Inhibition of Salmonella Enteritidis by Essential Oil Components and the Effect of Storage on the Quality of Chicken
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Minimum Inhibitory Concentration(MIC) Determinations
2.3. Sample Preparation
2.4. Microbial Indicators Determinations
2.5. Antimicrobial Effects
2.6. Physical and Chemical Indexes Determinations
2.6.1. pH
2.6.2. Moisture Content
2.6.3. TVB-N
2.6.4. Color
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results
3.1. MIC
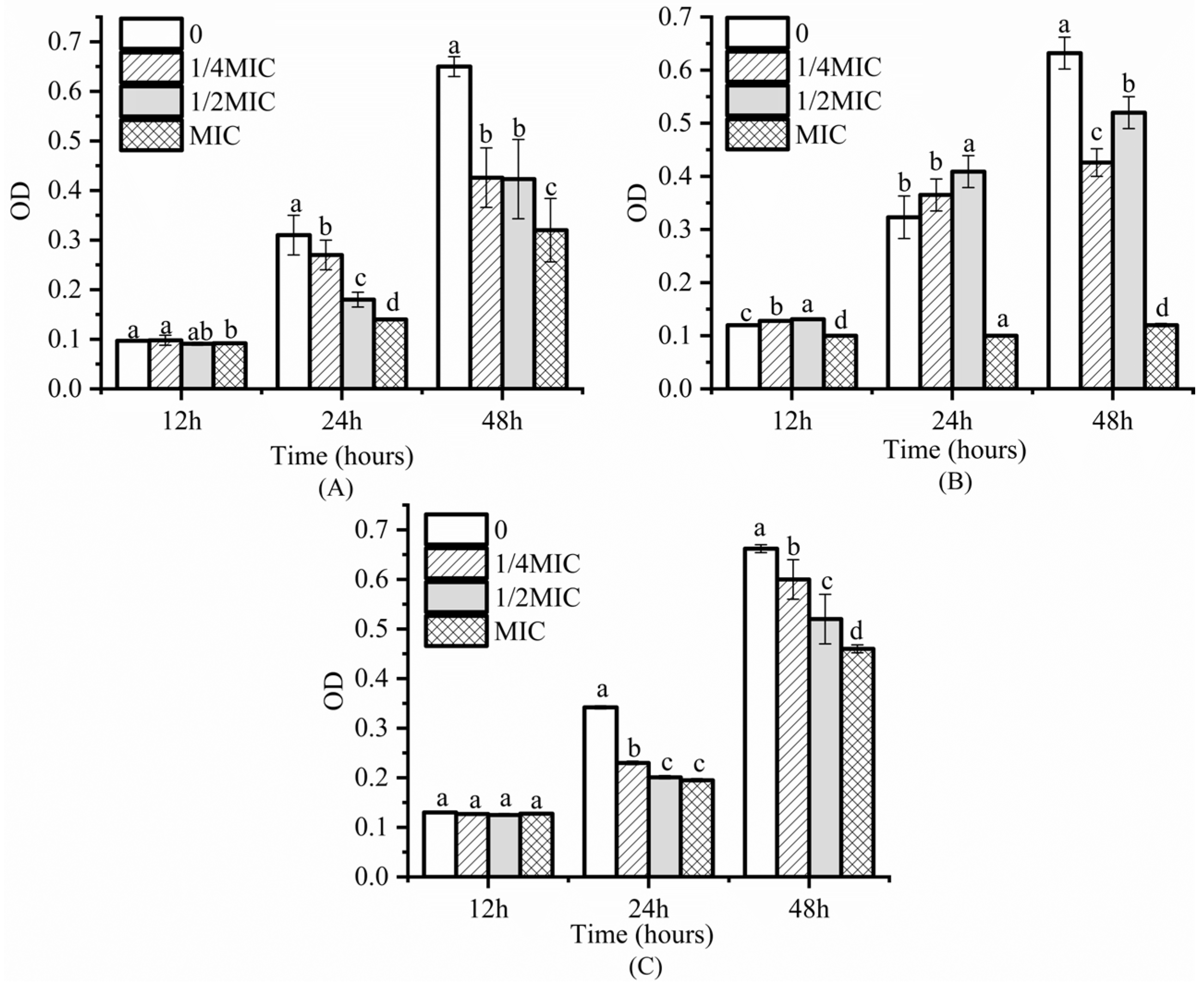
3.2. Inhibitory Effect on S. Enteritidis Biofilm
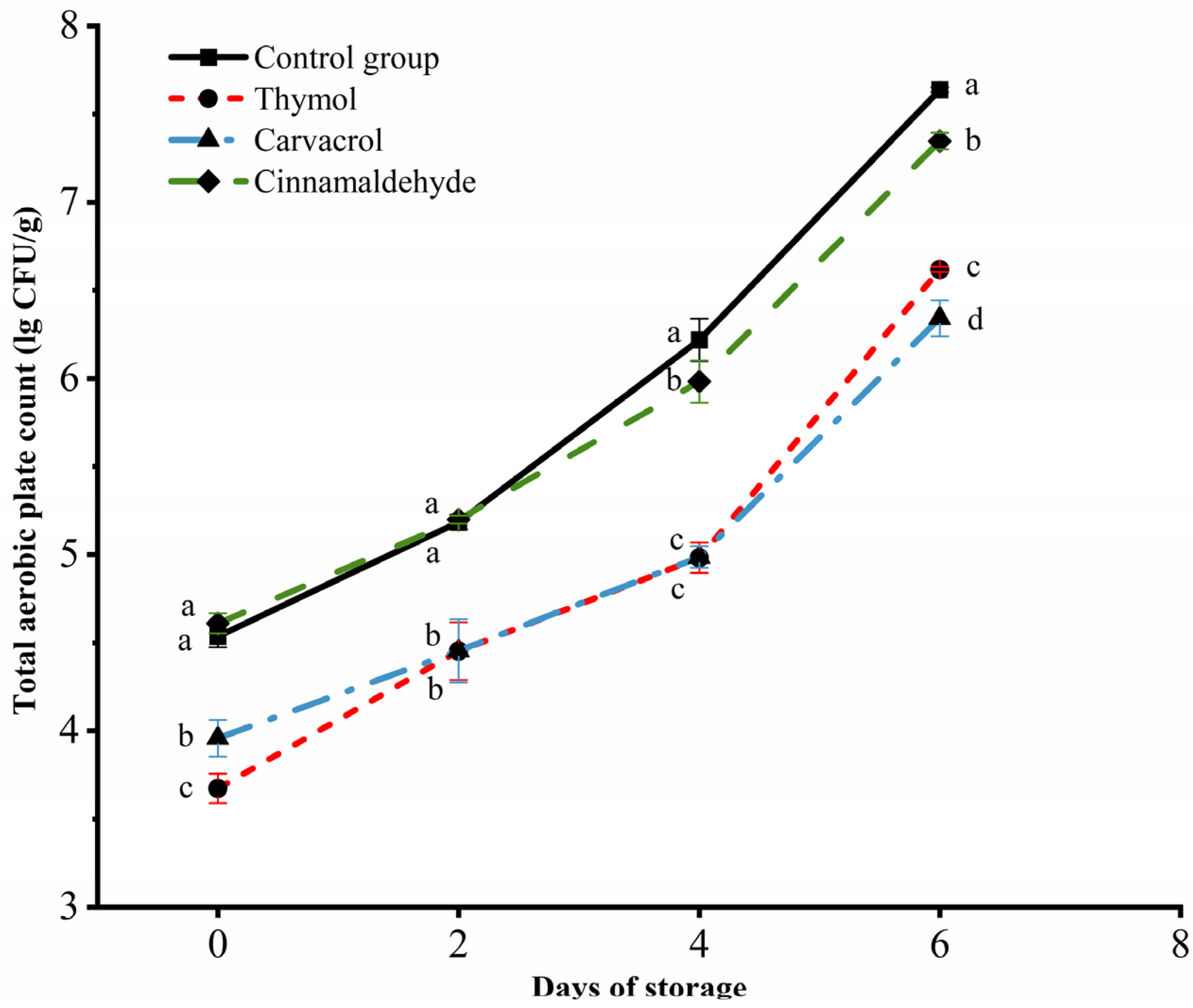
3.3. Changes in the Total Aerobic Plate Count of Chicken during Storage
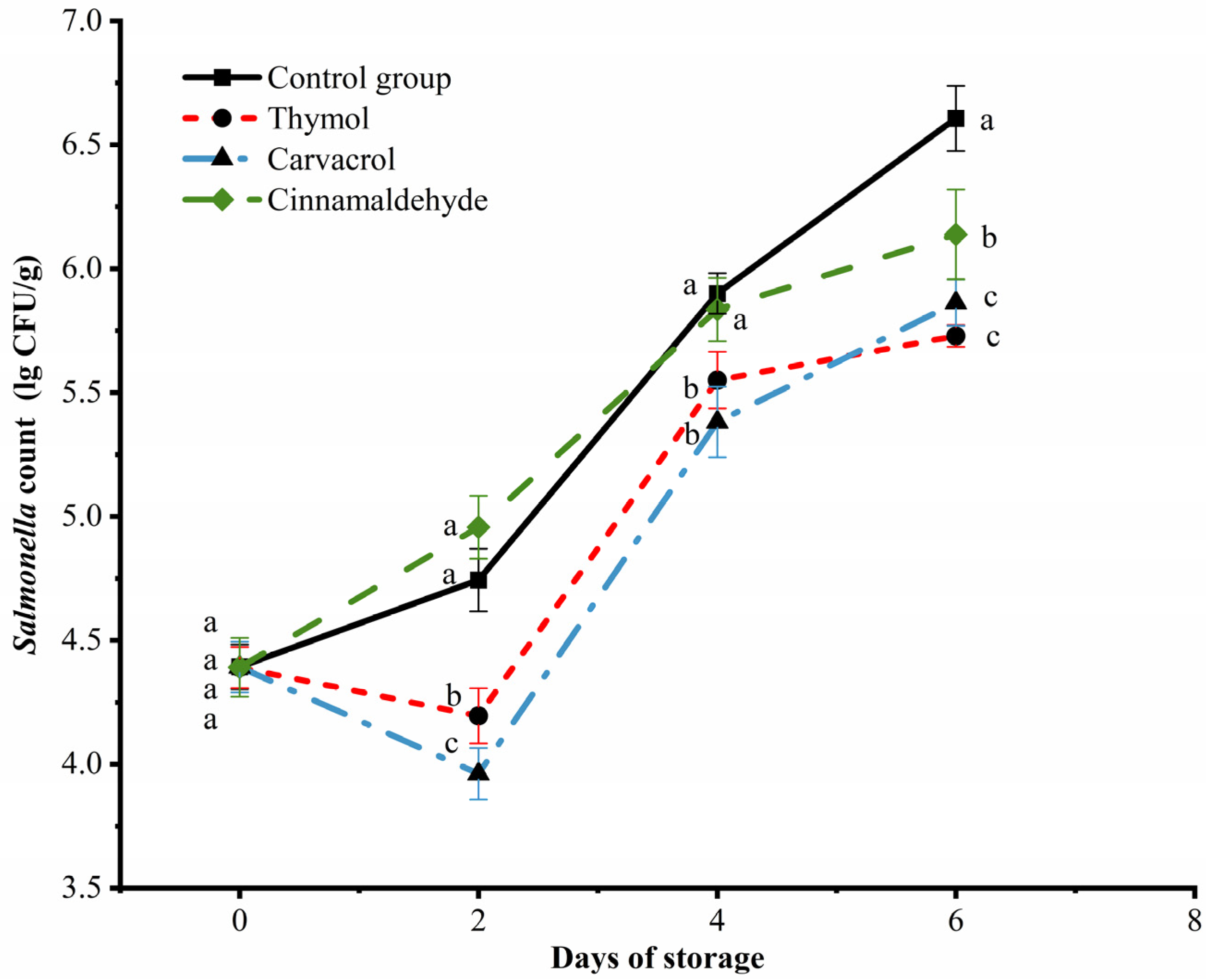
3.4. Changes in S. Enteritidis Count in Chicken Meat during Storage
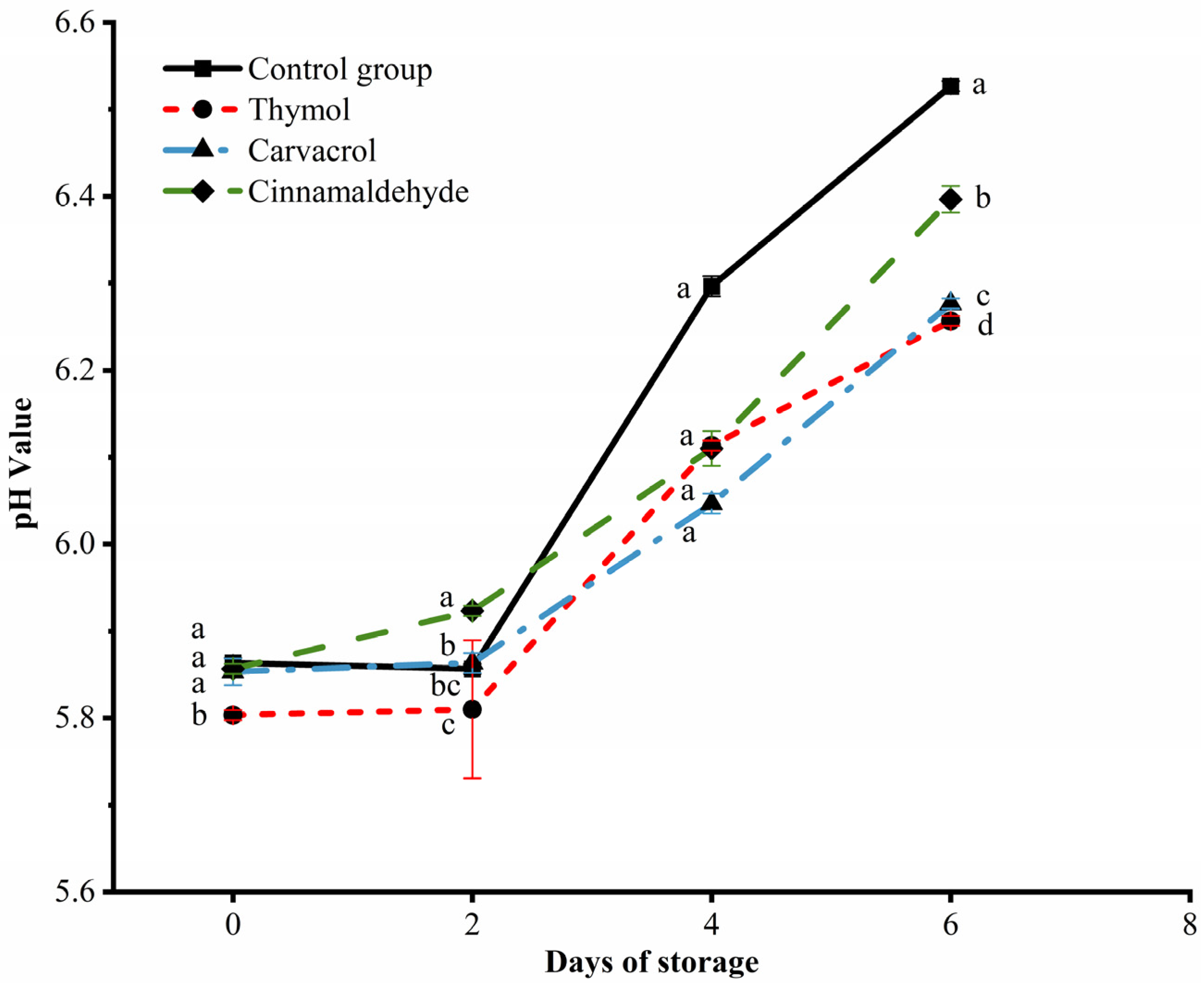
3.5. Changes in pH Values of Chicken during Storage
3.6. Changes of Moisture Content of Chicken during Storage
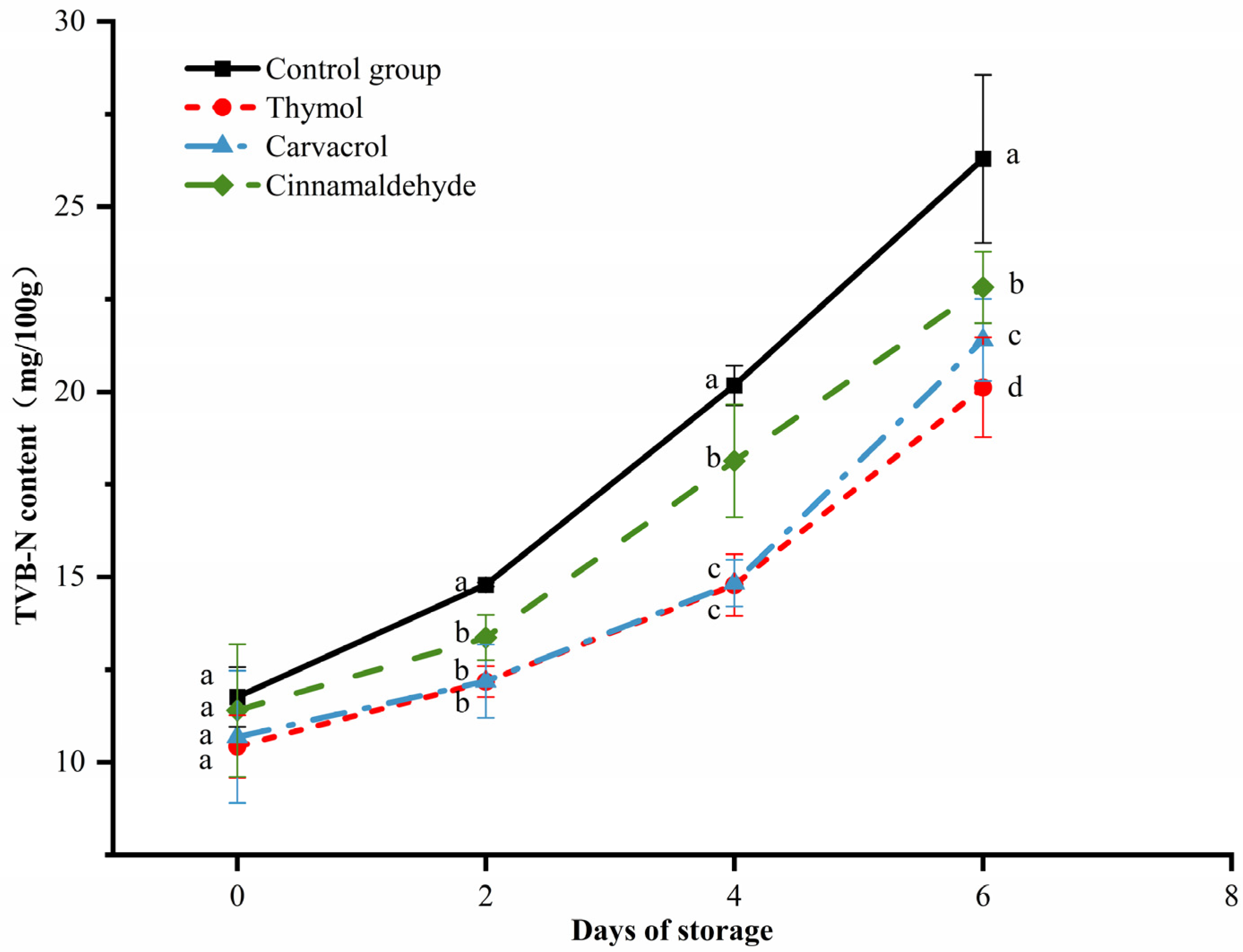
3.7. Changes in the TVB-N of Chicken during Storage
3.8. Changes in Chicken Color during Storage
3.9. Changes in the Sensory Indicators of Chicken during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Z.; Anwar, T.M.; Peng, X.; Biswas, S.; Elbediwi, M.; Li, Y.; Fang, W.; Yue, M. Prevalence and Antimicrobial Resistance of Salmonella Recovered from Pig-Borne Food Products in Henan, China. Food Control 2021, 121, 107535. [Google Scholar] [CrossRef]
- Hofer, U. Salmonella Enteritidis: Chicken or Egg? Nat. Rev. Microbiol. 2021, 19, 682. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Atwill, E.R.; Pitesky, M.; Huang, A.; Lavelle, K.; Rickard, M.; Shafii, M.; Hung-Fan, M.; Li, X. Antimicrobial Resistance Profiles of Non-Typhoidal Salmonella from Retail Meat Products in California, 2018. Front. Microbiol. 2022, 13, 835699. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration [FDA]. The National Antimicrobial Resistance Monitoring System. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef]
- Lin, L.; Mei, C.; Shi, C.; Li, C.; Abdel-Samie, M.A.; Cui, H. Preparation and Characterization of Gelatin Active Packaging Film Loaded with Eugenol Nanoparticles and Its Application in Chicken Preservation. Food Biosci. 2023, 53, 102778. [Google Scholar] [CrossRef]
