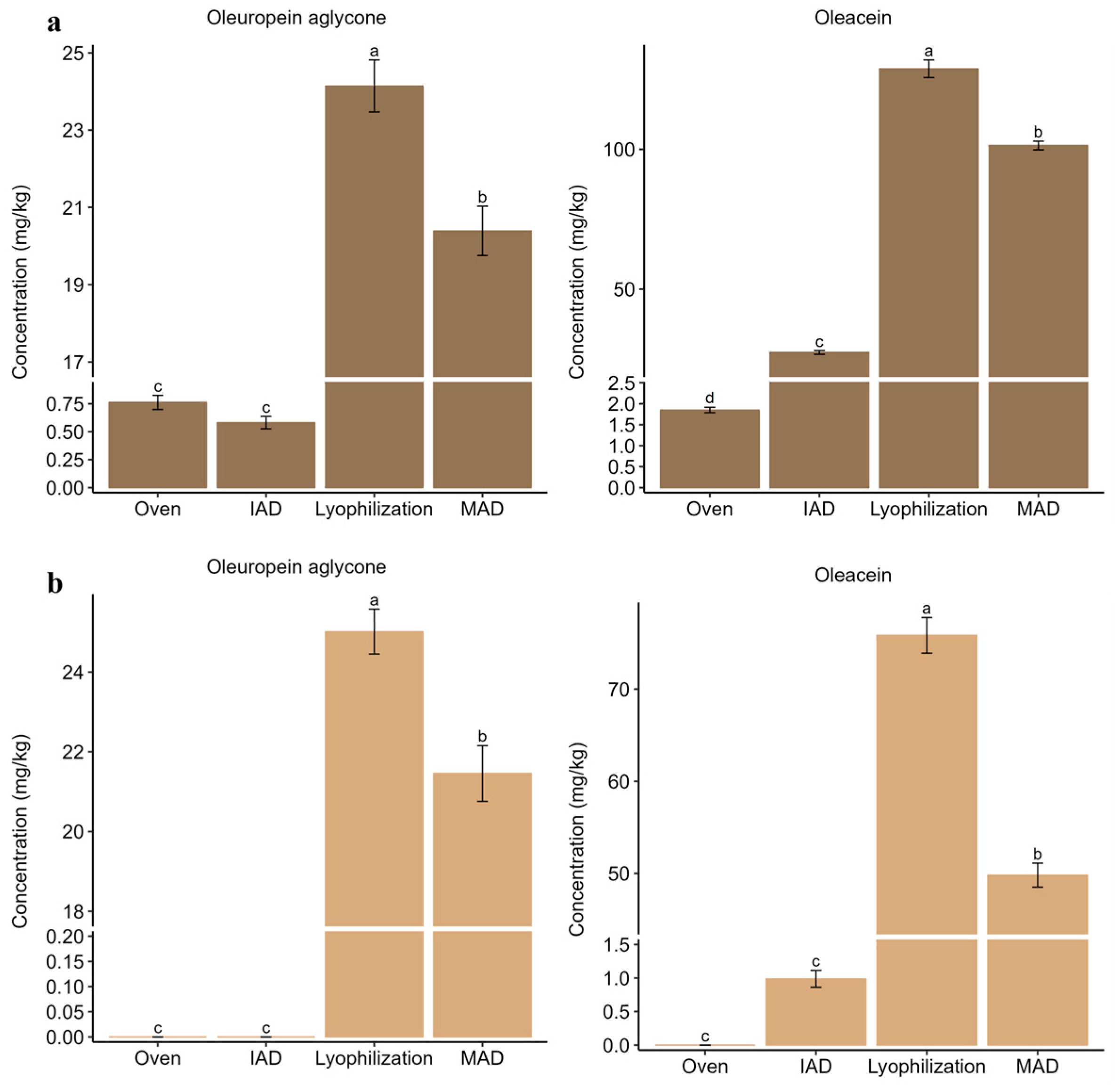
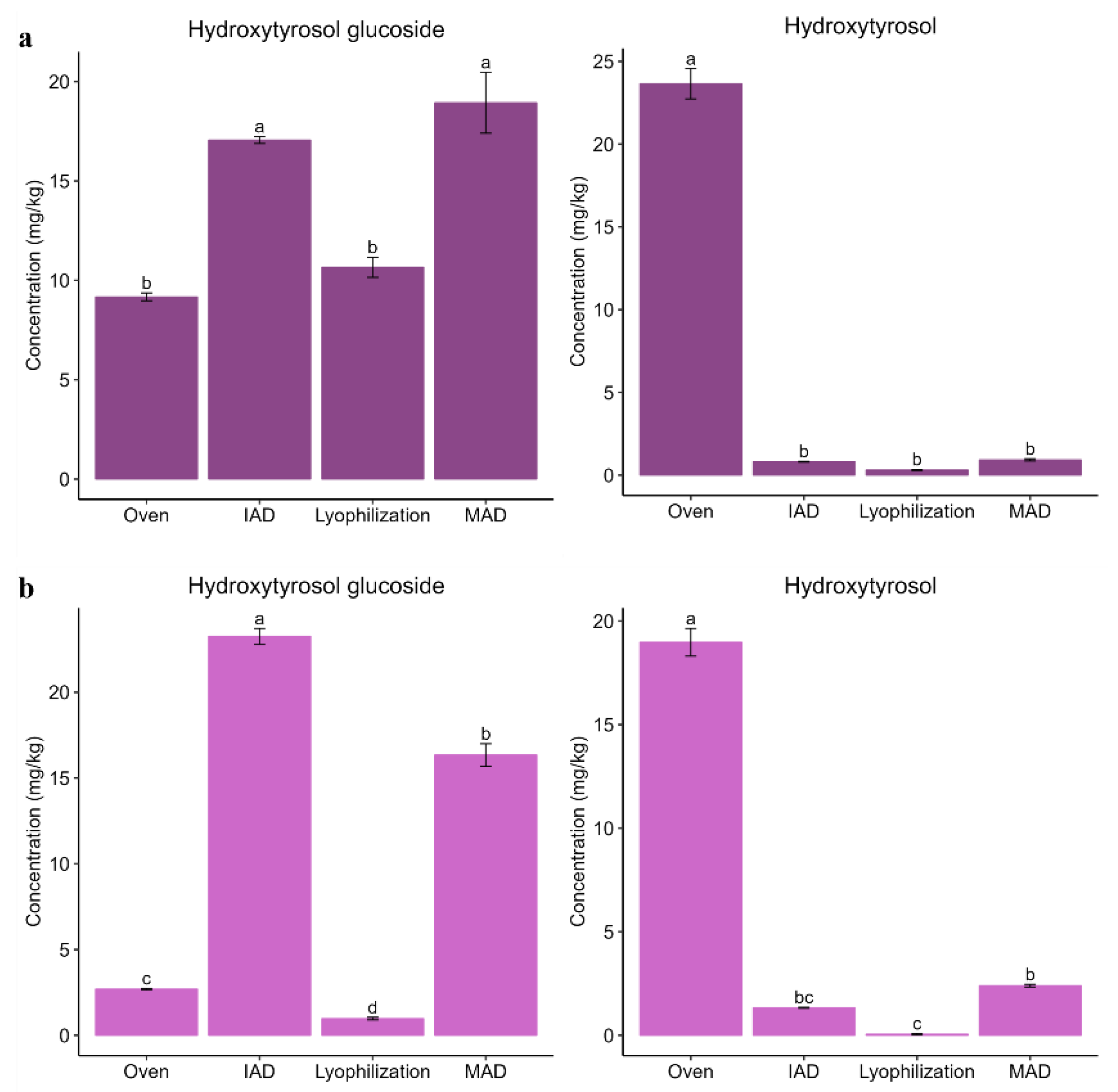
3.2. Influence of Drying on the Extraction of Bioactive Compounds from Olive Leaves
3.3. Influence of Drying on the Extraction of Bioactive Compounds from Olive Pomace
3.4. Influence of Drying on the Extraction of Bioactive Compounds from Olive Fruits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castillo-Luna, A.; Criado-Navarro, I.; Ledesma-Escobar, C.A.; López-Bascón, M.A.; Priego-Capote, F. The Decrease in the Health Benefits of Extra Virgin Olive Oil during Storage Is Conditioned by the Initial Phenolic Profile. Food Chem. 2021, 336, 127730. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Mainte. EFSA J. 2011, 9, 1–25. [Google Scholar] [CrossRef]
- del Mar Contreras, M.; Romero, I.; Moya, M.; Castro, E. Olive-Derived Biomass as a Renewable Source of Value-Added Products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Ryan, D.; Antolovich, M.; Prenzler, P.; Robards, K.; Lavee, S. Biotransformations of Phenolic Compounds in Olea europaea L. Sci. Hortic. 2002, 92, 147–176. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Manzanares, P.; Ruiz, E.; Ballesteros, M.; Negro, M.J.; Gallego, F.J.; López-Linares, J.C.; Castro, E. Residual Biomass Potential in Olive Tree Cultivation and Olive Oil Industry in Spain: Valorization Proposal in a Biorefinery Context. Span. J. Agric. Res. 2017, 15, e0206. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; de Castro, M.D.L. Coverage Exploitation of By-Products from the Agrofood Industry. Green Extr. Nat. Prod. Theory Pract. 2014, 265–306. [Google Scholar] [CrossRef]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive Tree (Olea europeae L.) Leaves: Importance and Advances in the Analysis of Phenolic Compounds. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Functional Oligosaccharides: Production, Properties and Applications. World J. Microbiol. Biotechnol. 2011, 27, 1119–1128. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive Profile, Dehydration, Extraction and Application of the Bioactive Components of Olive Leaves; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 42, ISBN 9190864174. [Google Scholar]
