Microbiota Composition during Fermentation of Broomcorn Millet Huangjiu and Their Effects on Flavor Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Collection
2.3. Physiochemical Characteristics and Volatile Compounds during Brewing
2.4. DNA Extraction and High-Throughput Sequencing
2.5. Operational Taxonomic Units (OTU) Cluster, Species Annotation, and Phylogenetic Analysis
2.6. Correlations between Microorganisms and Flavor Compounds
2.7. Preparation of Wheat Qu and Fortified Qu for Huangjiu
2.8. Huangjiu Brewing and Measured Indices
2.9. Statistical Analysis
3. Results
3.1. Changes of the Acid, Reducing Sugar, and Alcohol during Broomcorn Millet Huangjiu Primary Fermentation
3.2. Changes in Volatile Flavor Compounds during Broomcorn Millet Huangjiu’s Primary Fermentation
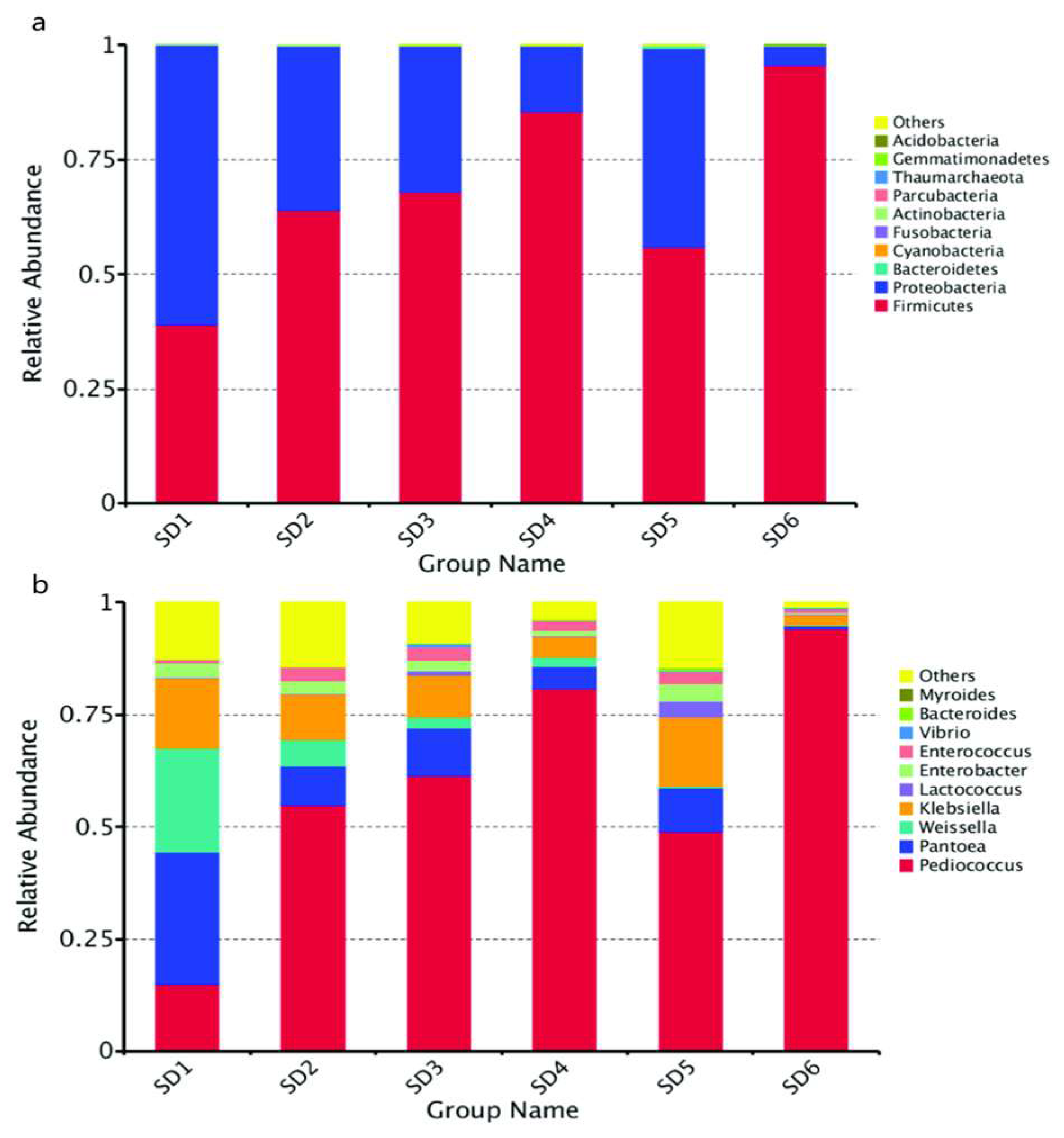
3.3. Bacterial Diversity of Broomcorn Millet Huangjiu during Primary Fermentation
3.3.1. Analysis of Bacterial Community Dynamics
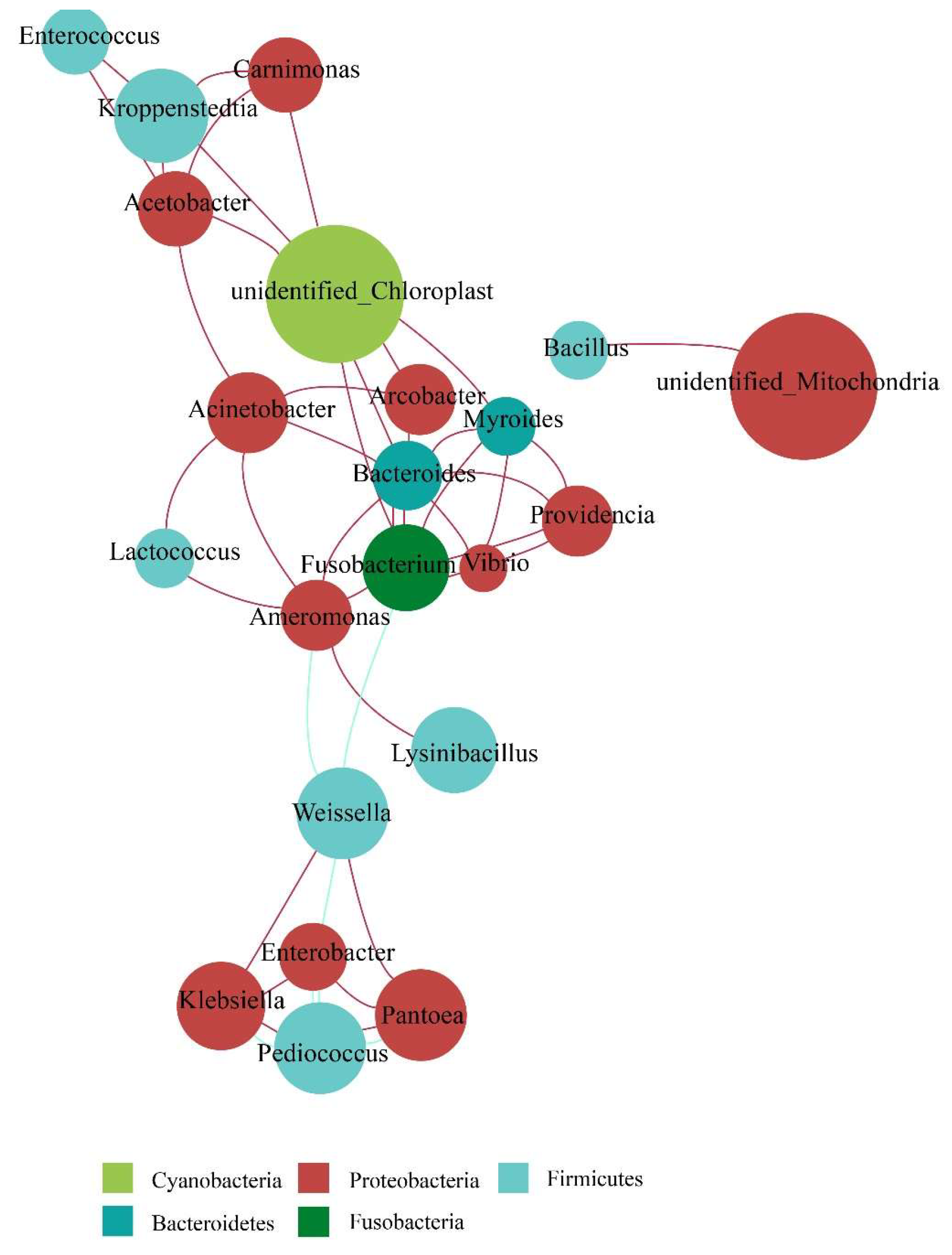
3.3.2. Bacterial Community Network Analysis during Fermentation
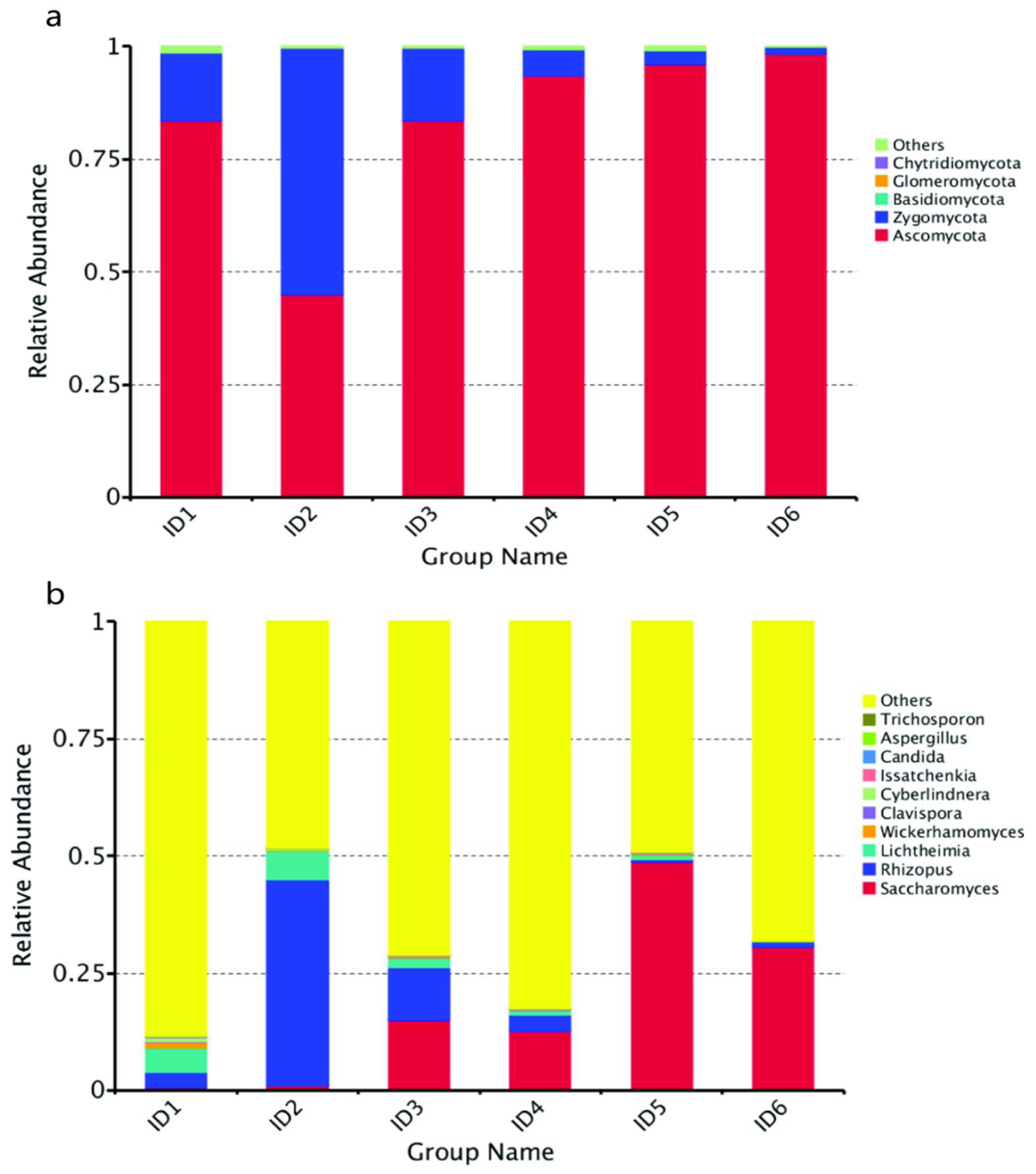
3.4. Fungal Diversity of Broomcorn Millet Huangjiu during Primary Fermentation
3.4.1. Analysis of Fungal Community Dynamics
3.4.2. Fungal Community Network Analysis during Fermentation
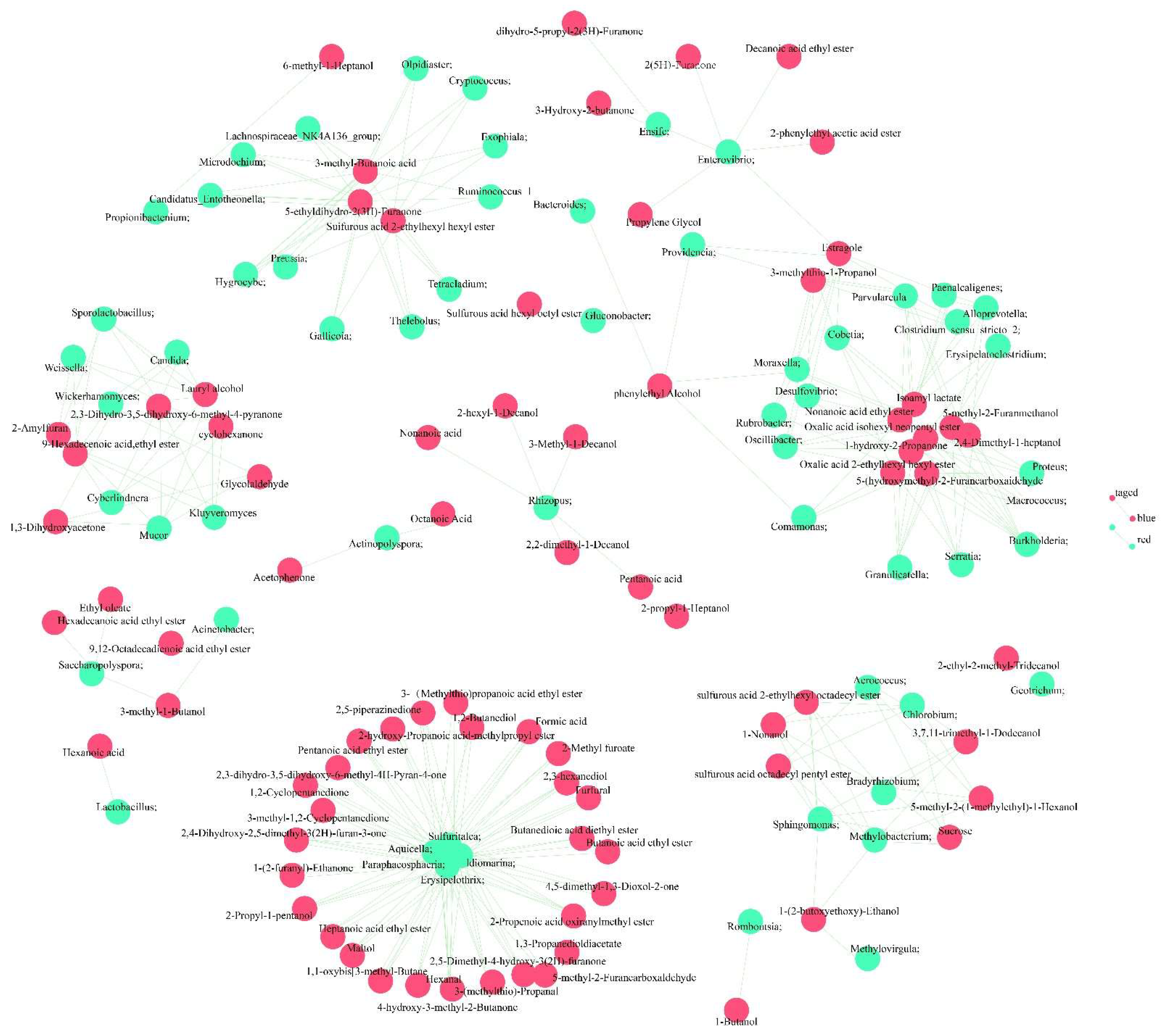
3.5. Correlation between Microorganisms and Volatile Flavor Compounds during Primary Fermentation
3.6. Effects of the Quality of Broomcorn Millet Huangjiu with Fortified Qu Fermentation
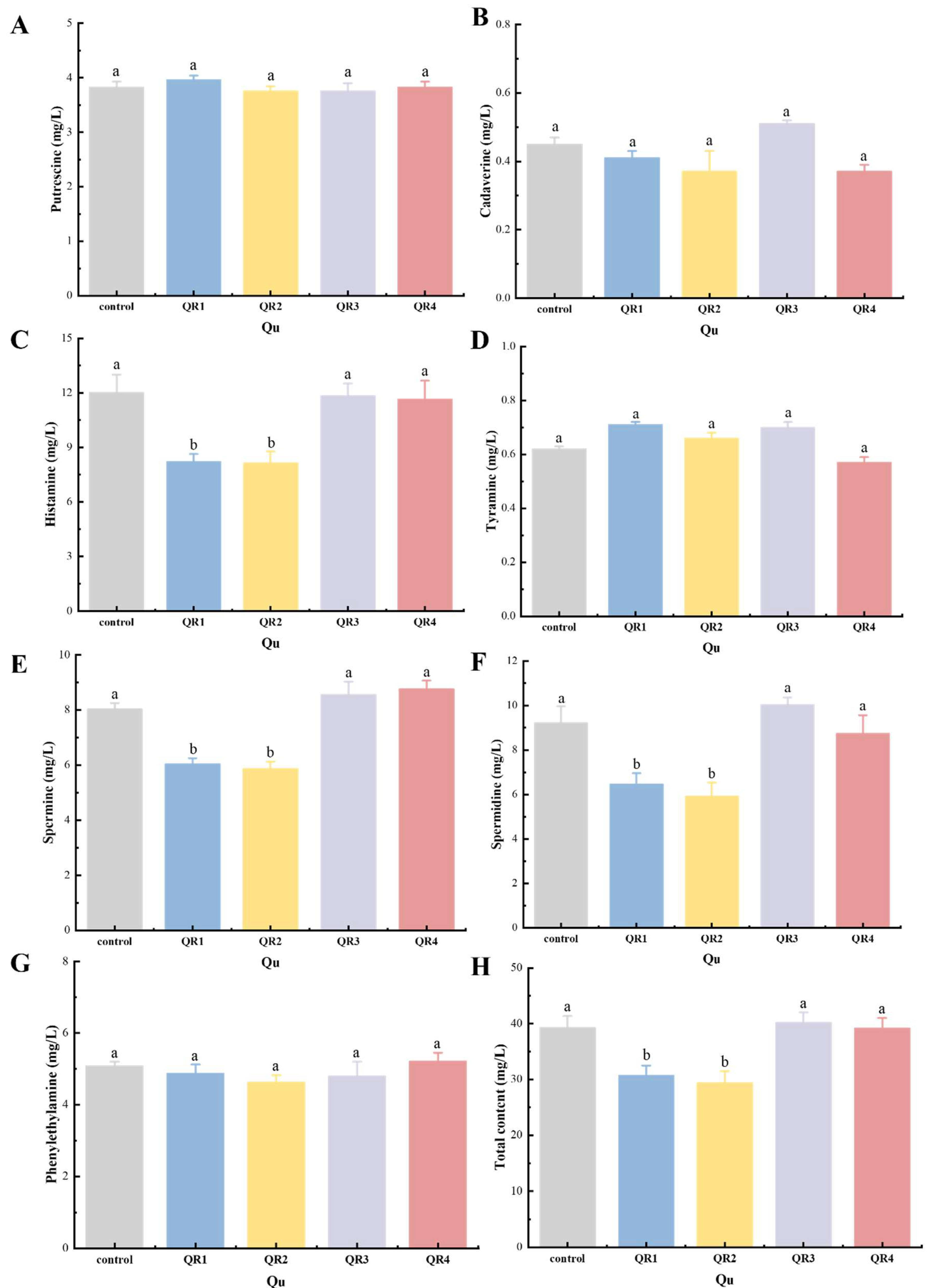
3.6.1. Effects of Biogenic Amine Content in Huangjiu
3.6.2. Analysis of Volatile Flavor Compounds in Huangjiu
