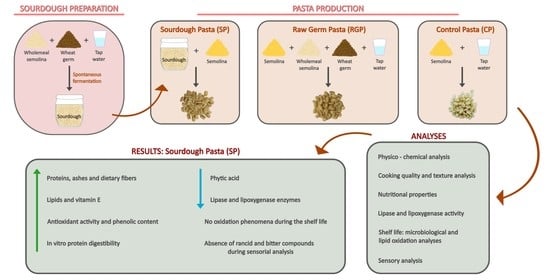
Quality Evaluation of Fresh Pasta Fortified with Sourdough Containing Wheat Germ and Wholemeal Semolina
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Physicochemical Composition of Raw Materials and Samples
- 0 < ΔE < 1—the difference is unnoticeable
- 1 < ΔE < 2—the difference is only noticed by an experienced observer
- 2 < ΔE < 3.5—the difference is also noticed by an unexperienced observer
- 3.5 < ΔE < 5—the difference is clearly noticeable
- 5 < ΔE—gives the impression that these are two different color
- where L*1, a*1, b*1 refer to the color parameters of the control sample, and L*2, a*2, b*2 refer to the color parameters of the experimental sample [21].
2.3. Sourdough Preparation, Refreshment and Analysis
2.4. Pasta Making and Analysis
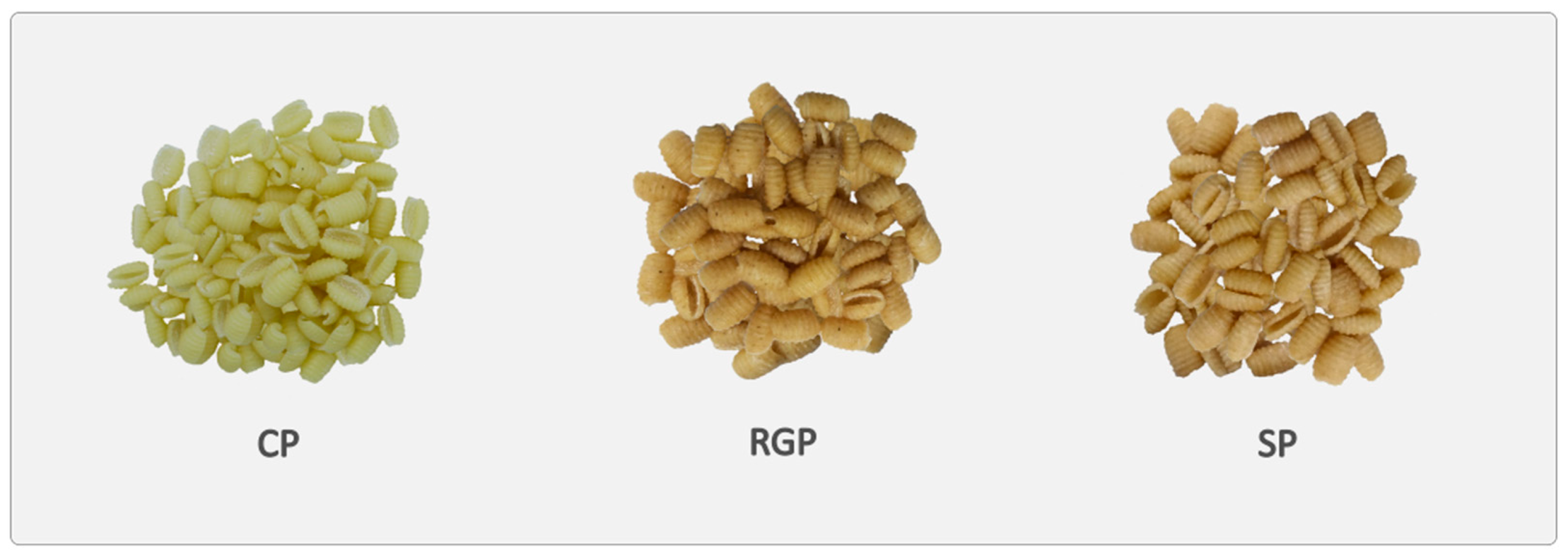
- CP (Control Pasta). Pasta made with semolina (1000 g) and water (300 g);
- RGP (Raw Germ Pasta). Pasta made with 300 g of unfermented GWS (which contained 40 g of wheat germ and 260 g of wholemeal semolina), 700 g of semolina, and 300 g of water;
- SP (Sourdough Pasta). Pasta made with 600 g of sourdough and 1000 g of semolina. The water (300 mL) was into the sourdough.