- Malik, N.S.A.; Bradford, J.M. Recovery and Stability of Oleuropein and Other Phenolic Compounds during Extraction and Processing of Olive (Olea europaea L.) Leaves. J. Food Agric. Environ. 2008, 6, 8–13. [Google Scholar]
- Erbay, Z.; Icier, F. Optimization of Drying of Olive Leaves in a Pilot-Scale Heat Pump Dryer. Dry. Technol. 2009, 27, 416–427. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. Thin-Layer Drying Behaviors of Olive Leaves (Olea europaea L.). J. Food Process Eng. 2010, 33, 287–308. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of Freezing and Dehydration of Olive Leaves (Var. Serrana) on Extract Composition and Antioxidant Potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Bahloul, N.; Ben Slimen, I.; Kechaou, N. Comparison on the Total Phenol Contents and the Color of Fresh and Infrared Dried Olive Leaves. Ind. Crops Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Bahloul, N.; Boudhrioua, N.; Kouhila, M.; Kechaou, N. Convective Solar Drying of Olive Leaves. J. Food Process Eng. 2011, 34, 1338–1362. [Google Scholar] [CrossRef]
- Garcia-Perez, J.V.; García-Alvarado, M.A.; Carcel, J.A.; Mulet, A. Extraction Kinetics Modeling of Antioxidants from Grape Stalk (Vitis vinifera Var. Bobal): Influence of Drying Conditions. J. Food Eng. 2010, 101, 49–58. [Google Scholar] [CrossRef]
- Souilem, S.; Fki, I.; Kobayashi, I.; Khalid, N.; Neves, M.A.; Isoda, H.; Sayadi, S.; Nakajima, M. Emerging Technologies for Recovery of Value-Added Components from Olive Leaves and Their Applications in Food/Feed Industries. Food Bioprocess Technol. 2017, 10, 229–248. [Google Scholar] [CrossRef]
- Ghanem, N.; Mihoubi, D.; Kechaou, N.; Mihoubi, N.B. Microwave Dehydration of Three Citrus Peel Cultivars: Effect on Water and Oil Retention Capacities, Color, Shrinkage and Total Phenols Content. Ind. Crops Prod. 2012, 40, 167–177. [Google Scholar] [CrossRef]