3.6.3. Analysis of Physicochemical Indexes in Huangjiu
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Hu, W.; Xia, Y.; Mu, Z.; Tao, L.; Song, X.; Zhang, H.; Ni, B.; Ai, L. Flavor Formation in Chinese Rice Wine (Huangjiu): Impacts of the Flavor-Active Microorganisms, Raw Materials, and Fermentation Technology. Front. Microbiol. 2020, 11, 580247. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.M.; Huang, Z.R.; Wu, L.; Wu, Q.; Guo, W.L.; Zhao, W.H.; Liu, B.; Zhang, W.; Rao, P.F.; Lv, X.C.; et al. Microbial diversity and flavor of Chinese rice wine (Huangjiu): An overview of current research and future prospects. Curr. Opin. Food Sci. 2021, 42, 37–50. [Google Scholar] [CrossRef]
- Ye, Y.T.; Wang, L.X.; Zhan, P.; Tian, H.L.; Liu, J.S.; Liu, J.S. Characterization of the aroma compounds of Millet Huangjiu at different fermentation stages. Food Chem. 2022, 366, 130691. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.L.; Ji, Z.W.; Liu, S.P.; Han, X.; Zheng, F.P.; Mao, J. Characterization of the volatile compounds of huangjiu using comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry (GC × GC-TOFMS). J. Food Process. Preserv. 2019, 43, e14159. [Google Scholar] [CrossRef]
- Liu, S.P.; Mao, J.; Liu, Y.Y.; Meng, X.Y.; Ji, Z.W.; Zhou, Z.L.; Ai-lati, A. Bacterial succession and the dynamics of volatile compounds during the fermentation of Chinese rice wine from Shaoxing region. World J. Microbiol. Biotechnol. 2015, 31, 1907–1921. [Google Scholar] [CrossRef]
- Yang, Y.J.; Xia, Y.J.; Hu, W.Y.; Tao, L.R.; Liu, H.D.; Xie, C.L.; Bai, W.D.; Ai, L.Z. Soaking induced discrepancies in oenological properties, flavor profiles, microbial community and sensory characteristic of Huangjiu (Chinese rice wine). LWT 2021, 139, 110575. [Google Scholar] [CrossRef]