2.5. Cooking Quality and Texture Analysis of Pasta
2.6. Nutritional Properties
2.6.1. In Vitro Protein Digestibility
2.6.2. Phytic Acid and Total Dietary Fiber Determination
2.6.3. Total Phenolic Content and Antioxidant Activity
2.7. Lipase and Lipoxygenase Activity
2.8. Determination of Vitamin E by HPLC Analysis
2.8.1. Materials and Standard Solution Preparation
2.8.2. HPLC Conditions
2.8.3. Preparation of Standards Solutions
2.8.4. Sample Extraction
2.9. Lipid Oxidation Analysis
2.10. GC Analysis of FAME
2.11. Sensory Analysis
2.12. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Characteristics of Pasta
3.2. Cooking Quality
3.3. Textural Properties
3.4. Nutritional Properties
3.4.1. In Vitro Protein Digestion Analysis
3.4.2. Phytic Acid Content
3.4.3. Total Dietary Fiber
3.4.4. Total Phenolic Content and Antioxidant Activity
3.4.5. Vitamin E Content
3.5. Lipase and Lipoxygenase Activity
3.6. Microbiological Analysis of Pasta
3.7. Oxidative Degradation Products of Lipids
3.8. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boukid, F.; Folloni, S.; Ranieri, R.; Vittadini, E. A compendium of wheat germ: Separation, stabilization and food applications. Trends Food Sci. Tech. 2018, 78, 120–133. [Google Scholar] [CrossRef]
- Brandolini, A.; Hidalgo, A. Wheat germ: Not only a by-product. Int. J. Food Sci. Nutr. 2012, 63 (Supp. 1), 71–74. [Google Scholar] [CrossRef]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Wheat (Triticum aestivum L.) bran in bread making: A critical review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef]
- Stevenson, L.E.O.; Phillips, F.; O’sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Willett, W.C.; Manson, J.A.E.; Rosner, B.; Hu, F.B.; Sun, Q. Intake of whole grain foods and risk of coronary heart disease in US men and women. BMC Med. 2022, 20, 192. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Özcan, M.M.; AL-Juhaımı, F.; Babıker, E.E.; Sarker, Z.I.; Ahmed, I.A.M.; Ahmed, M.A. Nutritional composition, extraction, and utilization of wheat germ oil: A review. Eur. J. Lipid Sci. Tech. 2017, 119, 1600160. [Google Scholar] [CrossRef]
- de Punder, K.; Pruimboom, L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef]
- Bin, Q.; Jiang, D.; Cho, I.H.; Peterson, D.G. Chemical markers for bitterness in wheat bread. Flavour Fagr. J. 2012, 27, 454–458. [Google Scholar] [CrossRef]
- Heiniö, R.L.; Noort, M.W.J.; Katina, K.; Alam, S.A.; Sozer, N.; De Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory characteristics of wholegrain and bran-rich cereal foods—A review. Trends Food Sci. Tech. 2016, 47, 25–38. [Google Scholar] [CrossRef]
- Laureati, M.; Conte, A.; Padalino, L.; Del Nobile, M.A.; Pagliarini, E. Effect of fiber information on consumer’s expectation and liking of wheat bran enriched pasta. J. Sens. Stud. 2016, 31, 348–359. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbial. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- Pontonio, E.; Lorusso, A.; Gobbetti, M.; Rizzello, C.G. Use of fermented milling by-products as functional ingredient to develop a low-glycaemic index bread. J. Cereal Sci. 2017, 77, 235–242. [Google Scholar] [CrossRef]