- Hui, X.; Yan, G.; Tian, F.-L.; Li, H.; Gao, W.-Y. Antimicrobial Mechanism of the Major Active Essential Oil Compounds and Their Structure–Activity Relationship. Med. Chem. Res. 2017, 26, 442–449. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, X.; Zhao, S.; Li, S.; Zhang, J. Research Note: Preservative Effect of Compound Spices Extracts on Marinated Chicken. Poult. Sci. 2022, 101, 101778. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Chen, X.; Li, W.; Wang, L.; Li, W.; Du, J.; Zhang, S. Effects of Cinnamon Essential Oil on the Physiological Metabolism of Salmonella Enteritidis. Front. Microbiol. 2022, 13, 1035894. [Google Scholar] [CrossRef]
- Alibi, S.; Selma, W.B.; Mansour, H.B.; Navas, J. Activity of Essential Oils Against Multidrug-Resistant Salmonella Enteritidis. Curr. Microbiol. 2022, 79, 273. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Analysis of the Synergistic Antifungal Mechanism of Eugenol and Citral. LWT 2020, 123, 109128. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal Effect of Cinnamaldehyde, Eugenol and Carvacrol Nanoemulsion against Penicillium digitatum and Application in Postharvest Preservation of Citrus Fruit. LWT 2021, 141, 110924. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The Antibacterial Properties of Phenolic Isomers, Carvacrol and Thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhao, J.; Yan, X.; Zeng, Z.; Wan, D.; Yu, P.; Xia, J.; Zhang, G.; Gong, D. Synergistic Effects of Monocaprin and Carvacrol against Escherichia coli O157:H7 and Salmonella Typhimurium in Chicken Meat Preservation. Food Control 2022, 132, 108480. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, J.; Zhang, T.; Hati, S.; Mo, H.; Xu, D.; Li, H.; Hu, L.; Liu, Z. Characterization of Carvacrol Incorporated Antimicrobial Film Based on Agar/Konjac Glucomannan and Its Application in Chicken Preservation. J. Food Eng. 2022, 330, 111091. [Google Scholar] [CrossRef]