- De, F.; Genovese, A.; Ferranti, P.; Gilbert, J.; Ercolini, D. Metatranscriptomics reveals temperature-driven functional changes in microbiome impacting cheese maturation rate. Sci. Rep. 2016, 6, 2045–2322. [Google Scholar] [CrossRef]
- Peng, Q.; Zheng, H.J.; Meng, K.; Yu, H.F.; Xie, G.F.; Zhang, Y.H.; Yang, X.Y.; Chen, J.L.; Xu, Z.Q.; Lin, Z.C.; et al. Quantitative study on core bacteria producing flavor substances in Huangjiu (Chinese yellow rice wine). LWT 2022, 168, 113900. [Google Scholar] [CrossRef]
- Wang, P.X.; Mao, J.; Meng, X.Y.; Li, X.Z.; Liu, Y.Y.; Feng, H. Changes in flavour characteristics and bacterial diversity during the traditional fermentation of Chinese rice wines from Shaoxing region. Food Control 2014, 44, 58–63. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.Y.; Sun, L.P.; Zhang, W.; Lu, X.; Li, Z.P.; Xu, J.L.; Ren, Q. The changes of microbial diversity and flavor compounds during the fermentation of millet Huangjiu, a traditional Chinese beverage. PLoS ONE 2022, 12, e0262353. [Google Scholar] [CrossRef]
- Huang, Z.R.; Hong, J.L.; Xu, J.X.; Li, L.; Guo, W.L.; Pan, Y.Y.; Chen, S.J.; Bai, W.D.; Rao, P.F.; Ni, L.; et al. Exploring core functional microbiota responsible for the production of volatile flavour during the traditional brewing of Wuyi Hong Qu glutinous rice wine. Food Microbiol. 2018, 76, 487–496. [Google Scholar] [CrossRef]