- Bayat, E.; Moosavi-Nasab, M.; Fazaeli, M.; Majdinasab, M.; Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M. Wheat germ fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum: Process optimization for enhanced composition and antioxidant properties in vitro. Foods 2022, 11, 1125. [Google Scholar] [CrossRef]
- Marti, A.; Torri, L.; Casiraghi, M.C.; Franzetti, L.; Limbo, S.; Morandin, F.; Quaglia, L.; Pagani, M.A. Wheat germ stabilization by heat-treatment or sourdough fermentation: Effects on dough rheology and bread properties. LWT-Food Sci. Technol. 2014, 59, 1100–1106. [Google Scholar] [CrossRef]
- Tovar, L.E.R.; Gänzle, M.G. Degradation of wheat germ agglutinin during sourdough fermentation. Foods 2021, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; Di Cagno, R.; Gobbetti, M. Use of sourdough fermented wheat germ for enhancing the nutritional, texture and sensory characteristics of the white bread. Eur. Food Res. Technol. 2010, 230, 645–654. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 2010. [Google Scholar]
- Fois, S.; Campus, M.; Piu, P.P.; Siliani, S.; Sanna, M.; Roggio, T.; Catzeddu, P. Fresh pasta manufactured with fermented whole wheat semolina: Physicochemical, sensorial, and nutritional properties. Foods 2019, 8, 422. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Teterycz, D.; Sobota, A.; Starek, A. Possibility of using wheat germ and wheat germ protein isolate for high-protein pasta production. Cereal Chem. 2022, 2, 10602. [Google Scholar] [CrossRef]
- Fois, S.; Piu, P.P.; Sanna, M.; Roggio, T.; Catzeddu, P. Starch digestibility and properties of fresh pasta made with semolina-based liquid sourdough. LWT-Food Sci. Technol. 2018, 89, 496–502. [Google Scholar] [CrossRef]
- Padalino, L.; Mastromatteo, M.; Lecce, L.; Spinelli, S.; Contò, F.; Del Nobile, M.A. Effect of durum wheat cultivars on physico-chemical and sensory properties of spaghetti. J. Sci. Food Agric. 2014, 94, 2196–2204. [Google Scholar] [CrossRef]
- Manthey, F.A.; Dick, T. Assessment of probe type for measuring pasta texture. Cereal Foods World 2012, 57, 56. [Google Scholar] [CrossRef]
- Pasini, G.; Simonato, B.; Giannattasio, M.; Peruffo, A.D.B.; Curioni, A. Modifications of wheat flour proteins during in vitro digestion of bread dough, crumb, and crust: An electrophoretic and immunological study. J. Agric. Food Chem. 2001, 49, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- De Marco, E.R.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT-Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Boroski, M.; de Aguiar, A.C.; Boeing, J.S.; Rotta, E.M.; Wibby, C.L.; Bonafé, E.G.; de Souza, N.E.; Visentainer, J.V. Enhancement of pasta antioxidant activity with oregano and carrot leaf. Food Chem. 2011, 125, 696–700. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Yaghoubi, A.S.; Hashemi, M. The impact of atmospheric cold plasma treatment on inactivation of lipase and lipoxygenase of wheat germs. Innov. Food Sci. Emerg. Tech. 2018, 47, 346–352. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A. Lipoxygenase activity in wholemeal flours from Triticum monococcum, Triticum turgidum and Triticum aestivum. Food Chem. 2012, 131, 1499–1503. [Google Scholar] [CrossRef]
- Siji, J. Analysis of fat-soluble vitamins from food matrix for nutrition labeling. In Agilent Application Note; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2011; Publication Number 5990-8668EN. [Google Scholar]
