Quercetin 3-O-Glucuronide from Aglianico Vine Leaves: A Selective Sustainable Recovery and Accumulation Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Leaf Harvesting
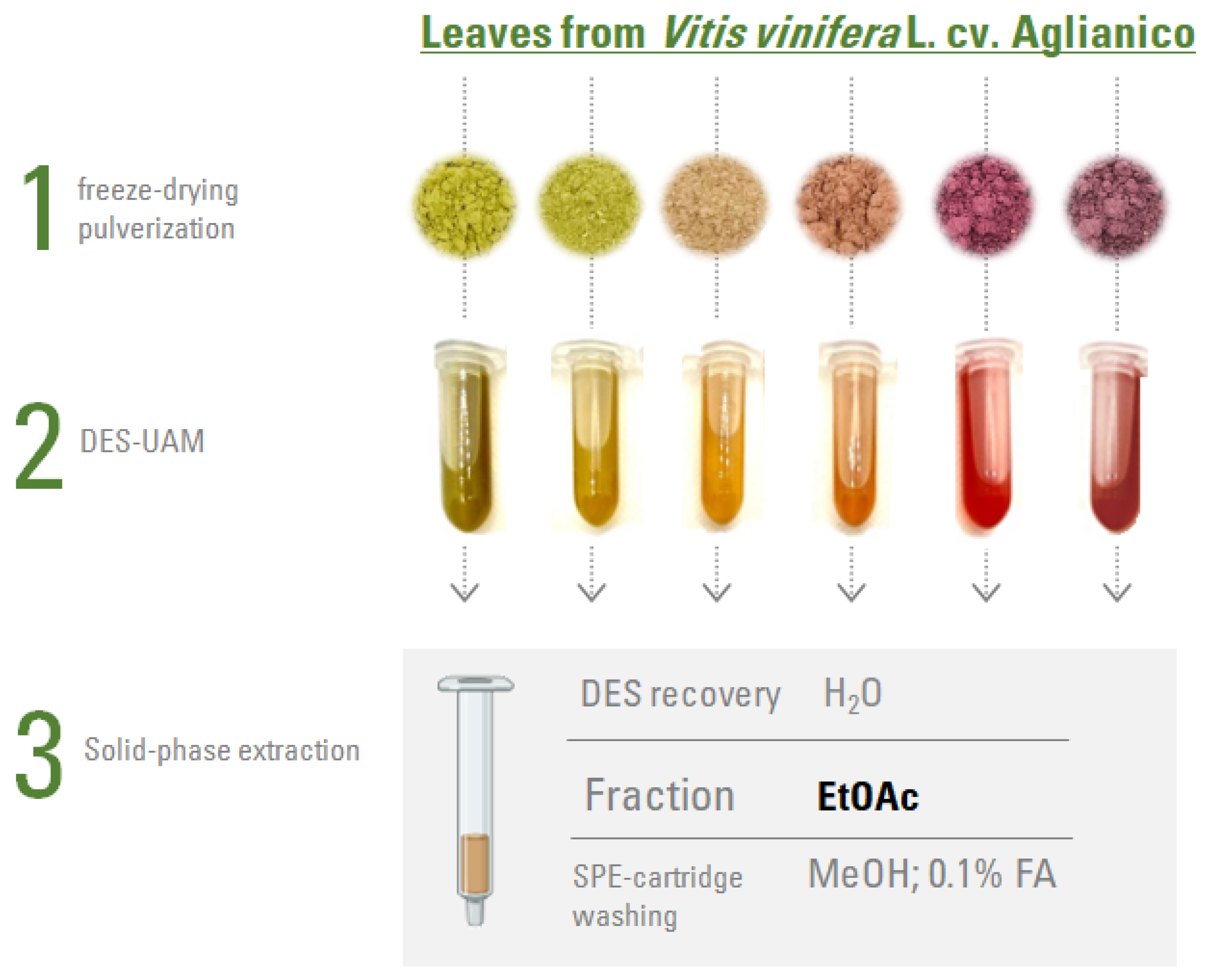
2.2. Leaf Extraction and Fractionation
2.2.1. Deep Eutectic Solvents (DES) Preparation
2.2.2. Extraction and Fractionation Process