- Lü, Y.; Gong, Y.; Li, Y.; Pan, Z.; Yao, Y.; Li, N.; Guo, J.L.; Gong, D.C.; Tian, Y.H.; Peng, C.Y. Characterization of microbial communities in Chinese rice wine collected at Yichang city and Suzhou city in China. J. Microbiol. Biotechnol. 2017, 27, 1409–1418. [Google Scholar] [CrossRef]
- Tian, S.F.; Zeng, W.Z.; Fang, F.; Zhou, J.W.; Du, G.C. The microbiome of Chinese rice wine (Huangjiu). Curr. Res. Food Sci. 2022, 5, 325–335. [Google Scholar] [CrossRef]
- Hong, X.T.; Chen, J.; Liu, L.; Wu, H.; Tan, H.Q.; Xie, G.F.; Xu, Q.; Zou, H.J.; Yu, W.J.; Wang, L.; et al. Metagenomic sequencing reveals the relationship between microbiota composition and quality of Chinese Rice Wine. Sci. Rep. 2016, 6, 26621. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.G.; Gao, X.L.; Jiang, X.Y.; Huang, M.Q.; Ye, H.; Wu, J.H.; Zhang, J.L.; Sun, X.T.; Wu, Q. Succession patterns of aroma components during brewing process of broomcorn millet (Panicum miliaceum L.) Huangjiu. Food Res. Int. 2022, 154, 110982. [Google Scholar] [CrossRef]
- Aboul-Maaty, N.A.-F.; Oraby, H.A.-S. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull. Natl. Res. Cent. 2019, 43, 25. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improve sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genom. Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OUT sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Green genes, a chimera-checked 16S rRNA gene database and work bench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Hao, S.Y.; Ren, Q.; Wang, J.X.; Li, L.Y.; Huang, M.Q. Two novel Planococcus species isolated from baijiu pit mud with potential application in brewing. Front. Microbiol. 2023, 11, 1139810. [Google Scholar] [CrossRef]