- Lian, H.; Shi, J.; Zhang, X.; Peng, Y. Effect of the Added Polysaccharide on the Release of Thyme Essential Oil and Structure Properties of Chitosan Based Film. Food Packag. Shelf Life 2020, 23, 100467. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; You, F.; Zhou, M.; Shi, S. Fabrication, Characterization, and Antimicrobial Activity of Carvacrol-Loaded Zein Nanoparticles Using the PH-Driven Method. IJMS 2022, 23, 9227. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Bellissimo, A.; Pinto, L.; Petrella, A.; De Vietro, N.; Iannaccone, G.; Baruzzi, F.; De Giglio, E. A Green Approach to Develop Zeolite-Thymol Antimicrobial Composites: Analytical Characterization and Antimicrobial Activity Evaluation. Heliyon 2022, 8, e09551. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, B.; Yan, J.; Qin, Y.; Yuan, M.; Cheng, G.; Yuan, M. Development of a Sodium Alginate-Based Active Package with Controlled Release of Cinnamaldehyde Loaded on Halloysite Nanotubes. Foods 2021, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, F.; Huang, J.; Li, Q.; Yang, Q.; Ju, J. Application of Essential Oils as Slow-Release Antimicrobial Agents in Food Preservation: Preparation Strategies, Release Mechanisms and Application Cases. Crit. Rev. Food Sci. Nutr. 2023, 1–26. [Google Scholar] [CrossRef]
- Duc, H.M.; Son, H.M.; Honjoh, K.; Miyamoto, T. Isolation and Application of Bacteriophages to Reduce Salmonella Contamination in Raw Chicken Meat. LWT 2018, 91, 353–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, J.; Ma, X.; Jia, C.; Han, J.; Song, C.; Liu, Y.; Wei, D.; Xu, H.; Qin, J.; et al. Nano-Emulsification Essential Oil of Monarda didyma L. to Improve Its Preservation Effect on Postharvest Blueberry. Food Chem. 2023, 417, 135880. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Waite-Cusic, J.; Huang, E. Control of Salmonella in Chicken Meat Using a Combination of a Commercial Bacteriophage and Plant-Based Essential Oils. Food Control 2020, 110, 106984. [Google Scholar] [CrossRef]
- Oliveira, G.R.; Oliveira, W.K.; Andrade, C.; Melo, A.D.B.; Luciano, F.B.; Macedo, R.E.F.; Costa, L.B. Natural Antimicrobials for Control of Salmonella Enteritidis in Feed and in Vitro Model of the Chicken Digestive Process. J. Anim. Physiol. Anim. Nutr. 2019, 103, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shen, Y.R.; Wu, S.; Xiao, Y.Q.; He, Q.; Shi, S.R. The Dietary Combination of Essential Oils and Organic Acids Reduces Salmonella Enteritidis in Challenged Chicks. Poult. Sci. 2019, 98, 6349–6355. [Google Scholar] [CrossRef]
