Dyes Used in Processed Meat Products in the Polish Market, and Their Possible Risks and Benefits for Consumer Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
- Score = 0 (no risk or benefits): the papers indicate that the dye has no genotoxicity or carcinogenicity, acute or chronic toxicity or hypersensitisation potential but also has no benefit to human health
- Score = 1 (occurrences of risk or benefits): the papers indicate genotoxicity, carcinogenicity, acute or chronic toxicity, or hypersensitisation potential or benefit for human health concerning a particular dye.
3. Results
3.1. Incidence
3.2. The Analysis of Label Incompliance
3.3. An Analysis of Dye Presence Prediction in Processed Meat Based on Product Qualities
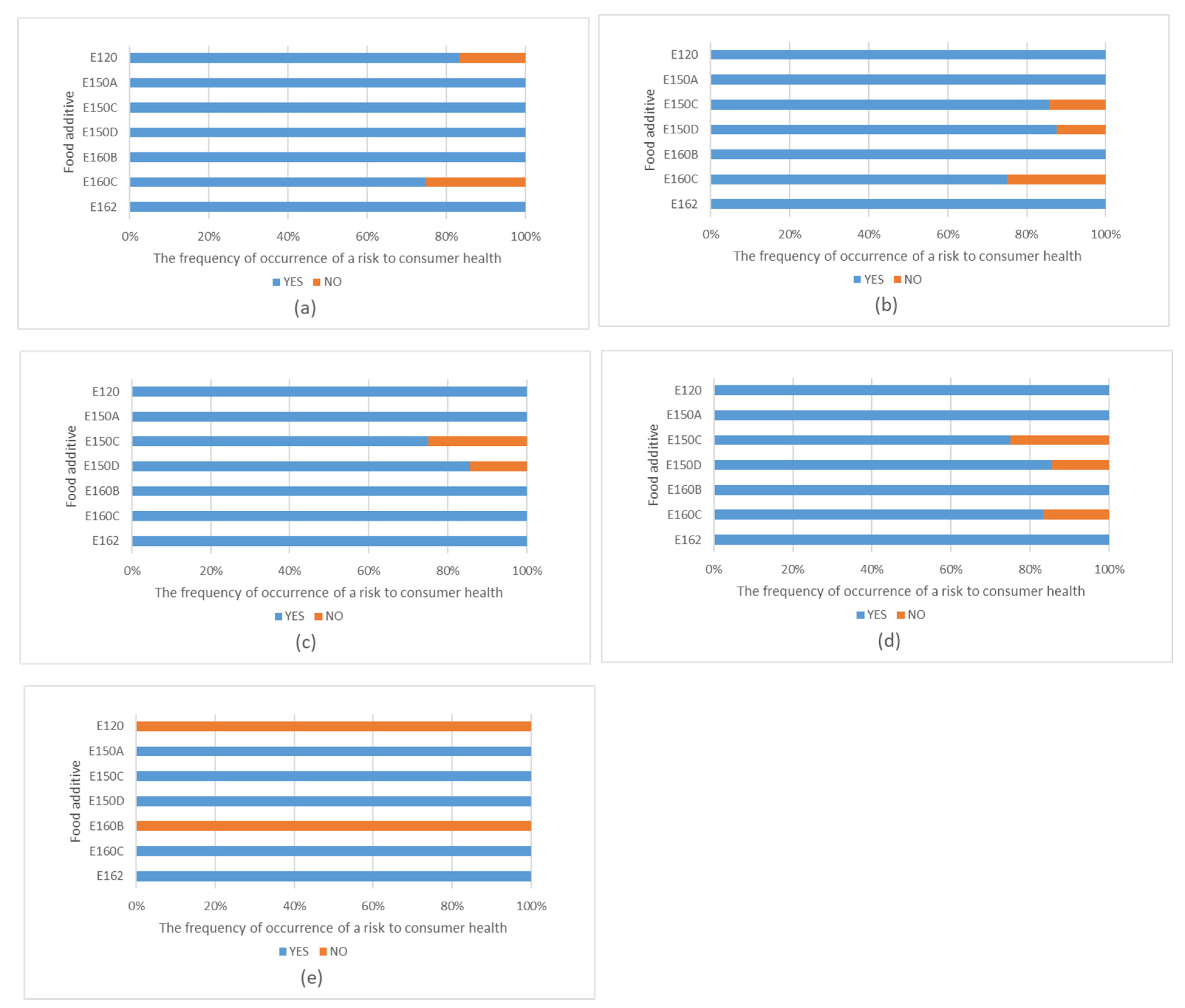
3.4. Evaluations of Risks and Benefits for Consumer Health
4. Discussion
4.1. Carmine, Carminic Acid, Cochineal Extract—E120
4.2. Paprika Extract (E160c), Capsicum Extract, Capsanthin and Capsorubin
4.3. Betanin, Beetroot Red—E162
4.4. Caramel Colours 150a, 150c and 150d
4.5. Annatto, Bixin, Norbixin—E160b
4.6. Assessment of the Frequency and Correctness of the Use of Dyes in Processed Meat Products
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koch, C.; Koch, E.C. Preconceptions of taste based on color. J. Psychol. 2003, 137, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wadhera, D.; Capaldi-Phillips, E.D. A review of visual cues associated with food on food acceptance and consumption. Eat. Behav. 2014, 15, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food Colour Additives: A Synoptical Overview on Their Chemical Properties, Applications in Food Products, and Health Side Effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Spence, C.; Wan, X.; Woods, A.; Velasco, C.; Deng, J.; Youssef, J.; Deroy, O. On tasty colours and colourful tastes? Assessing, explaining and utilizing crossmodal correspondences between colours and basic tastes. Flavour 2015, 4, 23. [Google Scholar] [CrossRef]
- Spence, C.; Levitan, C.A.; Shankar, M.U.; Zampini, M. Does Food Color Influence Taste and Flavor Perception in Humans? Chemosens. Percept. 2010, 3, 68–84. [Google Scholar] [CrossRef]
- EC. Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002. Laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Union 2002, L31, 1–24. [Google Scholar]
- EC. Regulation (EC) No. 1333/2008 of 16 December 2008. On Food Additives. Off. J. Eur. Union 2008, L354, 16–33. [Google Scholar]
- Guidance (EC) Guidance Document Describing the Food Categories in Part E of Annex II to Regulation (EC) No 1333/2008 on Food Additives. Available online: https://food.ec.europa.eu (accessed on 10 December 2022).
- EC. Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004. Laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, L139, 55–205. [Google Scholar]