- Xu, J.L.; Wu, H.J.; Wang, Z.W.; Zheng, F.P.; Lu, X.; Li, Z.P.; Ren, Q. Microbial dynamics and metabolite changes in Chinese Rice Wine fermentation from sorghum with different tannin content. Sci. Rep. 2018, 8, 4639. [Google Scholar] [CrossRef]
- Liu, S.P.; Zhou, Y.; Zhou, Z.L.; Zhou, Z.L.; Han, X.; Xu, Y.Z.; Mao, J. Environmental factors drive microbial succession and huangjiu flavor formation during raw wheat qu fermentation. Food Biosci. 2023, 51, 102342. [Google Scholar] [CrossRef]
- Yasuda, M.; Tachibana, S.; Kuba-Miyara, M. Biochemical aspects of red koji and tofuyo prepared using Monascus fungi. Appl. Microbiol. Biotechnol. 2012, 96, 49–60. [Google Scholar] [CrossRef]
- Cai, H.Y.; Zhang, T.; Zhang, Q.; Luo, J.; Cai, C.G.; Mao, J.W. Microbial diversity and chemical analysis of the starters used in traditional Chinese sweet rice wine. Food Microbiol. 2018, 73, 319–326. [Google Scholar] [CrossRef]
- Barzideh, Z.; Siddiqi, M.; Mohamed, H.M.; LaPointe, G. Dynamics of starter and non-starter lactic acid bacteria populations in long-ripened Cheddar cheese using propidium monoazide (PMA) treatment. Microorganisms 2022, 10, 1669. [Google Scholar] [CrossRef]
- Cázares-Vásquez, M.L.; Rodríguez-Herrera, R.; Aguilar-González, C.N.; Sáenz-Galindo, A.; Solanilla-Duque, J.F.; Contreras-Esquivel, J.C.; Flores-Gallegos, A.C. Microbial exopolysaccharides in traditional Mexican fermented beverages. Fermentation 2021, 7, 249. [Google Scholar] [CrossRef]