- Nurit, E.; Lyan, B.; Pujos-Guillot, E.; Branlard, G.; ve Piquet, A. Change in B and E vitamin and lutein, β-sitosterol contents in industrial milling fractions and during toasted bread production. J. Cereal Sci. 2016, 69, 290–296. [Google Scholar] [CrossRef]
- Karakas, F.P.; Keskin, C.N.; Agil, F.; Zencirci, N. Profiles of vitamin B and E in wheat grass and grain of einkorn (Triticum monococcum spp. monococcum), emmer (Triticum dicoccum ssp. dicoccum Schrank.), durum (Triticum durum Desf.), and bread wheat (Triticum aestivum L.) cultivars by LC-ESI-MS/MS analysis. J. Cereal Sci. 2021, 2021, 103177. [Google Scholar] [CrossRef]
- Siliani, S.; Melis, R.; Loi, B.; Guala, I.; Baroli, M.; Sanna, R.; Uzzau, S.; Roggio, T.; Addis, M.F.; Anedda, R. Influence of seasonal and environmental patterns on the lipid content and fatty acid profiles in gonads of the edible sea urchin Paracentrotus lividus from Sardinia. Mar. Environ. Res. 2016, 113, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Melis, R.; Vitangeli, I.; Anedda, R. Effect of fish diet and cooking mode on the composition and microstructure of ready-to-eat fish fillets of gilthead sea bream (Sparus aurata) juveniles. J. Food Compos. Anal. 2022, 114, 104847. [Google Scholar] [CrossRef]
- Pınarlı, I.; Öner, M.D.; İbanoğlu, Ş. Effect of wheat germ addition on the microbiological quality, in vitro protein digestibility, and gelatinization behavior of macaroni. Eur. Food Res. Technol. 2004, 219, 52–59. [Google Scholar] [CrossRef]
- Aktaş, K.; Bilgiçli, N.; Levent, H. Influence of wheat germ and β-glucan on some chemical and sensory properties of Turkish noodle. J. Food Sci. Technol. 2015, 52, 6055–6060. [Google Scholar] [CrossRef]
- Drabińska, N.; Nogueira, M.; Ciska, E.; Jelen, H. Effect of drying and broccoli leaves incorporation on the nutritional quality of durum wheat pasta. Pol. J. Food Nutr. Sci. 2022, 72, 273–285. [Google Scholar] [CrossRef]
- Fuad, T.; Prabhasankar, P. Role of ingredients in pasta product quality: A review on recent developments. Crit. Rev. Food Sci. Nutr. 2010, 50, 787–798. [Google Scholar] [CrossRef]
- Tarzi, B.G.; Shakeri, V.; Ghavami, M. Quality evaluation of pasta enriched with heated and unheated wheat germ during storage. Adv. Environ. Biol. 2012, 6, 1700–1708. Available online: https://link.gale.com/apps/doc/A299344556/AONE?u=anon~c57e5414&sid=googleScholar&xid=7d03a5b5 (accessed on 8 June 2023).
- Matsuo, R.R.; Dexter, J.E.; Boudreau, A.; Daun, J.K. The role of lipids in determining spaghetti cooking quality. Cereal Chem. 1986, 63, 484–489. [Google Scholar]
- Aravind, N.; Sissons, M.; Egan, N.; Fellows, C. Effect of insoluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012, 130, 299–309. [Google Scholar] [CrossRef]
- Vignola, M.B.; Bustos, M.C.; Pérez, G.T. In vitro dialyzability of essential minerals from white and whole grain pasta. Food Chem. 2018, 265, 128–134. [Google Scholar] [CrossRef]
- Östman, E.M.; Nilsson, M.; Elmståhl, H.L.; Molin, G.; Björck, I.M.E. On the effect of lactic acid on blood glucose and insulin responses to cereal products: Mechanistic studies in healthy subjects and in vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Schettino, R.; Pontonio, E.; Rizzello, C.G. Use of fermented hemp, chickpea and milling by-products to improve the nutritional value of semolina pasta. Foods 2019, 8, 604. [Google Scholar] [CrossRef]