- CFR. Color Additives. 21 CFR Part 70. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-70 (accessed on 10 December 2022).
- CFR. Listing of Color Additives Exempt from Certification. 21 CFR Part 73. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-73 (accessed on 10 December 2022).
- PKN. Polska Norma PN-A-82007; PKN: Warsaw, Poland, 1996; pp. 1–12. [Google Scholar]
- MF. Dane z Zeznań Podatkowych Podatników, o Których Mowa w Art. 27b Ustawy z Dnia 15 Lutego 1992 r. o Podatku Dochodowym od Osób Prawnych (Dz. U. z 2017 r. poz. 2343, ze zm.) Oraz w Ustawie z 24 Listopada 2017 r. o Zmianie Ustawy o Podatku Dochodowym od Osób Prawnych (Dz. U. poz. 2369). Available online: https://gov.pl (accessed on 16 June 2019).
- GUS. Demographic Yearbook of Poland 2018. Available online: https://stat.gov.pl (accessed on 17 June 2019).
- Dobson, A.J.; Adrian, G.B. An Introduction to Generalized Linear Models, 4th ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- EU. Commission Regulation (EU) No. 231/2012 of 22 March 2012. Laying Down Specifications for Food Additives Listed in Annexes II and III to Regulation (EC) No. 1333/2008 of the European Parliament and of the Council. J. Eur. Union 2012, L83, 1–294. [Google Scholar]
- FAO. Cochineal Extract. Monograph 1. Prepared at the 55th JECFA 2000. Available online: https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-137.pdf (accessed on 4 December 2022).
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of cochineal, carminic acid, carmines (E120) as a food additive. EFSA J. 2015, 13, 4288. [Google Scholar]
- Cooksey, C.J. The red insect dyes: Carminic, kermesic and laccaic acids and their derivatives. Biotech. Histochem. 2019, 94, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Merinas-Amo, R.; Martínez-Jurado, M.; Jurado-Güeto, S.; Alonso-Moraga, Á.; Merinas-Amo, T. Biological Effects of Food Coloring in In Vivo and In Vitro Model Systems. Foods 2019, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, R.; Selvi, M.; Erkoç, F. Evaluation of potential genotoxicity of five food dyes using the somatic mutation and recombination test. Chemosphere 2012, 88, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, Q.; Tan, J.; Hu, L.; Ge, C.; Xu, M. Carminic acid supplementation protects against fructose-induced kidney injury mainly through suppressing inflammation and oxidative stress via improving Nrf-2 signaling. Aging 2021, 13, 10326–10353. [Google Scholar] [CrossRef]
- Arif, A.; Ahmad, A.; Ahmad, M. Toxicity assessment of carmine and its interaction with calf thymus DNA. J. Biomol. Struct. Dyn. 2021, 39, 5861–5871. [Google Scholar] [CrossRef]
- Lucas, C.D.; Hallagan, J.B.; Taylor, S.L. The role of natural color additives in food allergy. Adv. Food Nutr. Res. 2001, 43, 195–216. [Google Scholar] [PubMed]