- Miho, H.; Moral, J.; Barranco, D.; Ledesma-Escobar, C.A.; Priego-Capote, F.; Díez, C.M. Influence of Genetic and Interannual Factors on the Phenolic Profiles of Virgin Olive Oils. Food Chem. 2021, 342, 128357. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Laboratory-Scale Optimization of Olive Oil Extraction: Simultaneous Addition of Enzymes and Microtalc Improves the Yield. Eur. J. Lipid Sci. Technol. 2014, 116, 1054–1062. [Google Scholar] [CrossRef]
- Trujillo, I.; Ojeda, M.A.; Urdiroz, N.M.; Potter, D.; Barranco, D.; Rallo, L.; Diez, C.M. Identification of the Worldwide Olive Germplasm Bank of Córdoba (Spain) Using SSR and Morphological Markers. Tree Genet. Genomes 2014, 10, 141–155. [Google Scholar] [CrossRef]
- AOAC. Official Methods for Analysis of the AOAC, 15th ed.; AOAC: Arlington, VA, USA, 1990; p. 771. [Google Scholar]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.A. MixOmics: An R Package for ‘omics Feature Selection and Multiple Data Integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Medina, E.; Mateo, M.A.; Brenes, M. Quantification of Bioactive Compounds in Picual and Arbequina Olive Leaves and Fruit. J. Sci. Food Agric. 2017, 97, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; García, A.; Medina, E.; Ruíz-Méndez, M.V.; de Castro, A.; Brenes, M. Triterpenic Acids in Table Olives. Food Chem. 2010, 118, 670–674. [Google Scholar] [CrossRef]
- García, A.; Brenes, M.; Dobarganes, M.C.; Romero, C.; Ruiz-Méndez, M.V. Enrichment of Pomace Olive Oil in Triterpenic Acids during Storage of “Alpeorujo” Olive Paste. Eur. J. Lipid Sci. Technol. 2008, 110, 1136–1141. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Innocenti, M.; Mulinacci, N. Whole Lyophilized Olives as Sources of Unexpectedly High Amounts of Secoiridoids: The Case of Three Tuscan Cultivars. J. Agric. Food Chem. 2015, 63, 1175–1185. [Google Scholar] [CrossRef]