2.3. Quali-Quantitative Analyses
2.3.1. UV–Vis Spectroscopy
2.3.2. UHPLC-ESI-QqTOF-MS Untargeted Profile
2.3.3. HPLC-UV-ESI-TQMS Quantitative Analysis
2.4. Statistical Analysis
3. Results and Discussion
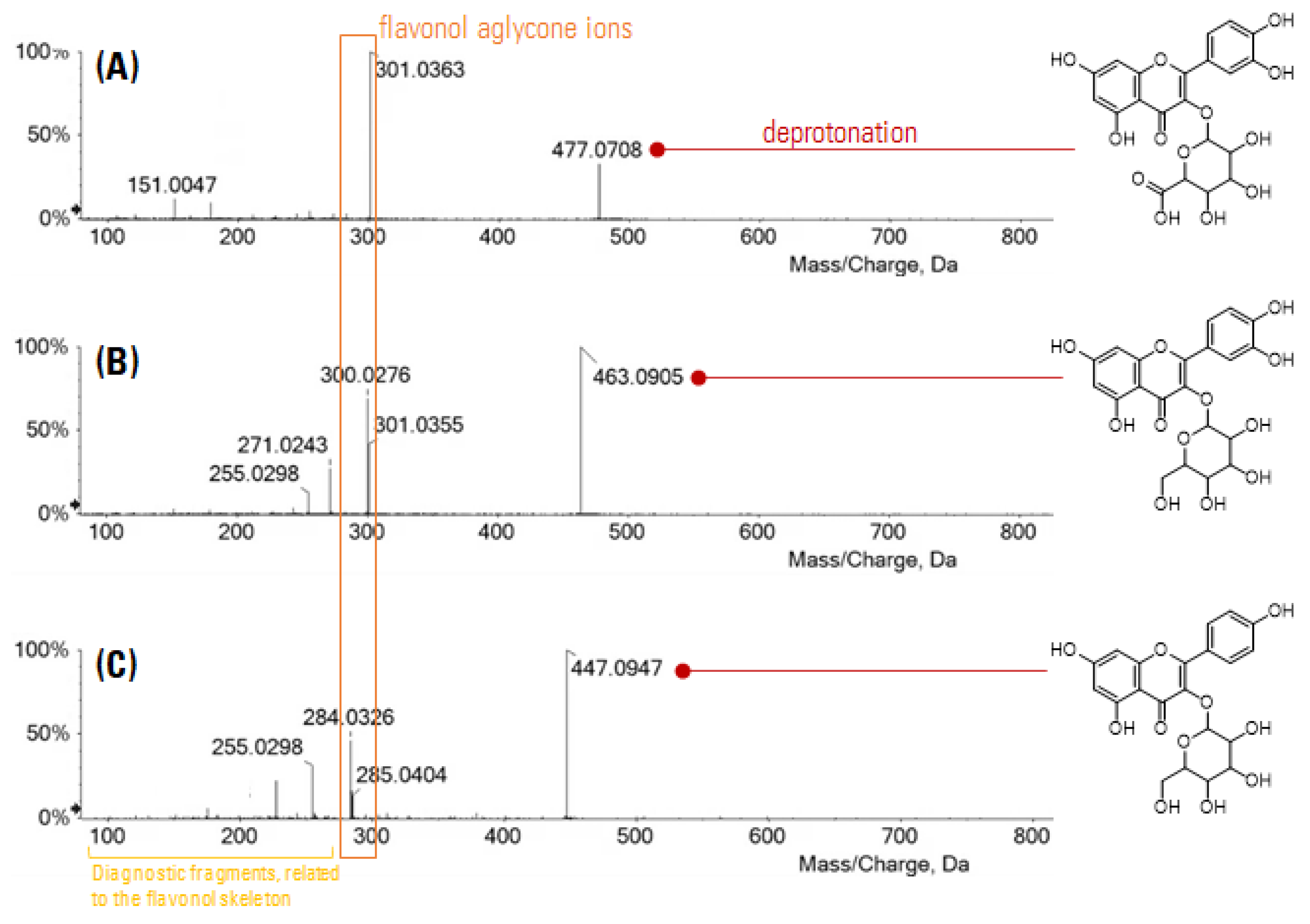
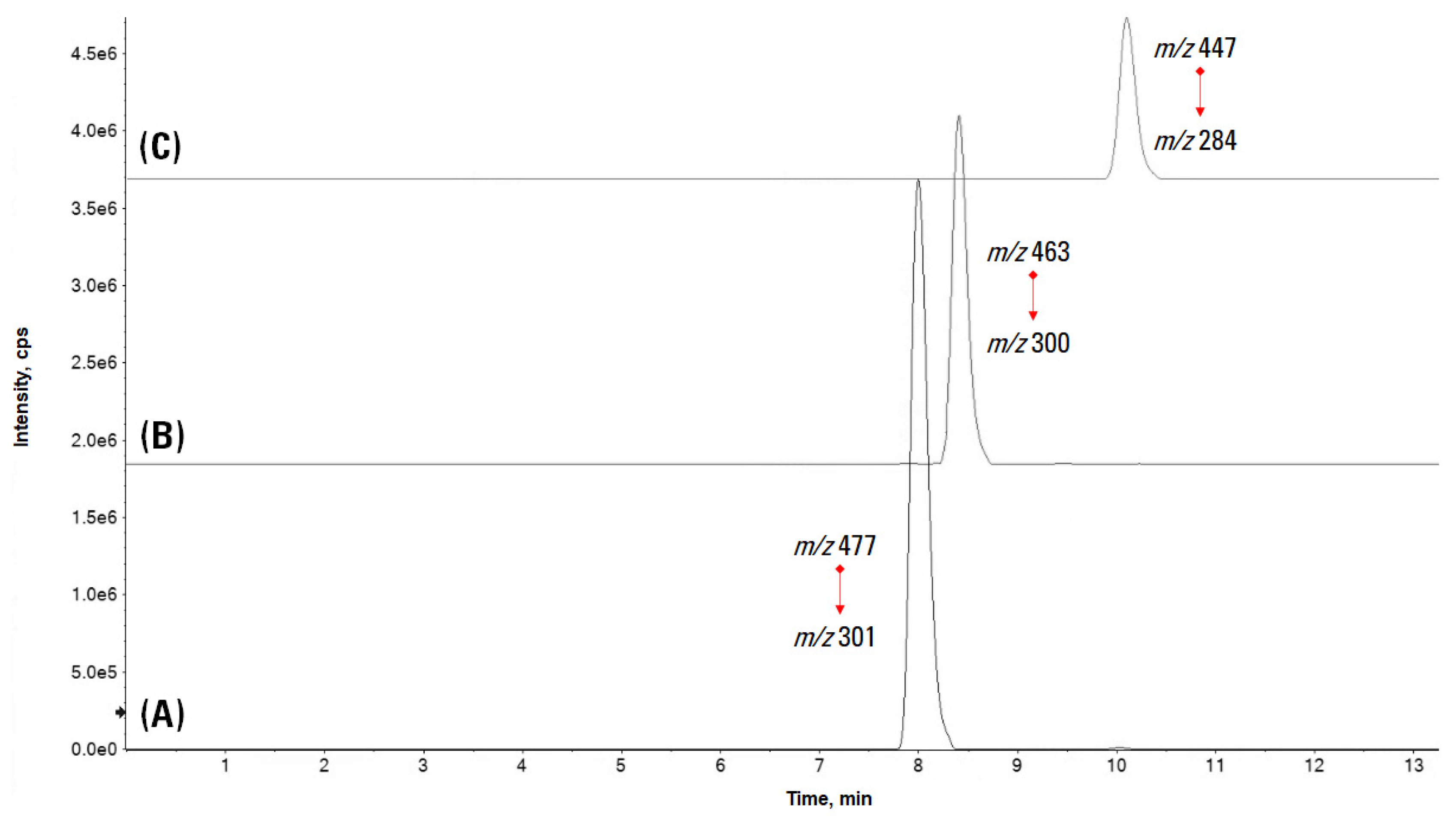
3.1. Leaf Extracts’ Qualitative Profile: An Untargeted Approach