- Andreozzi, L.; Giannetti, A.; Cipriani, F.; Caffarelli, C.; Mastrorilli, C.; Ricci, G. Hypersensitivity reactions to food and drug additives: Problem or myth? Acta Biomed. 2019, 90, 80–90. [Google Scholar]
- Chung, K.; Baker, J.R., Jr.; Baldwin, J.L.; Chou, A. Identification of carmine allergens among three carmine allergy patients. Allergy 2001, 56, 73–77. [Google Scholar] [CrossRef]
- Lemoine, A.; Pauliat-Desbordes, S.; Challier, P.; Tounian, P. Adverse reactions to food additives in children: A retrospective study and a prospective survey. Arch. Pediatr. 2020, 27, 368–371. [Google Scholar] [CrossRef]
- Beaudouin, E.; Kanny, G.; Lambert, H.; Fremont, S.; Moneret-Vautrin, D.A. Food anaphylaxis following ingestion of carmine. Ann. Allergy Asthma Immunol. 1995, 74, 427–430. [Google Scholar]
- Greenhawt, M.J.; Baldwin, J.L. Carmine dye and cochineal extract: Hidden allergens no more. Ann. Allergy Asthma Immunol. 2009, 103, 73–75. [Google Scholar] [CrossRef]
- Kägi, M.K.; Wüthrich, B.; Johansson, S.G. Campari-Orange anaphylaxis due to carmine allergy. Lancet 1994, 344, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Takeo, N.; Nakamura, M.; Nakayama, S.; Okamoto, O.; Sugimoto, N.; Sugiura, S.; Sato, N.; Harada, S.; Yamaguchi, M.; Mitsui, N.; et al. Cochineal dye-induced immediate allergy: Review of Japanese cases and proposed new diagnostic chart. Allergol. Int. 2018, 67, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, B.; Kägi, M.K.; Stücker, W. Anaphylactic reactions to ingested carmine (E120). Allergy 1997, 52, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Oosuna, H.; Yamakawa, T.; Aihara, M.; Ikezawa, Z. Cochineal extract-induced immediate allergy. J. Dermatol. 2009, 36, 72–74. [Google Scholar] [CrossRef]
- Gultekin, F.; Doguc, D.K. Allergic and immunologic reactions to food additives. Clin. Rev. Allergy Immunol. 2013, 45, 6–29. [Google Scholar] [CrossRef]
- Añíbarro, B.; Seoane, J.; Vila, C.; Múgica, V.; Lombardero, M. Occupational asthma induced by inhaled carmine among butchers. Int. J. Occup. Med. Environ. Health 2003, 16, 133–137. [Google Scholar]
- DiCello, M.C.; Myc, A.; Baker, J.R., Jr.; Baldwin, J.L. Anaphylaxis after ingestion of carmine colored foods: Two case reports and a review of the literature. Allergy Asthma Proc. 1999, 20, 377–382. [Google Scholar] [CrossRef]
- Ohgiya, Y.; Arakawa, F.; Akiyama, H.; Yoshioka, Y.; Hayashi, Y.; Sakai, S.; Ito, S.; Yamakawa, Y.; Ohgiya, S.; Ikezawa, Z.; et al. Molecular cloning, expression, and characterization of a major 38-kd cochineal allergen. J. Allergy Clin. Immunol. 2009, 123, 1157–1162. [Google Scholar] [CrossRef]
- FAO. Paprika Extract. Monograph 14. Prepared at the 77th JECFA 2013. Available online: https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/monograph16/additive-510-m16.pdf (accessed on 14 September 2022).