- Paparella, A.; Mazzarrino, G.; Chaves-López, C.; Rossi, C.; Sacchetti, G.; Guerrieri, O.; Serio, A. Chitosan Boosts the Antimicrobial Activity of Origanum vulgare Essential Oil in Modified Atmosphere Packaged Pork. Food Microbiol. 2016, 59, 23–31. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Shao, X.; Huang, P.; Zha, J.; Ye, Y. Occurrence and Antimicrobial Resistance of Salmonella Isolated from Retail Meats in Anhui, China. Food Sci. Nutr. 2021, 9, 4701–4710. [Google Scholar] [CrossRef]
- Tang, X.; Shen, Y.; Song, X.; Benghezal, M.; Marshall, B.J.; Tang, H.; Li, H. Reassessment of the Broth Microdilution Method for Susceptibility Testing of Helicobacter pylori. J. Infect. Dis. 2022, 226, S486–S492. [Google Scholar] [CrossRef]
- Tu, Q.; Li, S.; Zeng, Z.; Liu, Y.; Wang, C.; Chen, S.; Hu, B.; Li, C. Cinnamon Essential Oil Liposomes Modified by Sodium Alginate-chitosan: Application in Chilled Pork Preservation. Int. J. Food Sci. Technol. 2023, 58, 939–953. [Google Scholar] [CrossRef]
- Catford, A.; Ganz, K.; Tamber, S. Enumerative Analysis of Salmonella in Outbreak-Associated Breaded and Frozen Comminuted Raw Chicken Products. J. Food Prot. 2017, 80, 814–818. [Google Scholar] [CrossRef]
- Reid, A. 2009 MFHPB-20: Isolation and Identification of Salmonella from Food and Environmental Samples. In HPB Methods for the Microbiologcal Analysis of Foods, Vol. 2. The Compendium of Analytical Methods. Health Canada, Ottawa, Ontario, Canada. World J. Microbiol. Biotechnol. 2020, 36, 24. [Google Scholar]
- Shatila, F.; Yaşa, İ.; Yalçın, H.T. Biofilm Formation by Salmonella Enterica Strains. Curr. Microbiol. 2021, 78, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Zduńczyk, W.; Tkacz, K.; Modzelewska-Kapituła, M. The Effect of Superficial Oregano Essential Oil Application on the Quality of Modified Atmosphere-Packed Pork Loin. Foods 2023, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Kang, T.; Sun, J.; Wang, J.; Zhao, W.; Gao, S.; Wang, W.; Ma, Q. Improving TVB-N Prediction in Pork Using Portable Spectroscopy with Just-in-Time Learning Model Updating Method. Meat Sci. 2022, 188, 108801. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Ding, J.; Dwibedi, V.; Huang, H.; Ge, Y.; Li, Y.; Li, Q.; Sun, T. Preparation and Antibacterial Mechanism of Cinnamaldehyde/Tea Polyphenol/Polylactic Acid Coaxial Nanofiber Films with Zinc Oxide Sol to Shewanella putrefaciens. Int. J. Biol. Macromol. 2023, 237, 123932. [Google Scholar] [CrossRef]