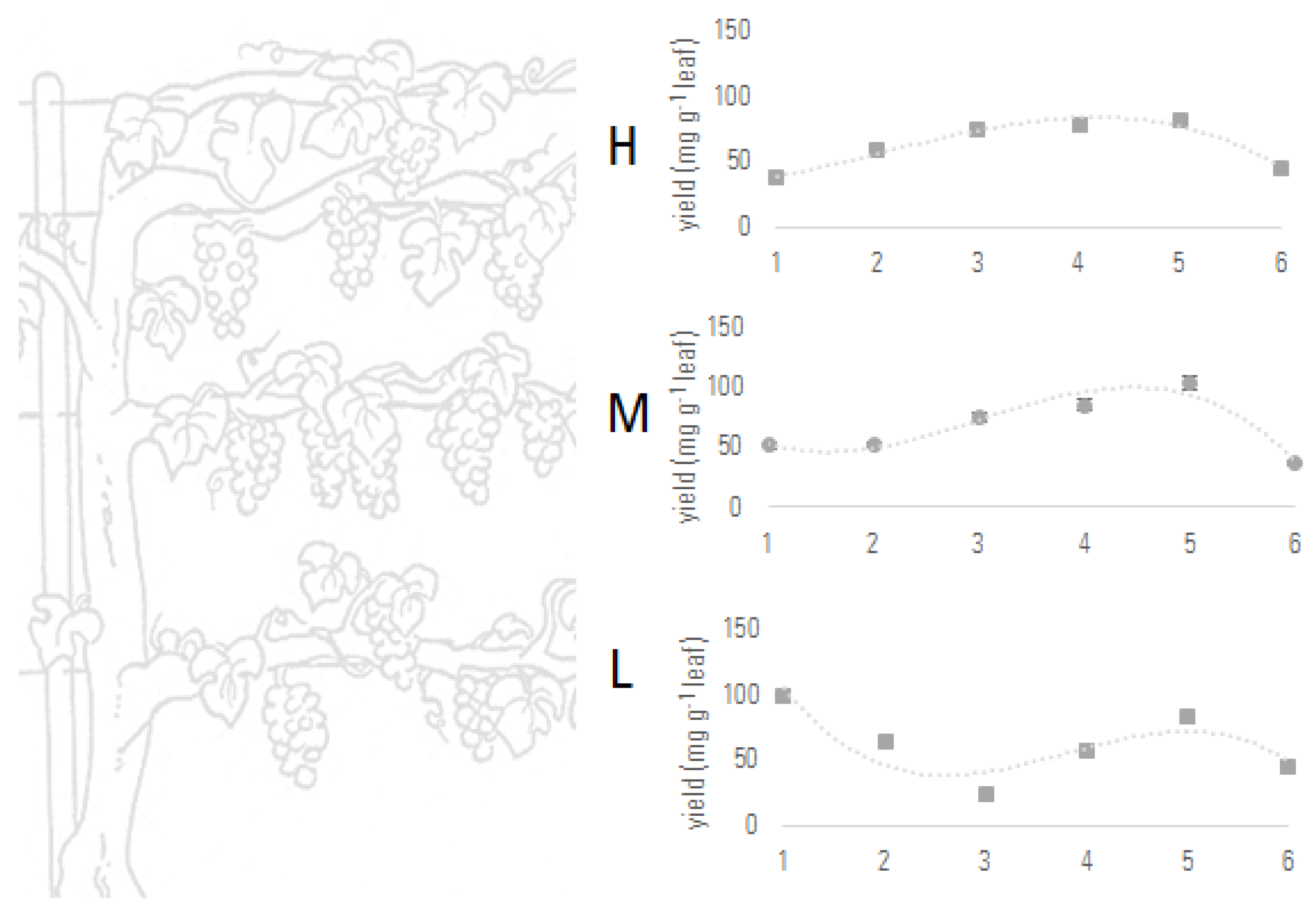
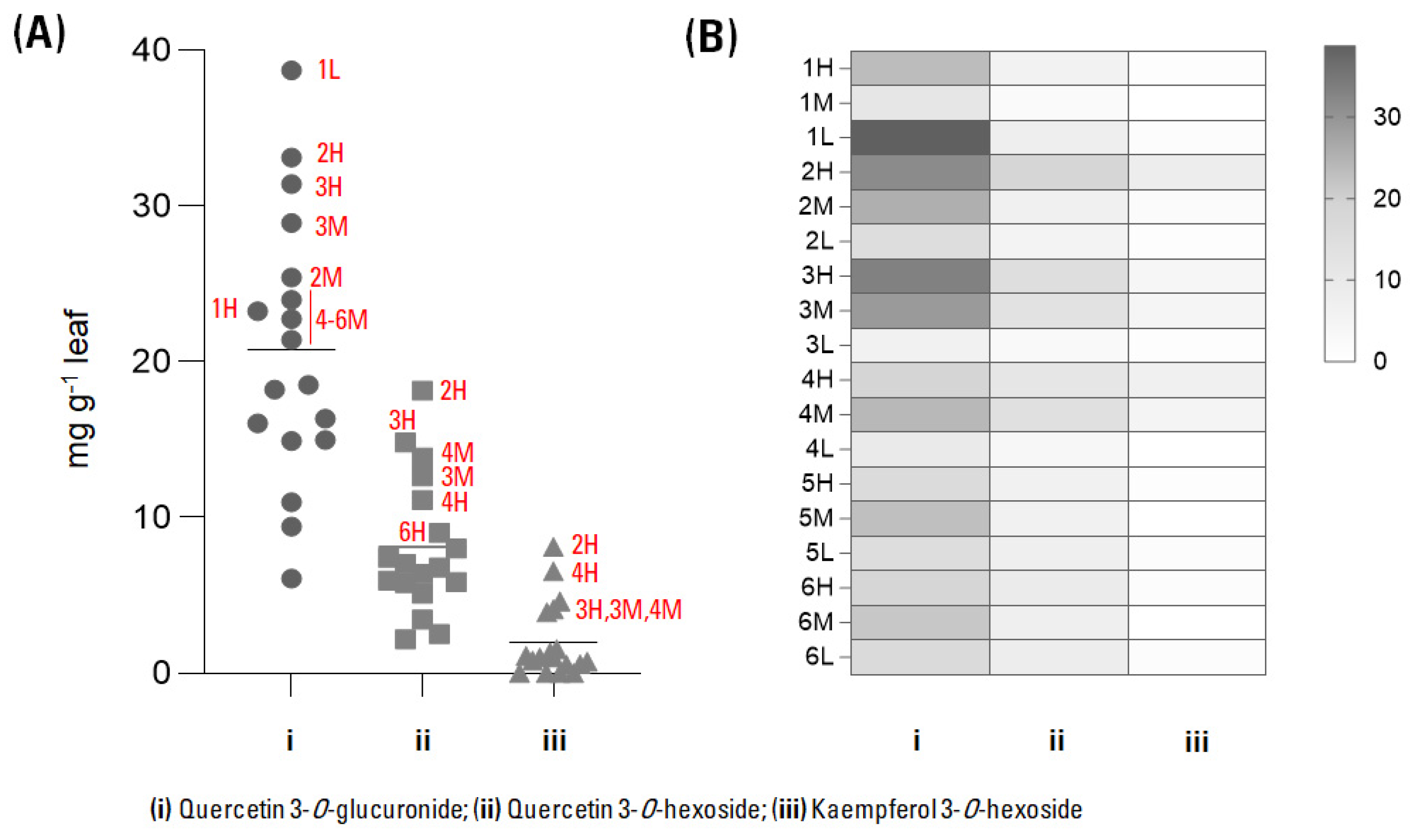
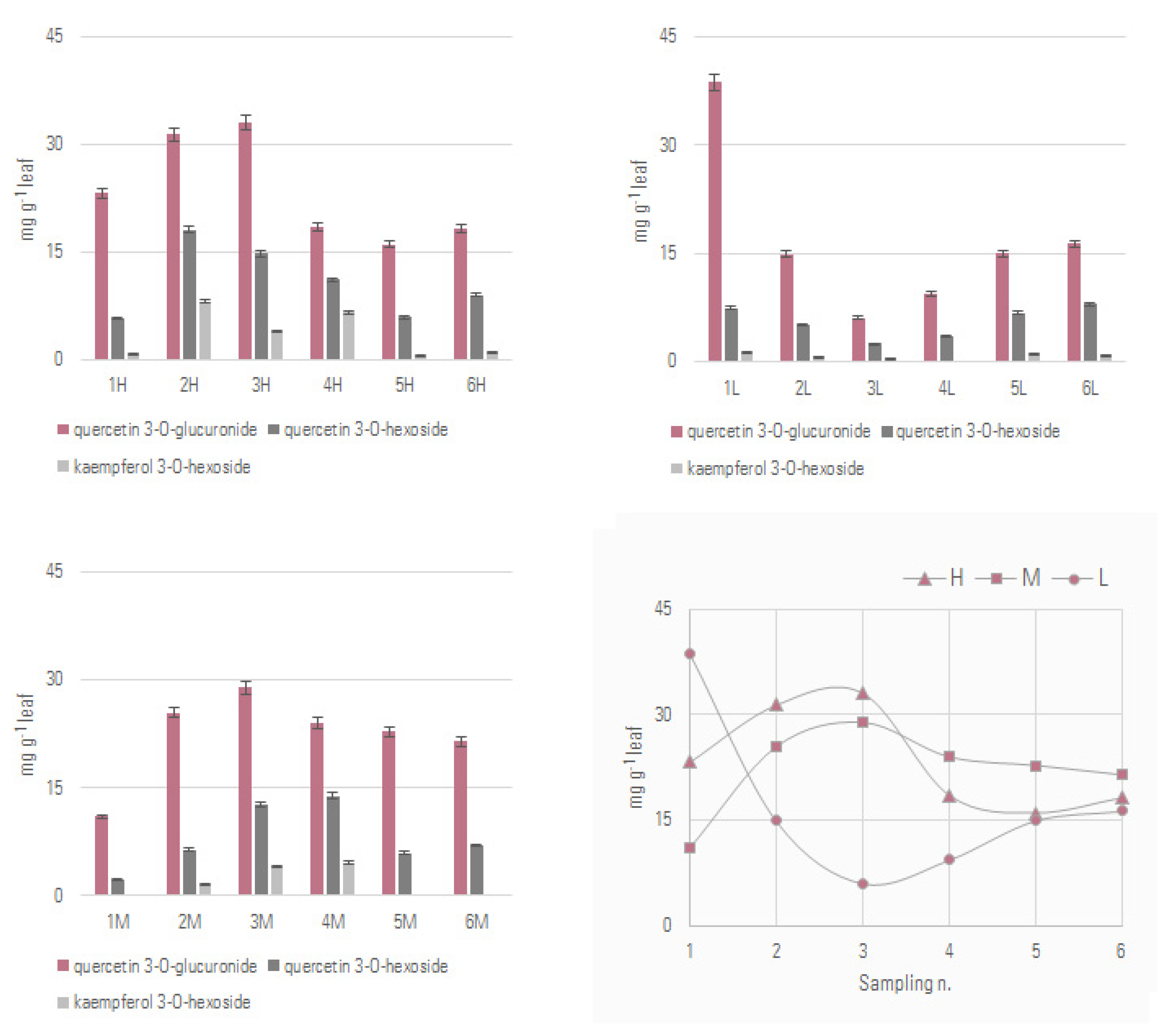
3.2. Leaf Extracts Quantitative Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, O.; Nier, S.; Tamásy, C. Towards a Circular Bioeconomy? Pathways and Spatialities of Agri-Food Waste Valorisation. Tijdschr. Voor Econ. Soc. Geogr. 2022, 113, 194–210. [Google Scholar] [CrossRef]
- Chauhan, C.; Dhir, A.; Ul Akram, M.; Salo, J. Food loss and waste in food supply chains. A systematic literature review and framework development approach. J. Clean. Prod. 2021, 295, 126438. [Google Scholar] [CrossRef]
- Kiss, K.; Ruszkai, C.; Takács-György, K. Examination of Short Supply Chains Based on Circular Economy and Sustainability Aspects. Resources 2019, 8, 161. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Voss, G.B.; Coelho, M.C.; Pintado, M.E. Chapter 33—Food waste and by-product valorization as an integrated approach with zero waste: Future challenges. In Future Foods: Global Trends, Opportunities, and Sustainability Challenges, 1st ed.; Bhat, R., Ed.; Academic Press: London, UK, 2022; pp. 569–596. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. Sci. Total Environ. 2020, 706, 136033. [Google Scholar] [CrossRef]
- OIV. The International Organisation of Vine and Wine. 2021. Available online: https://www.oiv.int/what-we-do/global-report?oiv (accessed on 11 June 2023).