- Scientific Opinion. On the re-evaluation of paprika extract (E160c) as a food additive. In: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA J. 2017, 13, 4320. [Google Scholar]
- Akagi, A.; Sano, N.; Uehara, H.; Minami, T.; Otsuka, H.; Izumi, K. Non-carcinogenicity of capsaicinoids in B6C3F1 mice. Food Chem. Toxicol. 1998, 36, 1065–1071. [Google Scholar] [CrossRef]
- Inoue, T.; Umemura, T.; Maeda, M.; Ishii, Y.; Okamura, T.; Tasaki, M.; Nishikawa, A. Safety assessment of dietary administered paprika color in combined chronic toxicity and carcinogenicity studies using F344 rats. Food Chem. Toxicol. 2008, 46, 2689–2693. [Google Scholar] [CrossRef]
- Kanki, K.; Nishikawa, A.; Furukawa, F.; Kitamura, Y.; Imazawa, T.; Umemura, T.; Hirose, M. A 13-week subchronic toxicity study of paprika color in F344 rats. Food Chem. Toxicol. 2003, 41, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Bley, K.; Boorman, G.; Mohammad, B.; McKenzie, D.; Babbar, S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol. Pathol. 2012, 40, 847–873. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Erexson, G.; Riach, C.; Innes, D.; Stevenson, F.; Murli, H.; Bley, K. Genotoxicity studies with pure trans-capsaicin. Mutat. Res. 2004, 557, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Díaz Barriga Arceo, S.; Madrigal-Bujaidar, E.; Calderón Montellano, E.; Ramírez Herrera, L.; Díaz García, B.D. Genotoxic effects produced by capsaicin in mouse during subchronic treatment. Mutat. Res. 1995, 345, 105–109. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef]
- Baskaran, P.; Markert, L.; Bennis, J.; Zimmerman, L.; Fox, J.; Thyagarajan, B. Assessment of Pharmacology, Safety, and Metabolic activity of Capsaicin Feeding in Mice. Sci. Rep. 2019, 9, 8588. [Google Scholar] [CrossRef]
- Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef]
- Cho, S.C.; Lee, H.; Choi, B.Y. An updated review on molecular mechanisms underlying the anticancer effects of capsaicin. Food Sci. Biotechnol. 2017, 26, 1–13. [Google Scholar] [CrossRef]
- Chung, Y.C.; Baek, J.Y.; Kim, S.R.; Ko, H.W.; Bok, E.; Shin, W.H.; Won, S.Y.; Jin, B.K. Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2017, 49, 298. [Google Scholar] [CrossRef] [PubMed]
- Höper, J.; Helfert, S.; Heskamp, M.L.; Maihöfner, C.G.; Baron, R. High concentration capsaicin for treatment of peripheral neuropathic pain: Effect on somatosensory symptoms and identification of treatment responders. Curr. Med. Res. Opin. 2014, 30, 565–574. [Google Scholar] [CrossRef]
- Kim, C.S.; Kawada, T.; Kim, B.S.; Han, I.S.; Choe, S.Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell. Signal. 2003, 15, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Bázan-Lugo, E.; García-Martínez, I.; Alfaro-Rodríguez, R.H.; Totosaus, A. Color compensation in nitrite-reduced meat batters incorporating paprika or tomato paste. J. Sci. Food Agric. 2012, 92, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Chin, K.B. Characteristics of low-nitrite pork emulsified-sausages with paprika oleoresin solution during refrigerated storage. J. Anim. Sci. Technol. 2021, 63, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion. On the re-evaluation of beetroot red (E 162) as a food additive. In: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA J. 2015, 13, 4318. [Google Scholar]
- FAO. Beet Red. Monograph. Prepared at the 31st JECFA 1987. Available online: https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-052.pdf (accessed on 14 September 2022).
- Haveland-Smith, R.B. Evaluation of the genotoxicity of some natural food colours using bacterial assays. Mutat. Res. 1981, 91, 285–290. [Google Scholar] [CrossRef]
- Reynoso, R.C.; Giner, T.V.; de Mejia, E.G. Safety of a filtrate of fermented garambullo fruit: Biotransformation and toxicity studies. Food Chem. Toxicol. 1999, 37, 825–830. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- von Elbe, J.H.; Schwartz, S.J. Absence of mutagenic activity and a short-term toxicity study of beet pigments as food colorants. Arch. Toxicol. 1981, 49, 93–98. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef]
- Gliszczyńska-Swigło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef]
- Khan, M.I. Plant Betalains: Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.F.; Stoner, G.D. Red Beetroot and Betalains as Cancer Chemopreventative Agents. Molecules 2019, 24, 1602. [Google Scholar] [CrossRef]
- Zielińska-Przyjemska, M.; Olejnik, A.; Dobrowolska-Zachwieja, A.; Grajek, W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytother. Res. 2009, 23, 49–55. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, X.; Tian, Z.; Ma, Y.; Sun, C. Betalain exerts cardioprotective and anti-inflammatory effects against the experimental model of heart failure. Hum. Exp. Toxicol. 2021, 40, 16–28. [Google Scholar] [CrossRef]
- Vieira Teixeira da Silva, D.; Dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.; Paschoalin, V.M.F. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef]
- FAO. Caramel Colours. Monograph 11. Prepared at the 74th JECFA 2011. Available online: https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/monograph11/additive-329-m11.pdf (accessed on 14 September 2022).