- García-Vico, L.; García-Rodríguez, R.; Sanz, C.; Pérez, A.G. Biochemical Aspects of Olive Freezing-Damage: Impact on the Phenolic and Volatile Profiles of Virgin Olive Oil. LWT 2017, 86, 240–246. [Google Scholar] [CrossRef]
- Jiang, B.; Lyles, J.T.; Reynertson, K.A.; Kronenberg, F.; Kennelly, E.J. Stability Evaluation of Selected Polyphenols and Triterpene Glycosides in Black Cohosh. J. Agric. Food Chem. 2008, 56, 9510–9519. [Google Scholar] [CrossRef]
- Pu, H.; Li, Z.; Hui, J.; Raghavan, G.S.V. Effect of Relative Humidity on Microwave Drying of Carrot. J. Food Eng. 2016, 190, 167–175. [Google Scholar] [CrossRef]

| Leaves | Pomace | Fruit | |
|---|---|---|---|
| Lyophilization | 24 h | 48 h | 48 h |
| Under vacuum | Under vacuum | Under vacuum | |
| (1.70%) | (1.60%) | (2.00%) | |
| Oven-drying | 24 h | 48 h | 48 h |
| 45 °C | 45 °C | 45 °C | |
| (3.30%) | (3.80%) | (3.80%) | |
| MAD | 40 min | 50 min | 50–80 min |
| 90 W | 90 W | 90 W | |
| (2.10%) | (2.50%) | (3.20%) | |
| IAD | 2 h | 12 h | 24 h |
| 60 °C | 60 °C | 60 °C | |
| (2.00%) | (2.50%) | (3.30%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Luna, A.; Miho, H.; Ledesma-Escobar, C.A.; Priego-Capote, F. Comparison of Drying Techniques for Extraction of Bioactive Compounds from Olive-Tree Materials. Foods 2023, 12, 2684. https://doi.org/10.3390/foods12142684
Castillo-Luna A, Miho H, Ledesma-Escobar CA, Priego-Capote F. Comparison of Drying Techniques for Extraction of Bioactive Compounds from Olive-Tree Materials. Foods. 2023; 12(14):2684. https://doi.org/10.3390/foods12142684
Chicago/Turabian StyleCastillo-Luna, Ana, Hristofor Miho, Carlos A. Ledesma-Escobar, and Feliciano Priego-Capote. 2023. "Comparison of Drying Techniques for Extraction of Bioactive Compounds from Olive-Tree Materials" Foods 12, no. 14: 2684. https://doi.org/10.3390/foods12142684
APA StyleCastillo-Luna, A., Miho, H., Ledesma-Escobar, C. A., & Priego-Capote, F. (2023). Comparison of Drying Techniques for Extraction of Bioactive Compounds from Olive-Tree Materials. Foods, 12(14), 2684. https://doi.org/10.3390/foods12142684