- Christ, K.L.; Burritt, R.L. Critical environmental concerns in wine production: An integrative review. J. Clean. Prod. 2013, 53, 232–242. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef]
- Niculescu, V.-C.; Ionete, R.-E. An Overview on Management and Valorisation of Winery Wastes. Appl. Sci. 2023, 13, 5063. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Yadav, V.; Yadav, A.; Rahman, M.U.; Yuan, W.Z.; Li, Z.; Wang, X. Integrated biorefinery approach to valorize winery waste: A review from waste to energy perspectives. Sci. Total Environ. 2020, 719, 137315. [Google Scholar] [CrossRef] [PubMed]
- Tacchini, M.; Burlini, I.; Maresca, I.; Grandini, A.; Bernardi, T.; Guerrini, A.; Lerin, L.; Sacchetti, G. Polyphenols From Vitis vinifera Lambrusco By-Products (Leaves From Pruning): Extraction Parameters Evaluation Through Design of Experiment. Nat. Prod. Commun. 2019, 14, 1934578X1986290. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Imazio, S.; Biagini, B.; Failla, O.; Scienza, A. Pedigree Reconstruction of the Italian Grapevine Aglianico (Vitis vinifera L.) from Campania. Mol. Biotechnol. 2013, 54, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Labanca, F.; Faraone, I.; Nolè, M.R.; Hornedo-Ortega, R.; Russo, D.; García-Parrilla, M.C.; Chiummiento, L.; Bonomo, M.G.; Milella, L. New Insights into the Exploitation of Vitis vinifera L. cv. Aglianico Leaf Extracts for Nutraceutical Purposes. Antioxidants 2020, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-HerreraB, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC Trends Anal. Chem. 2021, 134, 116108. [Google Scholar] [CrossRef]
- Brahmi-Chendouh, N.; Piccolella, S.; Gravina, C.; Fiorentino, M.; Formato, M.; Kheyar, N.; Pacifico, S. Ready-to-Use Nutraceutical Formulations from Edible and Waste Organs of Algerian Artichokes. Foods 2022, 11, 3955. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep Eutectic Solvents as Extraction Media for Valuable Flavonoids from Natural Sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- European Chemicals Agency: Information on Chemicals. Available online: https://echa.europa.eu/it/information-on-chemicals (accessed on 4 July 2023).
- Food and Drug Administration, Department of Health and Human Services. Code of Federal Regulations. Available online: https://www.ecfr.gov/current/title-21/chapter-I (accessed on 4 July 2023).