- Scientific Opinion. On the re-evaluation of caramel colours (E 150 a,b,c,d) as food additives. In: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA J. 2011, 9, 2004. [Google Scholar]
- Adams, K.; Allen, J.A.; Brooker, P.C.; Jones, E.; Proudlock, R.J. Assessment of the genotoxic potential of Caramel Colour I in four short-term tests. Food Chem. Toxicol. 1992, 30, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.A.; Brooker, P.C.; Jones, E.; Adams, K.; Richold, M. Absence of mutagenic activity in Salmonella and of clastogenic activity in CHO cells of Caramel Colours I, II, III and IV. Food Chem. Toxicol. 1992, 30, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Brusick, D.J.; Jagannath, D.R.; Galloway, S.M.; Nestmann, E.R. Genotoxicity hazard assessment of Caramel Colours III and IV. Food Chem. Toxicol. 1992, 30, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Houben, G.F.; Penninks, A.H. Immunotoxicity of the colour additive caramel colour III; a review on complicated issues in the safety evaluation of a food additive. Toxicology 1994, 91, 289–302. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and carcinogenesis studies of 4-methylimidazole (Cas No. 822-36-6) in F344/N rats and B6C3F1 mice (feed studies). Natl. Toxicol. Program Tech. Rep. Ser. 2007, 535, 1–274. [Google Scholar]
- Sengar, G.; Sharma, H.K. Food caramels: A review. J. Food Sci. Technol. 2014, 51, 1686–1696. [Google Scholar] [CrossRef]
- Vollmuth, T.A. Caramel color safety—An update. Food Chem. Toxicol. 2018, 111, 578–596. [Google Scholar] [CrossRef]
- Houben, G.F.; Abma, P.M.; van den Berg, H.; van Dokkum, W.; van Loveren, H.; Penninks, A.H.; Seinen, W.; Spanhaak, S.; Vos, J.G.; Ockhuizen, T. Effects of the colour additive caramel colour III on the immune system: A study with human volunteers. Food Chem. Toxicol. 1992, 30, 749–757. [Google Scholar] [CrossRef]
- Hengel, M.; Shibamoto, T. Carcinogenic 4(5)-methylimidazole found in beverages, sauces, and caramel colors: Chemical properties, analysis, and biological activities. J. Agric. Food Chem. 2013, 61, 780–789. [Google Scholar] [CrossRef]
- Jacobson, M.F. Carcinogenicity and regulation of caramel colorings. Int. J. Occup. Med. Environ. Health 2012, 18, 254–259. [Google Scholar] [CrossRef]
- Liang, J.; Cao, P.; Wang, X.; Gao, P.; Xu, H.; Ma, N. Dietary intake assessment of caramel colours and their processing by-products 4-methylimidazole and 2-acetyl-4-tetrahydroxy-butylimidazole for the Chinese population. Food Addit. Contam. 2019, 36, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G.; Rosati, C.; Bramley, P.M. To dye or not to dye: Biochemistry of annatto unveiled. Trends Biotechnol. 2003, 21, 513–516. [Google Scholar] [CrossRef] [PubMed]
- FAO. Annatto Extract (Solvent-Extracted Bixin). Monograph 17. Prepared at the 80th JECFA 2015. Available online: https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/monograph17/additive-040-m17.pdf (accessed on 14 September 2022).
- Younes, M.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; Mennes, W.; et al. Safety of annatto E and the exposure to the annatto colouring principles bixin and norbixin (E 160b) when used as a food additive. In: EFSA Panel on Food Additives and Flavourings (FAF). EFSA J. 2019, 17, 5626. [Google Scholar]