- Xiao, R.; Chen, S.Q.; Wang, X.Q.; Chen, K.Q.; Hu, J.; Wei, K.; Ning, Y.; Xiong, T.; Lu, F.G. Microbial community starters affect the profiles of volatile compounds in traditional Chinese Xiaoqu rice wine: Assement via high-throughput sequencing and gas chromatography-ion mobility spectrometry. LWT 2022, 170, 114000. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Qi, Y.N.; Ma, H.X.; Li, W.; Dai, L.H.; Xiao, D.G. Decreased production of higher alcohols by Saccharomyces cerevisiae for Chinese rice wine fermentation by deletion of Bat aminotransferases. J. Ind. Microbiol. Biotechnol. 2015, 42, 617–625. [Google Scholar] [CrossRef]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–89. [Google Scholar] [CrossRef]
- Li, S.S.; Jia, X.Q.; Wen, J.P. Improved 2-methyl-1-propanol production in an engineered Bacillus subtilis by constructing inducible pathways. Biotechnol. Lett. 2012, 34, 2253–2258. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, Q.W.; Xie, J.R.; Chen, C.; Lou, X.M.; Ai, L.Z.; Tian, H.X. Characterization of Bitter Compounds in Shaoxing Huangjiu by Quantitative Measurements, Taste Recombination, and Omission Experiments. J. Agric. Food Chem. 2022, 70, 12907–12915. [Google Scholar] [CrossRef]
- Cha, J.W.; Jang, S.H.; Son, J.; Kim, J.; Jung, I.; Kim, K.H.; Chang, Y.K.; Jeong, K.J. Engineering of Klebsiella oxytoca for the production of 2,3-butanediol from high concentration of xylose. ACS Sustain. Chem. Eng. 2021, 9, 14395–14404. [Google Scholar] [CrossRef]
- Yang, Y.J.; Ai, L.Z.; Mu, Z.Y.; Liu, H.; Yan, X.; Ni, L.; Zhang, H.; Xia, Y.J. Flavor compounds with high odor activity values (OAV > 1) dominate the aroma of aged Chinese rice wine (Huangjiu) by molecular association. Food Chem. 2022, 383, 132370. [Google Scholar] [CrossRef]
- Pinu, F.R.; Villas-Boas, S.G. Rapid quantification of major volatile metabolites in fermented food and beverages using gas chromatography-mass spectrometry. Metabolites 2017, 7, 37. [Google Scholar] [CrossRef]