- Labuckas, D.O.; Maestri, D.M.; Perelló, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Sze-Tao, K.W.C.; Sathe, S.K. Walnuts (Juglans regia L): Proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J. Sci. Food Agric. 2000, 80, 1393–1401. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Barnes, P.J. Cereal tocopherols. In Proceedings of the 7th World Cereal and Bread Congress, Prague, Czech, 2–28 July 1982. [Google Scholar]
- Piironen, V.; Syvaoja, E.L.; Varo, P.; Salminen, K.; Koivistoinen, P. Tocopherols and tocotrienols in Finnish foods: Vegetables, fruits, and berries. J. Agric. Food Chem. 1986, 34, 742–746. [Google Scholar] [CrossRef]
- Nielsen, M.M.L.; Hansen, Å. Stability of vitamin E in wheat flour and whole wheat flour during storage. Cereal Chem. 2008, 85, 716–720. [Google Scholar] [CrossRef]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef]
- Fratianni, A.; Di Criscio, T.; Mignogna, R.; Panfili, G. Carotenoids, tocols and retinols evolution during egg pasta–making processes. Food Chem. 2012, 131, 590–595. [Google Scholar] [CrossRef]
- Ateş Sönmezoğlu, Ö.; Balkan, A.S. Molecular and biochemical analysis of durum wheat genotypes to examine carotenoid pigment content and lipoxygenase enzyme activity. Cereal Res. Commun. 2014, 42, 218–228. [Google Scholar] [CrossRef]
- Rose, D.J.; Pike, O.A. A simple method to measure lipase activity in wheat and wheat bran as an estimation of storage quality. J. Am. Oil Chem. Soc. 2006, 83, 415–419. [Google Scholar] [CrossRef]
- Bahal, G.; Sudha, M.L.; Ramasarma, P.R. Wheat germ lipoxygenase: Its effect on dough rheology, microstructure, and bread making quality. Int. J. Food Prop. 2013, 16, 1730–1739. [Google Scholar] [CrossRef]
- Arslan, D.; Demir, K.M.; Acar, A.; Arslan, F.N. Investigation of wheat germ and oil characteristics with regard to different stabilization techniques. Food Technol. Biotech. 2020, 58, 348–355. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Syrokou, M.K.; Tziompra, S.; Psychogiou, E.E.; Mpisti, S.D.; Paramithiotis, S.; Bosnea, L.; Mataragas, M.; Skandamis, P.N.; Drosinos, E.H. Technological and safety attributes of lactic acid bacteria and yeasts isolated from spontaneously fermented greek wheat sourdoughs. Microorganisms 2021, 9, 671. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A.; Cioccia, G.; Iride, A. Lipolytic activity of Williopsis californica and Saccharomyces cerevisiae in extra virgin olive oil. Int. J. Food Microbiol. 2006, 107, 27–32. [Google Scholar] [CrossRef]
- Goicoechea, E.; Guillen, M.D. Analysis of hydroperoxides, aldehydes and epoxides by 1H nuclear magnetic resonance in sunflower oil oxidized at 70 and 100 C. J. Agric. Food Chem. 2010, 58, 6234–6245. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A Review of thermo-oxidative degradation of food lipids studied by 1H NMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Ibargoitia, M.L.; Guillén, M.D. 1H NMR study of the in vitro digestion of highly oxidized soybean oil and the effect of the presence of ovalbumin. Foods 2021, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Caño-Ochoa, S.D.; Ruiz-Aracama, A.; Guillén, M.D. Alpha-Tocopherol, a powerful molecule, leads to the formation of oxylipins in polyunsaturated oils differently to the temperature increase: A detailed study by proton nuclear magnetic resonance of walnut oil oxidation. Antioxidants 2022, 11, 604. [Google Scholar] [CrossRef]