- EFSA; Tard, A. Exposure assessment of annatto colouring principles bixin and norbixin (E 160b) when used as food additives. EFSA J. 2017, 15, 4966. [Google Scholar]
- Agner, A.R.; Barbisan, L.F.; Scolastici, C.; Salvadori, D.M. Absence of carcinogenic and anticarcinogenic effects of annatto in the rat liver medium-term assay. Food Chem. Toxicol. 2004, 42, 1687–1693. [Google Scholar] [CrossRef]
- Bautista, A.R.; Moreira, E.L.; Batista, M.S.; Miranda, M.S.; Gomes, I.C. Subacute toxicity assessment of annatto in rat. Food Chem. Toxicol. 2004, 42, 625–629. [Google Scholar] [CrossRef]
- Júnior, A.C.; Asad, L.M.; Oliveira, E.B.; Kovary, K.; Asad, N.R.; Felzenszwalb, I. Antigenotoxic and antimutagenic potential of an annatto pigment (norbixin) against oxidative stress. Genet. Mol. Res. 2005, 4, 94–99. [Google Scholar]
- Paumgartten, F.J.; De-Carvalho, R.R.; Araujo, I.B.; Pinto, F.M.; Borges, O.O.; Souza, C.A.; Kuriyama, S.N. Evaluation of the developmental toxicity of annatto in the rat. Food Chem. Toxicol. 2002, 40, 1595–1601. [Google Scholar] [CrossRef]
- Scientific Opinion. On the safety of annatto extracts (E 160b) as a food additive. In: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA J. 2016, 14, 4544. [Google Scholar]
- Myles, I.A.; Beakes, D. An Allergy to Goldfish? Highlighting the Labeling Laws for Food Additives. World Allergy Organ. J. 2009, 2, 314–316. [Google Scholar] [CrossRef]
- Ramsey, N.B.; Tuano, K.T.; Davis, C.M.; Dillard, K.; Hanson, C. Annatto seed hypersensitivity in a pediatric patient. Ann. Allergy Asthma Immunol. 2016, 117, 331–333. [Google Scholar] [CrossRef]
- Sadowska, B.; Sztormowska, M.; Chełmińska, M. Annatto hypersensitivity after oral ingestion confirmed by placebo-controlled oral challenge. Ann. Allergy Asthma Immunol. 2021, 127, 510–511. [Google Scholar] [CrossRef]
- Ebo, D.G.; Ingelbrecht, S.; Bridts, C.H.; Stevens, W.J. Allergy for cheese: Evidence for an IgE-mediated reaction from the natural dye annatto. Allergy 2009, 64, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Nish, W.A.; Whisman, B.A.; Goetz, D.W.; Ramirez, D.A. Anaphylaxis to annatto dye: A case report. Ann. Allergy 1991, 66, 129–131. [Google Scholar] [PubMed]
- Randhawa, S.; Bahna, S.L. Hypersensitivity reactions to food additives. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.L. Annatto and IBS. J. Clin. Gastroenterol. 2009, 43, 1014–1015. [Google Scholar] [CrossRef]
- Beni, A.A.; Rodrigues, R.F.; Conte, L.; Costa, I.F.; Delalibera, É.A.; Roehrs, M.; Rampelotto, C.; Emanuelli, T.; Somacal, S. Dietary supplementation with annatto food-coloring extracts increases the resistance of human erythrocytes to hemolysis. Nutr. Res. 2020, 76, 71–81. [Google Scholar] [CrossRef]
- Cuong, T.V.; Chin, K.B. Effects of Annatto (Bixa orellana L.) Seeds Powder on Physicochemical Properties, Antioxidant and Antimicrobial Activities of Pork Patties during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2016, 36, 476–486. [Google Scholar] [CrossRef]
- Zarringhalami, S.; Sahari, M.A.; Hamidi-Esfehani, Z. Partial replacement of nitrite by annatto as a colour additive in sausage. Meat Sci. 2009, 81, 281–284. [Google Scholar] [CrossRef]
- Halagarda, M.; Wójciak, K.M. Health and safety aspects of traditional European meat products. A review. Meat Sci. 2022, 184, 108623. [Google Scholar] [CrossRef]