- Stefan, D.S.; Popescu, M.; Luntraru, C.M.; Suciu, A.; Belcu, M.; Ionescu, L.E.; Popescu, M.; Iancu, P.; Stefan, M. Comparative study of useful compounds extracted from lophanthus anisatus by green extraction. Molecules 2022, 27, 7737. [Google Scholar] [CrossRef] [PubMed]

| Compounds (g/L) | Fermentation Time (Days) | |||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | |
| acid | 1.71 ± 0.07 | 2.43 ± 0.07 | 2.71 ± 0.04 | 4.90 ± 0.15 | 5.32 ± 0.11 | 6.41 ± 0.16 |
| reducing sugar | 27.65 ± 0.97 | 32.35 ± 0.88 | 26.55 ± 0.91 | 24.25 ± 1.09 | 22.45 ± 0.89 | 19.14 ± 0.62 |
| alcohol | 2.32 ± 0.13 | 10.62 ± 1.35 | 18.54 ± 1.27 | 29.83 ± 1.45 | 53.16 ± 2.19 | 61.33 ± 3.25 |
| Flavor Component | Control | QR1 | QR2 | QR3 | QR4 |
|---|---|---|---|---|---|
| Alkane | 85.51 ± 3.67 a | 90.25 ± 3.64 a | 95.37 ± 5.85 b | 88.43 ± 3.58 a | 90.52 ± 3.61 a |
| Alcohol | 151.25 ± 4.38 a | 164.37 ± 4.83 b | 166.23 ± 5.45 b | 147.64 ± 5.09 a | 152.85 ± 4.69 a |
| Ester | 52.02 ± 3.11 a | 70.35 ± 2.84 b | 68.38 ± 3.72 b | 54.07 ± 4.19 a | 55.09 ± 3.24 a |
| Ketone | 1.43 ± 0.02 a | 1.71 ± 0.13 a | 1.74 ± 0.04 a | 1.60 ± 0.05 a | 1.51 ± 0.04 a |
| Aldehyde | 1.08 ± 0.01 a | 0.92 ± 0.02 a | 0.95 ± 0.01 a | 0.86 ± 0.02 a | 0.98 ± 0.02 a |
| Acid | 2.34 ± 0.04 a | 9.54 ± 0.27 b | 11.25 ± 0.18 b | 3.05 ± 0.08 a | 2.19 ± 0.05 a |
| Pyrazine | 0.03 ± 0.002 a | 0.12 ± 0.003 a | 0.06 ± 0.001 a | 0.04 ± 0.002 a | 0.03 ± 0.001 a |
| Phenol | 8.05 ± 0.41 a | 10.85 ± 0.74 a | 9.54 ± 0.36 a | 8.37 ± 0.42 a | 8.86 ± 0.59 a |