- Zhang, J.; Du, C.; Li, Q.; Hu, A.; Peng, R.; Sun, F.; Zhang, W. Inhibition Mechanism and Antibacterial Activity of Natural Antibacterial Agent Citral on Bamboo Mould and Its Anti-Mildew Effect on Bamboo. R. Soc. Open Sci. 2021, 8, 202244. [Google Scholar] [CrossRef]
- Čabarkapa, I.; Čolović, R.; Đuragić, O.; Popović, S.; Kokić, B.; Milanov, D.; Pezo, L. Anti-Biofilm Activities of Essential Oils Rich in Carvacrol and Thymol against Salmonella Enteritidis. Biofouling 2019, 35, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Mrenoshki, D.; Pellegrini, F.; Capozzi, L.; Cordisco, M.; Del Sambro, L.; Trotta, A.; Camero, M.; Tempesta, M.; Buonavoglia, D.; et al. Antibacterial and Biofilm Production Inhibition Activity of Thymus vulgaris L. Essential Oil against Salmonella Spp. Isolates from Reptiles. Pathogens 2023, 12, 804. [Google Scholar] [CrossRef]
- Misiou, O.; Van Nassau, T.J.; Lenz, C.A.; Vogel, R.F. The Preservation of Listeria -Critical Foods by a Combination of Endolysin and High Hydrostatic Pressure. Int. J. Food Microbiol. 2018, 266, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, Y.; Chen, H.; Song, R.; Li, Y. Performance of Eugenol Emulsion/Chitosan Edible Coating and Application in Fresh Meat Preservation. Food Process. Preserv. 2022, 46, e16407. [Google Scholar] [CrossRef]
- Moon, H.; Kim, N.H.; Kim, S.H.; Kim, Y.; Ryu, J.H.; Rhee, M.S. Teriyaki Sauce with Carvacrol or Thymol Effectively Controls Escherichia coli O157:H7, Listeria monocytogenes, Salmonella typhimurium, and Indigenous Flora in Marinated Beef and Marinade. Meat Sci. 2017, 129, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Pennesi, D.; Wu, C. The Effect of Thymol and Thyme Oil Feed Supplementation on Growth Performance, Serum Antioxidant Levels, and Cecal Salmonella Population in Broilers. J. Appl. Poult. Res. 2010, 19, 432–443. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Zhao, R.; Wang, J.; Cheng, Y.; Liu, Q.; Wang, Y.; Yang, S. Synergistic Interaction Between Paired Combinations of Natural Antimicrobials Against Poultry-Borne Pathogens. Front. Microbiol. 2022, 13, 811784. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, H.; Liu, K.; Cao, H.; Mao, H.; Dong, X.; Yin, Z. Analysis of the Physical Meat Quality in Partridge (Alectoris chukar) and Its Relationship with Intramuscular Fat. Poult. Sci. 2020, 99, 1225–1231. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, J.; Li, S.; Hamzah, S.S.; Jiang, H.; Zhou, A.; Zeng, S.; Lin, S. Curcumin-Based Photodynamic Sterilization for Preservation of Fresh-Cut Hami Melon. Molecules 2019, 24, 2374. [Google Scholar] [CrossRef]
- Sari, T.V.; Hasanah, U.; Harahap, R.I.H.; Zalukhu, P.; Trisna, A. Chemical and Physical Quality of Broiler Meat with Drinking Water Containing Boiled and Fermented Water of Various Cooking Spices as Phytobiotics. IOP Conf. Ser. Earth Environ. Sci. 2022, 977, 012137. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total Volatile Basic Nitrogen (TVB-N) and Its Role in Meat Spoilage: A Review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Majdinasab, M.; Niakousari, M.; Shaghaghian, S.; Dehghani, H. Antimicrobial and Antioxidant Coating Based on Basil Seed Gum Incorporated with Shirazi Thyme and Summer Savory Essential Oils Emulsions for Shelf-Life Extension of Refrigerated Chicken Fillets. Food Hydrocoll. 2020, 108, 106011. [Google Scholar] [CrossRef]
- Hematizad, I.; Khanjari, A.; Basti, A.A.; Karabagias, I.K.; Noori, N.; Ghadami, F.; Gholami, F.; Teimourifard, R. In Vitro Antibacterial Activity of Gelatin-Nanochitosan Films Incorporated with Zataria multiflora Boiss Essential Oil and Its Influence on Microbial, Chemical, and Sensorial Properties of Chicken Breast Meat during Refrigerated Storage. Food Packag. Shelf Life 2021, 30, 100751. [Google Scholar] [CrossRef]
- Tomasevic, I.; Djekic, I.; Font-i-Furnols, M.; Terjung, N.; Lorenzo, J.M. Recent Advances in Meat Color Research. Curr. Opin. Food Sci. 2021, 41, 81–87. [Google Scholar] [CrossRef]
- Ijaz, M.; Li, X.; Zhang, D.; Hussain, Z.; Ren, C.; Bai, Y.; Zheng, X. Association between Meat Color of DFD Beef and Other Quality Attributes. Meat Sci. 2020, 161, 107954. [Google Scholar] [CrossRef] [PubMed]