- Brighenti, F.; Valtuena, S.; Pellegrini, N.; Ardigo, D.; Del Rio, D.; Salvatore, S.; Piatti, P.M.; Serafini, M.; Zavaroni, I. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Brit. J. Nutr. 2005, 93, 619–625. [Google Scholar] [CrossRef]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 2002, 76, 93–99. [Google Scholar] [CrossRef]
- Raczyk, M.; Polanowska, K.; Kruszewski, B.; Grygier, A.; Michałowska, D. Effect of spirulina (Arthrospira platensis) supplementation on physical and chemical properties of semolina (Triticum durum) based fresh pasta. Molecules 2022, 27, 355. [Google Scholar] [CrossRef]
- Cankurtaran, T.; Bilgiçli, N. Influence of wheat milling by-products on some physical and chemical properties of filled and unfilled fresh pasta. J. Food Sci. Technol. 2019, 56, 2845–2854. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, Z.; Hu, X.; Xing, Q.; Zhuo, W. Effect of wheat germ flour addition on wheat flour, dough and Chinese steamed bread properties. J. Cereal Sci. 2015, 64, 153–158. [Google Scholar] [CrossRef]

| Moisture | Proteins | Ashes | Lipids | pH | TTA | Color | ΔE | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | (g/100 g) | (g/100 g d.b.) | (mL NaOH) | L* | b* | a* | ||||
| CP | 24.09 ± 2.49 c | 11.82 ± 0.29 b | 0.84 ± 0.06 c | 1.17 ± 0.08 b | 6.84 ± 0.04 a | 1.43 ± 0.10 c | 66.94 ± 1.62 a | 27.24 ± 0.22 a | 1.06 ± 1.23 c | |
| RGP | 25.47 ± 1.38 b | 13.07 ± 0.37 a | 1.27 ± 0.06 b | 2.06 ± 0.16 a | 6.83 ± 0.06 a | 1.98 ± 0.19 b | 62.68 ± 1.48 c | 26.01 ± 0.38 b | 3.45 ± 1.51 b | 5.04 |
| SP | 26.32 ± 2.20 a | 13.31 ± 0.23 a | 1.31 ± 0.08 a | 2.09 ± 0.06 a | 4.82 ± 0.07 b | 7.72 ± 0.81 a | 64.56 ± 1.96 b | 25.01 ± 0.41 c | 3.63 ± 1.48 a | 4.15 |
| OCT 1 | CL 2 | SI 3 | WAI 4 | |
|---|---|---|---|---|
| (min.) | (%) | (%) | (%) | |
| CP | 4.8 ± 0.32 a | 16.11 ± 0.74 c | 2.73 ± 0.01 a | 90.22 ± 0.61 a |
| RGP | 3.9 ± 0.60 ab | 17.65 ± 0.86 b | 2.61 ± 0.03 b | 82.53 ± 4.78 b |
| SP | 3.3 ± 0.52 bc | 18.72 ± 0.94 a | 2.51 ± 0.05 c | 71.38 ± 7.84 c |
| Hardness | Adhesiveness | Springiness | Cohesiveness | Gumminess | Chewiness | |
|---|---|---|---|---|---|---|
| N | N × s | s | n/a | N | N | |
| CP | 11.82 ± 1.14 a | −0.03 ± 0.01 a | 0.91 ± 0.02 a | 0.62 ± 0.02 a | 7.21 ± 0.65 a | 6.56 ± 0.55 a |
| RGP | 11.81 ± 1.59 a | −0.03 ± 0.01 a | 0.87 ± 0.05 b | 0.63 ± 0.01 a | 7.49 ± 0.62 a | 6.51 ± 0.19 a |
| SP | 10.86 ± 0.60 b | −0.09 ± 0.04 b | 0.82 ± 0.07 c | 0.57 ± 0.02 b | 6.40 ± 0.15 b | 5.20 ± 0.51 b |
| CP | RGP | SP | |
|---|---|---|---|
| Protein Digestibility (%) * | 49.58 c | 57.67 a | 54.10 b |
| Protein Availability (g/100 pasta d.b.) | 5.88 c | 7.59 a | 7.22 b |
| Phytic acid (g/100 g d.b.) | 0.17 ± 0.01 c | 0.36 ± 0.01 a | 0.18 ± 0.01 b |
| Total dietary fiber (g/100 g d.b.) | 6.32 ± 0.12 c | 8.34 ± 0.14 b | 8.66 ± 0.04 a |
| Total Phenolic Content (mg/100 g d.b.) | 46.79 ± 1.13 c | 60.47 ± 7.27 b | 76.77 ± 5.76 a |
| Antioxidant activity (%) ** | 2.86 ± 0.83 c | 4.42 ± 0.54 b | 7.37 ± 0.72 a |
| α-TP | α–TT | β-TP | β-TT | Total | ||
|---|---|---|---|---|---|---|
| (μg/g d.b.) | (μg/g d.b.) | (μg/g d.b.) | (μg/g d.b.) | (μg/g d.b.) | ||