- Kalyniukova, A.; Holuša, J.; Musiolek, D.; Sedlakova-Kadukova, J.; Płotka-Wasylka, J.; Andruch, V. Application of deep eutectic solvents for separation and determination of bioactive compounds in medicinal plants. Ind. Crop Prod. 2021, 172, 114047. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Volpe, M.G.; Paolucci, M.; Pacifico, S. UHPLC-HR-MS/MS Guided Recovery of bioactive flavonol compounds from Greco di Tufo Vine leaves. Molecules 2019, 24, 3630. [Google Scholar] [CrossRef]
- Xu, M.; Huang, H.; Mo, X.; Zhu, Y.; Chen, X.; Li, X.; Peng, X.; Xu, Z.; Chen, L.; Rong, S.; et al. Quercetin-3-O-Glucuronide Alleviates Cognitive Deficit and Toxicity in Aβ1-42 -Induced AD-Like Mice and SH-SY5Y Cells. Mol. Nutr. Food Res. 2021, 65, e2000660. [Google Scholar] [CrossRef]
- Ha, A.T.; Rahmawati, L.; You, L.; Hossain, M.A.; Kim, J.-H.; Cho, J.Y. Anti-Inflammatory, Antioxidant, Moisturizing, and Antimelanogenesis Effects of Quercetin 3-O-β-D-Glucuronide in Human Keratinocytes and Melanoma Cells via Activation of NF-κB and AP-1 Pathways. Int. J. Mol. Sci. 2022, 23, 433. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Pacifico, F.; Pacifico, S. Wild aromatic plants bioactivity: A function of their (poly)phenol seasonality? A case study from Mediterranean area. Phytochem. Rev. 2018, 17, 785–799. [Google Scholar] [CrossRef]
- Esposito, T.; Paolucci, M.; Sansone, F.; Mencherini, T.; Pacifico, S.; Volpe, M.G. Exploitation and Valorization of Agro-Food Wastes from Grape Harvesting: Production, Characterization of MAE-Extracts from Vitis vinifera Leaves and Stabilization in Microparticulate Powder Form. Appl. Sci. 2021, 11, 5827. [Google Scholar] [CrossRef]
- Regione Campania Assessorato all’Agricoltura (Se.SIRCA). D.O.C.G. Taurasi. 2003. Available online: http://www.agricoltura.regione.campania.it/pubblicazioni/pdf/DOCGTAU1.PDF (accessed on 20 June 2023).
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Mattii, G.B. Effects of Defoliation at Fruit Set on Vine Physiology and Berry Composition in Cabernet Sauvignon Grapevines. Plants 2021, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Schoedl, K.; Schuhmacher, R.; Forneck, A. Correlating physiological parameters with biomarkers for UV-B stress indicators in leaves of grapevine cultivars Pinot noir and Riesling. J. Agric. Sci. 2013, 151, 189–200. [Google Scholar] [CrossRef]
- Bouderias, S.; Teszlaik, P.; Jakab, G.; Karapsi, L. Age- and season-dependent pattern of flavonol glycosides in Cabernet Sauvignon grapevine leaves. Sci. Rep. 2020, 10, 14241. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef]
- Csepregi, K.; Teszlák, P.; Kőrösi, L.; Hideg, É. Changes in grapevine leaf phenolic profiles during the day are temperature rather than irradiance driven. Plant Physiol. Biochem. 2019, 137, 169–178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioffi, E.; Comune, L.; Piccolella, S.; Buono, M.; Pacifico, S. Quercetin 3-O-Glucuronide from Aglianico Vine Leaves: A Selective Sustainable Recovery and Accumulation Monitoring. Foods 2023, 12, 2646. https://doi.org/10.3390/foods12142646
Cioffi E, Comune L, Piccolella S, Buono M, Pacifico S. Quercetin 3-O-Glucuronide from Aglianico Vine Leaves: A Selective Sustainable Recovery and Accumulation Monitoring. Foods. 2023; 12(14):2646. https://doi.org/10.3390/foods12142646
Chicago/Turabian StyleCioffi, Elena, Lara Comune, Simona Piccolella, Mario Buono, and Severina Pacifico. 2023. "Quercetin 3-O-Glucuronide from Aglianico Vine Leaves: A Selective Sustainable Recovery and Accumulation Monitoring" Foods 12, no. 14: 2646. https://doi.org/10.3390/foods12142646
APA StyleCioffi, E., Comune, L., Piccolella, S., Buono, M., & Pacifico, S. (2023). Quercetin 3-O-Glucuronide from Aglianico Vine Leaves: A Selective Sustainable Recovery and Accumulation Monitoring. Foods, 12(14), 2646. https://doi.org/10.3390/foods12142646