| Indicators | Score | Scoring Criteria |
|---|---|---|
| Color | 8–10 points | Light red, glossy surface |
| 6–7 points | Slightly dim, slightly shiny surface | |
| 6 points or fewer | Dull, surface matte | |
| Odor | 8–10 points | Normal smell of chicken, no peculiar smell |
| 6–7 points | Slightly smelly | |
| 6 points or fewer | Fishy odor or ammonia odor | |
| Stickiness | 8–10 points | Moist, not sticky |
| 6–7 points | Wetter, not sticky | |
| 6 points or fewer | Sticky hands | |
| Overall acceptability | 8–10 points | Willing to accept |
| 6–7 points | Acceptable | |
| 6 points or fewer | Unacceptable |
| Essential Oil Main Ingredients | Thymol | Carvacrol | Limonene | Citral | Cinnamaldehyde | β-Pinene |
|---|---|---|---|---|---|---|
| MIC (μg/mL) | 128 | 256 | 1024 | 512 | 128 | 2048 |
| Parameter | Processing Groups | Days of Storage | |||
|---|---|---|---|---|---|
| 0 d | 2 d | 4 d | 6 d | ||
| Moisture content | 0 | 0.75 Aa | 0.74 Bb | 0.74 Bb | 0.74 Bb |
| Thymol | 0.75 ABa | 0.75 Bab | 0.76 Aa | 0.75 ABa | |
| Carvacrol | 0.75 Aa | 0.75 Aab | 0.76 Aa | 0.76 Aa | |
| Cinnamaldehyde | 0.74 Ba | 0.75 ABa | 0.75 ABb | 0.76 Aa | |
| Parameter | Processing Groups | Days of Storage | |||
|---|---|---|---|---|---|
| 0 d | 2 d | 4 d | 6 d | ||
| L* values | 0 | 54.87 Da | 56.37 Cb | 57.40 Bb | 55.27 Ab |
| Thymol | 54.61 Da | 58.09 Ca | 58.42 Ba | 57.03 Aa | |
| Carvacrol | 54.86 Da | 57.93 Ca | 58.34 Ba | 56.21 Aab | |
| Cinnamaldehyde | 54.69 Da | 55.45 Cc | 57.36 Bb | 55.38 Abc | |
| a* values | 0 | 5.13 Da | 4.55 Cb | 4.10 Bb | 3.49 Ab |
| Thymol | 5.12 Da | 4.91 Ca | 4.78 Ba | 4.10 Aa | |
| Carvacrol | 5.07 Da | 4.99 Ca | 4.73 Ba | 3.74 Ab | |
| Cinnamaldehyde | 5.14 Da | 4.84 Ca | 4.13 Bb | 3.58 Ab | |
| b* values | 0 | 31.54 Da | 32.71 Ca | 34.50 Ba | 35.93 Aa |
| Thymol | 31.14 Dab | 32.12 Cab | 33.11 Bb | 34.60 Ac | |
| Carvacrol | 30.92 Db | 31.51 Cb | 32.31 Bc | 35.30 Ab | |
| Cinnamaldehyde | 31.29 Dab | 32.49 Ca | 34.34 Ba | 35.86 Aa | |
| Sensory Indicators | Processing Groups | 0 d | 2 d | 4 d | 6 d |
|---|---|---|---|---|---|
| Color | Control group | 9.50 ± 0.84 Aa | 8.67 ± 1.21 Aa | 6.00 ± 0.89 Bb | 4.33 ± 0.82 Cb |
| Thymol | 9.50 ± 0.84 Aa | 9.17 ± 0.98 Aa | 7.33 ± 0.82 Ba | 6.17 ± 0.75 Ca | |