- Halagarda, M.; Kędzior, W.; Pyrzyńska, E. Nutritional Value and Potential Chemical Food Safety Hazards of Selected Traditional and Conventional Pork Hams from Poland. J. Food Qual. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Halagarda, M.; Kędzior, W.; Pyrzyńska, E. Nutritional value and potential chemical food safety hazards of selected Polish sausages as influenced by their traditionality. Meat Sci. 2018, 139, 25–34. [Google Scholar] [CrossRef]
- Crehan, C.M.; Hughes, E.; Troy, D.J.; Buckley, D.J. Effects of fat level and maltodextrin on the functional properties of frankfurters formulated with 5, 12 and 30% fat. Meat Sci. 2000, 55, 463–469. [Google Scholar] [CrossRef]
- Hughes, E.; Cofrades, S.; Troy, D.J. Effects of fat level, oat fibre and carrageenan on frankfurters formulated with 5, 12 and 30% fat. Meat Sci. 1997, 45, 273–281. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Effects of protein level and fat/oil on emulsion stability, texture, microstructure and color of meat batters. Meat Sci. 2009, 82, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Sarıçoban, C.; Yılmaz, M.T. Modelling the Effects of Processing Factors on the Changes in Colour Parameters of Cooked Meatballs Using Response Surface Methodology. World Appl. Sci. J. 2010, 9, 14–22. [Google Scholar]
- Słowiński, M.; Miazek, J.; Dasiewicz, K.; Chmiel, M. The Effect of the Addition of Fiber Preparations on the Color of Medium-Grounded Pasteurized and Sterilized Model Canned Meat Products. Molecules 2021, 26, 2247. [Google Scholar] [CrossRef]
- Barbut, S.; Wood, J.; Marangoni, A. Potential use of organogels to replace animal fat in comminuted meat products. Meat Sci. 2016, 122, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans fatty acids and lipid profile: A serious risk factor to cardiovascular disease, cancer and diabetes. Diabetes Metab. Syndr. 2019, 13, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Babji, A.S.; Nuri, M.N.; Suherman, J.; Seri Chempaka, M.Y. Quality assessment of local and franchise beef and chicken burgers. Pertanika J. Trop. Agric. 2000, 23, 103–112. [Google Scholar]
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Wideman, N.; O’bryan, C.A.; Crandall, P.G. Factors affecting poultry meat colour and consumer preferences-A review. Worlds Poult. Sci. J. 2016, 72, 353–366. [Google Scholar] [CrossRef]
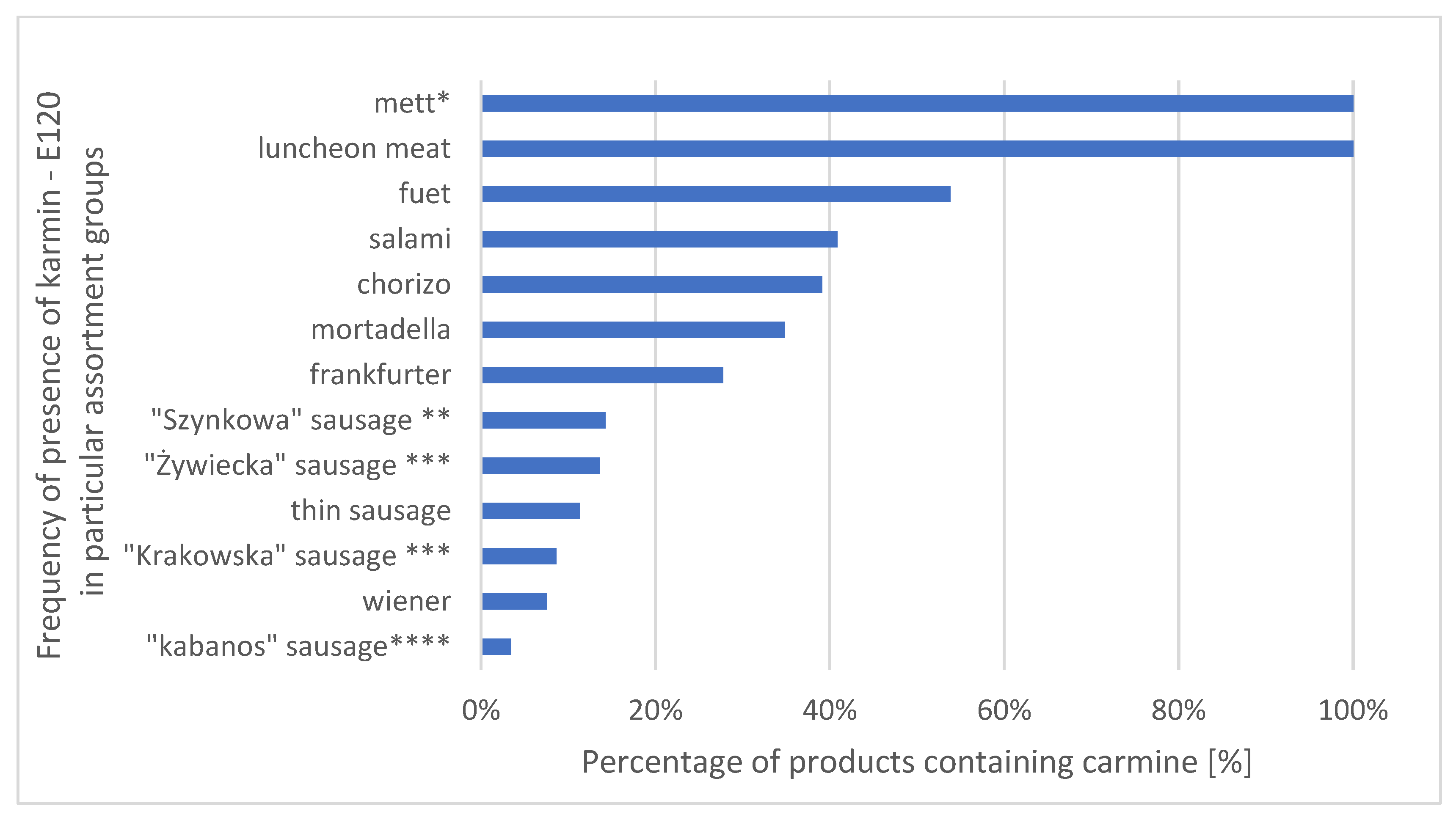


| Assortment Group | Food Colouring (Number of Examples) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E100 | E101 | E120 | E150A | E150C | E150D | E153 | E160A | E160B | E160C | E162 | E171 | |
| smoked meats | 0 | 1 | 3 | 2 | 2 | 8 | 0 | 0 | 0 | 1 | 0 | 1 |
| sausages | 0 | 1 | 164 | 3 | 7 | 5 | 0 | 1 | 8 | 31 | 25 | 0 |
| offal meats, including pâtés | 2 | 2 | 2 | 6 | 1 | 5 | 0 | 0 | 0 | 6 | 2 | 0 |
| other meat products | 1 | 0 | 14 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| meat preparations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| all products | 3 | 4 | 183 | 11 | 10 | 18 | 1 | 1 | 8 | 39 | 29 | 1 |
| Assortment Group | Food Colouring | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E100 | E101 | E120 | E150A | E150C | E150D | E153 | E160A | E160B | E160C | E162 | E171 | |