| Raw materials | Semolina | 10.85 ± 3.42 | 2.89 ± 0.80 | 10.14 ± 3.23 | 12.8 ± 0.98 | 36.68 ± 6.15 |
| Wholemeal semolina | 26.71 ± 2.15 | 2.00 ± 1.02 | 17.62 ± 1.62 | 18.10 ± 2.47 | 64.43 ± 9.04 | |
| GWS | 30.24 ± 3.46 | 6.00 ± 2.95 | 25.68 ± 1.01 | 15.95 ± 2.95 | 77.87 ± 8.26 | |
| Sourdough | 28.52 ± 5.70 | 2.63 ± 0.53 | 29.97 ± 5.09 | 16.38 ± 3.03 | 77.50 ± 12.35 | |
| Raw pasta | CP | 7.96 ± 1.32 b | 0.36 ± 0.05 b | 6.88 ± 1.11 c | 6.93 ± 1.03 b | 22.13 ± 2.63 b |
| RGP | 27.69 ± 4.44 a | 0.73 ± 0.15 a | 17.32 ± 0.34 b | 8.79 ± 0.61 a | 54.53 ± 4.31 a | |
| SP | 23.34 ± 2.58 a | 0.73 ± 0.18 a | 23.83 ± 3.36 a | 7.97 ± 0.84 a | 55.86 ± 5.03 a | |
| Cooked pasta | CP | 4.02 ± 1.72 a | 0.38 ± 0.09 a | 5.67 ± 0.79 b | 6.16 ± 1.24 a | 16.23 ± 2.52 c |
| RGP | 4.36 ± 1.78 a | 0.40 ± 0.12 a | 8.42 ± 1.81 b | 5.90 ± 1.63 a | 19.09 ± 5.10 b | |
| SP | 6.31 ± 1.36 a | 0.20 ± 0.07 b | 22.58 ± 3.26 a | 7.08 ± 0.96 a | 36.17 ± 5.53 a |
| Lipase (%) | Lipoxygenase (%) | |
|---|---|---|
| GWS | 100 a | 100 b |
| S | 31.00 ± 6.08 | 59.11 ± 10.93 |
| CP | 0 | 20.04 ± 3.57 |
| RGP | 0 | 27.13 ± 4.14 |
| SP | 18.00 ± 3.65 | 29.88 ± 5.57 |
| Samples | Days of Storage | Appearance | Smell | Taste | Texture | Overall Liking |
|---|---|---|---|---|---|---|
| CP | 0 | 7.70 ± 1.18 aA | 7.11 ± 1.03 aA | 7.13 ± 1.05 aA | 7.00 ± 1.09 aA | 6.88 ± 1.03 aA |
| RGP | 0 | 6.76 ± 1.45 bA | 6.25 ± 0.93 bA | 6.09 ± 1.78 bA | 6.43 ± 1.31 bA | 6.60 ± 1.28 aA |
| SP | 0 | 7.70 ± 1.18 aA | 7.09 ± 1.02 aA | 7.03 ± 1.14 aA | 7.01 ± 1.10 aA | 7.00 ± 1.09 aA |
| CP | 30 | 7.11 ± 0.88 aA | 6.62 ± 0.72 aB | 6.62 ± 0.69 aA | 6.58 ± 0.63 aA | 6.66 ± 0.75 aA |
| RGP | 30 | 6.17 ± 1.43 bAB | 5.96 ± 0.89 bAB | 5.62 ± 1.62 bAB | 6.01 ± 1.15 bAB | 5.60 ± 1.29 bB |
| SP | 30 | 6.98 ± 0.94 aB | 6.54 ± 0.70 aB | 6.49 ± 0.88 aA | 6.45 ± 0.67 bB | 6.56 ± 0.76 aA |
| CP | 60 | 7.64 ± 1.03 aA | 6.96 ± 0.87 aAB | 6.96 ± 0.89 aA | 6.94 ± 0.94 aA | 6.72 ± 0.98 aA |
| RGP | 60 | 5.82 ± 1.24 bB | 5.58 ± 1.05 bB | 5.27 ± 1.47 bB | 5.72 ± 0.96 bB | 5.58 ± 1.11 bB |
| SP | 60 | 7.31 ± 1.14 aAB | 6.88 ± 0.86 aAB | 6.76 ± 1.06 aA | 6.76 ± 0.99 aAB | 6.70 ± 0.83 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catzeddu, P.; Fois, S.; Tolu, V.; Sanna, M.; Braca, A.; Vitangeli, I.; Anedda, R.; Roggio, T. Quality Evaluation of Fresh Pasta Fortified with Sourdough Containing Wheat Germ and Wholemeal Semolina. Foods 2023, 12, 2641. https://doi.org/10.3390/foods12142641
Catzeddu P, Fois S, Tolu V, Sanna M, Braca A, Vitangeli I, Anedda R, Roggio T. Quality Evaluation of Fresh Pasta Fortified with Sourdough Containing Wheat Germ and Wholemeal Semolina. Foods. 2023; 12(14):2641. https://doi.org/10.3390/foods12142641
Chicago/Turabian StyleCatzeddu, Pasquale, Simonetta Fois, Valentina Tolu, Manuela Sanna, Angela Braca, Ilaria Vitangeli, Roberto Anedda, and Tonina Roggio. 2023. "Quality Evaluation of Fresh Pasta Fortified with Sourdough Containing Wheat Germ and Wholemeal Semolina" Foods 12, no. 14: 2641. https://doi.org/10.3390/foods12142641
APA StyleCatzeddu, P., Fois, S., Tolu, V., Sanna, M., Braca, A., Vitangeli, I., Anedda, R., & Roggio, T. (2023). Quality Evaluation of Fresh Pasta Fortified with Sourdough Containing Wheat Germ and Wholemeal Semolina. Foods, 12(14), 2641. https://doi.org/10.3390/foods12142641