| Carvacrol | 9.33 ± 0.82 Aa | 9.17 ± 0.95 Aa | 7.83 ± 0.75 Ba | 5.83 ± 0.75 Ca | |
| Cinnamaldehyde | 9.33 ± 0.82 Aa | 8.83 ± 0.98 Aa | 6.83 ± 0.75 Bab | 4.16 ± 0.75 Cb | |
| Odor | Control group | 9.67 ± 0.52 Aa | 8.67 ± 1.03 Aa | 4.00 ± 0.89 Bb | 2.33 ± 0.82 Cb |
| Thymol | 9.83 ± 0.41 Aa | 9.00 ± 0.89 Aa | 6.50 ± 1.05 Ba | 3.17 ± 0.75 Cab | |
| Carvacrol | 9.67 ± 0.82 Aa | 9.17 ± 0.75 Aa | 5.67 ± 0.82 Ba | 3.50 ± 0.55 Ca | |
| Cinnamaldehyde | 9.50 ± 0.84 Aa | 8.83 ± 0.75 Aa | 3.83 ± 0.75 Bb | 2.50 ± 0.84 Cb | |
| Stickiness | Control group | 9.83 ± 0.41 Aa | 8.33 ± 0.52 Bb | 6.83 ± 0.75 Cb | 5.83 ± 0.75 Da |
| Thymol | 9.67 ± 0.52 Aa | 9.50 ± 0.84 Aa | 8.50 ± 1.05 Ba | 6.83 ± 0.75 Ca | |
| Carvacrol | 9.83 ± 0.41 Aa | 9.33 ± 1.03 ABab | 8.33 ± 1.37 Ba | 6.67 ± 1.03 Ca | |
| Cinnamaldehyde | 9.83 ± 0.41 Aa | 9.17 ± 0.98 ABab | 8.17 ± 0.98 Ba | 6.50 ± 0.84 Ca | |
| Overall acceptability | Control group | 9.84 ± 0.41 Aa | 8.33 ± 1.03 Ba | 5.33 ± 0.82 Cb | 2.83 ± 0.75 Da |
| Thymol | 9.67 ± 0.52 Aa | 9.33 ± 0.82 Aa | 7.17 ± 0.75 Ba | 3.50 ± 1.05 Ca | |
| Carvacrol | 9.67 ± 0.82 Aa | 9.00 ± 0.89 Aa | 7.00 ± 0.63 Ba | 3.50 ± 0.84 Ca | |
| Cinnamaldehyde | 9.67 ± 0.52 Aa | 9.00 ± 0.89 Aa | 5.67 ± 1.03 Bb | 3.00 ± 1.26 Ca |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, T.; Chen, J.; Ye, Y. Inhibition of Salmonella Enteritidis by Essential Oil Components and the Effect of Storage on the Quality of Chicken. Foods 2023, 12, 2560. https://doi.org/10.3390/foods12132560
Wang W, Li T, Chen J, Ye Y. Inhibition of Salmonella Enteritidis by Essential Oil Components and the Effect of Storage on the Quality of Chicken. Foods. 2023; 12(13):2560. https://doi.org/10.3390/foods12132560
Chicago/Turabian StyleWang, Wu, Tingting Li, Jing Chen, and Yingwang Ye. 2023. "Inhibition of Salmonella Enteritidis by Essential Oil Components and the Effect of Storage on the Quality of Chicken" Foods 12, no. 13: 2560. https://doi.org/10.3390/foods12132560
APA StyleWang, W., Li, T., Chen, J., & Ye, Y. (2023). Inhibition of Salmonella Enteritidis by Essential Oil Components and the Effect of Storage on the Quality of Chicken. Foods, 12(13), 2560. https://doi.org/10.3390/foods12132560