| smoked meats | 0% | 100% | 33% | 50% | 50% | 63% | 0% | 0% | 0% | 100% | 0% | 100% |
| sausages | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| offal meats, including pâtés | 0% | 0% | 0% | 0% | 100% | 0% | 0% | 0% | 0% | 0% | 50% | 0% |
| other meat products | 0% | 0% | 43% | 0% | 0% | 0% | 100% | 0% | 0% | 0% | 0% | 0% |
| meat preparations | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| all products | 0% | 25% | 4% | 9% | 20% | 28% | 100% | 0% | 0% | 3% | 3% | 100% |
| Source | B | SE | Wald Chi2 | p | Exp(B) | Lower CI | Upper CI |
|---|---|---|---|---|---|---|---|
| Intercept | −2.413 | 0.2748 | 77.117 | 0.000 | 0.090 | 0.052 | 0.153 |
| PRODUCT (sausage) | 1.901 | 0.2636 | 51.995 | <0.001 | 6.690 | 3.991 | 11.215 |
| PRODUCT (other meat products) | 1.068 | 0.3787 | 7.955 | 0.005 | 2.910 | 1.385 | 6.112 |
| PRODUCT (meat preparations) | 0.702 | 0.7899 | 0.790 | 0.374 | 2.018 | 0.429 | 9.490 |
| PRODUCT (offal meat) | 0.409 | 0.3853 | 1.125 | 0.289 | 1.505 | 0.707 | 3.203 |
| PRODUCT (smoked meats) | 0* | ||||||
| WATER (absent) | −0.591 | 0.1746 | 11.462 | <0.001 | 0.554 | 0.393 | 0.780 |
| WATER (present) | 0* | ||||||
| FAVOURS (absent) | −0.494 | 0.1555 | 10.082 | 0.001 | 0.610 | 0.450 | 0.828 |
| FAVOURS (present) | 0* | ||||||
| FAT | 0.031 | 0.0063 | 25.184 | <0.001 | 1.032 | 1.019 | 1.045 |
| CARBOHYDRATES | 0.092 | 0.0282 | 10.568 | 0.001 | 1.096 | 1.037 | 1.158 |
| PROTEIN | −0.070 | 0.0118 | 35.571 | <0.001 | 0.932 | 0.911 | 0.954 |
| Source | B | SE | Wald Chi2 | p | Exp(B) | Lower CI | Upper CI |
|---|---|---|---|---|---|---|---|
| Intercept | −0.534 | 0.2026 | 6.959 | 0.008 | 0.586 | 0.394 | 0.872 |
| WATER (absent) | −0.561 | 0.1967 | 8.119 | 0.004 | 0.571 | 0.388 | 0.839 |
| WATER (present) | 0* | ||||||
| FAVOURS (absent) | −0.466 | 0.1825 | 6.510 | 0.011 | 0.628 | 0.439 | 0.898 |
| FAVOURS (present) | 0* | ||||||
| FAT | 0.058 | 0.0106 | 30.192 | <0.001 | 1.060 | 1.038 | 1.082 |
| CARBOHYDRES | 0.093 | 0.0367 | 6.395 | 0.011 | 1.097 | 1.021 | 1.179 |
| PROTEIN | −0.111 | 0.0182 | 37.128 | <0.001 | 0.895 | 0.863 | 0.927 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czech-Załubska, K.; Klich, D.; Jackowska-Tracz, A.; Didkowska, A.; Bogdan, J.; Anusz, K. Dyes Used in Processed Meat Products in the Polish Market, and Their Possible Risks and Benefits for Consumer Health. Foods 2023, 12, 2610. https://doi.org/10.3390/foods12132610
Czech-Załubska K, Klich D, Jackowska-Tracz A, Didkowska A, Bogdan J, Anusz K. Dyes Used in Processed Meat Products in the Polish Market, and Their Possible Risks and Benefits for Consumer Health. Foods. 2023; 12(13):2610. https://doi.org/10.3390/foods12132610
Chicago/Turabian StyleCzech-Załubska, Katarzyna, Daniel Klich, Agnieszka Jackowska-Tracz, Anna Didkowska, Janusz Bogdan, and Krzysztof Anusz. 2023. "Dyes Used in Processed Meat Products in the Polish Market, and Their Possible Risks and Benefits for Consumer Health" Foods 12, no. 13: 2610. https://doi.org/10.3390/foods12132610
APA StyleCzech-Załubska, K., Klich, D., Jackowska-Tracz, A., Didkowska, A., Bogdan, J., & Anusz, K. (2023). Dyes Used in Processed Meat Products in the Polish Market, and Their Possible Risks and Benefits for Consumer Health. Foods, 12(13), 2610. https://doi.org/10.3390/foods12132610