| Others | 9.04 ± 0.76 a | 12.73 ± 1.86 b | 13.21 ± 0.96 b | 8.78 ± 0.33 a | 9.43 ± 0.37 a |
| Total | 310.75 ± 7.39 a | 360.84 ± 5.36 b | 366.73 ± 6.81 b | 312.84 ± 6.26 a | 321.19 ± 8.04 a |
| Indexes (g/L) | Control | QR1 | QR2 | QR3 | QR4 |
|---|---|---|---|---|---|
| Total acid | 4.07 ± 0.61 a | 6.82 ± 0.39 b | 6.47 ± 0.39 b | 4.32 ± 0.52 a | 4.21 ± 0.54 a |
| Total sugar | 77.28 ± 2.87 a | 76.68 ± 3.19 a | 76.51 ± 2.82 a | 80.02 ± 2.92 a | 76.38 ± 2.51 a |
| Non-sugar solid | 19.36 ± 2.01 a | 18.35 ± 2.14 a | 18.06 ± 1.74 a | 20.04 ± 1.87 a | 20.04 ± 2.53 a |
| Alcohol | 11.25 ± 1.34 a | 12.06 ± 1.25 a | 11.88 ± 1.54 a | 10.93 ± 1.28 a | 11.27 ± 1.45 a |
| Amino acid nitrogen | 0.33 ± 0.02 a | 0.52 ± 0.02 b | 0.54 ± 0.01 b | 0.35 ± 0.02 a | 0.35 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wu, H.; Wang, J.; Ren, Q. Microbiota Composition during Fermentation of Broomcorn Millet Huangjiu and Their Effects on Flavor Quality. Foods 2023, 12, 2680. https://doi.org/10.3390/foods12142680
Wang K, Wu H, Wang J, Ren Q. Microbiota Composition during Fermentation of Broomcorn Millet Huangjiu and Their Effects on Flavor Quality. Foods. 2023; 12(14):2680. https://doi.org/10.3390/foods12142680
Chicago/Turabian StyleWang, Ke, Huijun Wu, Jiaxuan Wang, and Qing Ren. 2023. "Microbiota Composition during Fermentation of Broomcorn Millet Huangjiu and Their Effects on Flavor Quality" Foods 12, no. 14: 2680. https://doi.org/10.3390/foods12142680
APA StyleWang, K., Wu, H., Wang, J., & Ren, Q. (2023). Microbiota Composition during Fermentation of Broomcorn Millet Huangjiu and Their Effects on Flavor Quality. Foods, 12(14), 2680. https://doi.org/10.3390/foods12142680





